Abstract
An original design and synthesis of fluorescent ligands for melatonin receptor studies is presented and consists in the fusion of the endogenous ligand with the fluorescent BODIPY core. Probes I–IV show high affinities for MT1 and MT2 melatonin receptors and exhibit fluorescence properties compatible with cell observation.
Keywords: GPCRs, melatonin, fluorescence, molecular probes, BODIPY
Fluorescence is one of the most sensitive spectroscopic methods and many fluorescent ligands have been reported for locating G-protein-coupled receptors (GPCRs), for studying ligand/receptor interactions, and more generally for better understanding their pharmacology and physiological process.1−4 The history of fluorescent ligands is linked to the development of commercially available fluorophores. Organic dyes have been designed and synthesized to exhibit excitation and emission wavelengths, which are compatible with biological observation and are associated to ligands in conjugation reactions. The addition of such a distinct fluorescent molecule may alter both the chemical (while remaining within a spectrum of lipophilicity to hydrophilicity) and pharmacological (affinity, functionality, etc.) properties of the resulting fluorescent ligand that will modify its cellular behavior.
The melatonin receptors MT1 and MT2 are members of the GPCR family. They are involved in the regulation of the circadian rhythm and seasonal functions in mammals. They are also implicated in many biological processes ranging from anti-inflammatory to antioxidant effects including anti-Parkinson effects5,6 and were recently reported as part of the mechanism of action of the antidepressant agomelatine, an MT1 and MT2 receptor agonist and 5-HT2C antagonist.7,8 Despite the discovery of the high affinity agonist and nonselective 2-[125I]-MLT radioligand9 research on the pharmacology and the functionality/physiological impact of melatonin receptors suffers from the lack of selective probes for these receptors due to their very low level of expression. Moreover, the main disadvantages of this method are the radioactive hazards and the limitations of studying the molecular dynamics of receptor activation. To offer alternative probes, we have developed a concept aiming at using the aromatic core of an endogenous ligand as the source of fluorescence after slight chemical modification and without loss of biological activity. Herein, we report the design and synthesis of fluorescent ligands for melatonin receptor studies thanks to the fusion of the endogenous ligand with the fluorescent BODIPY core.
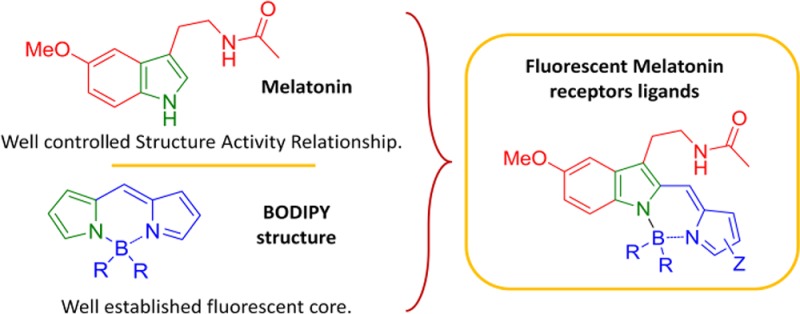
Melatonin presents in its chemical structure an indole ring possessing fluorescent properties that are unfortunately inappropriate for biological analysis due to interferences from other biochromophores (such as tryptophan).10,11 Our expertise on the melatonin structure/activity relationship12−16 prompts us to investigate the extension of the π-conjugation of the indole scaffold at position C-2 in order to obtain biologically compatible photophysical properties. The original idea consists in the fusion of the pyrrole ring of melatonin with one of the pyrrole rings of the highly stable and bright difluoro-boraindacene (BODIPY) fluorophore17−19 (Figure 1). The fluorescence of the resulting indole-based BODIPY was expected as recently described by Zhao, Zhu, and co-workers.20,21
Figure 1.
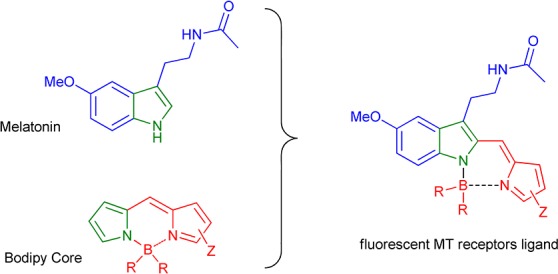
Fused melatonin-BODIPY.
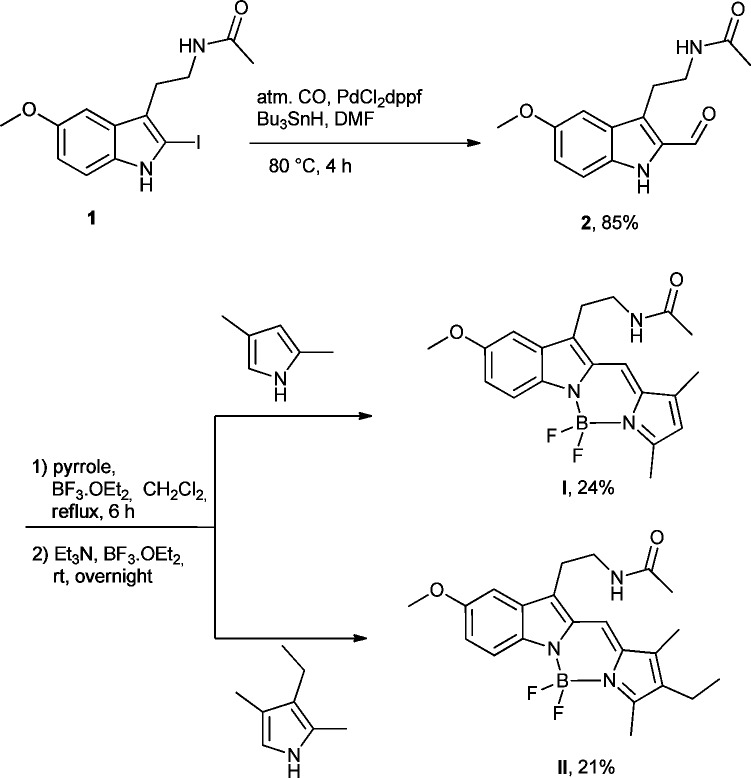
To attempt the fused melatonin-BODIPY core, 2-iodomelatonin 1,22−24 was converted into 2-formylmelatonin 2 by a palladium catalyzed carbonylative coupling reaction in the presence of tributyltin hydride in good yields using the Fukuyama procedure.25 Condensation of the pyrroles with 2 under traditional experimental conditions using acidic catalyst (POCl3, HBr) was unable to furnish the desired product. Considering the final difluoroborane complex, we envisaged the direct activation of the carbonyl function in compound 2 with boron trifluoroborate etherate.26 The Lewis acid was added slowly at low temperature to the solution of 2-formylmelatonin 2, and after 15 min the pyrrole was added. Synthesis of the final boron complex was achieved by adding triethylamine with six extra equivalents of BF3·Et2O. The first two desired structures I and II were isolated in 21% and 24% yield, respectively (Scheme 1).
Scheme 1. Synthetic Pathway to Molecules I and II.
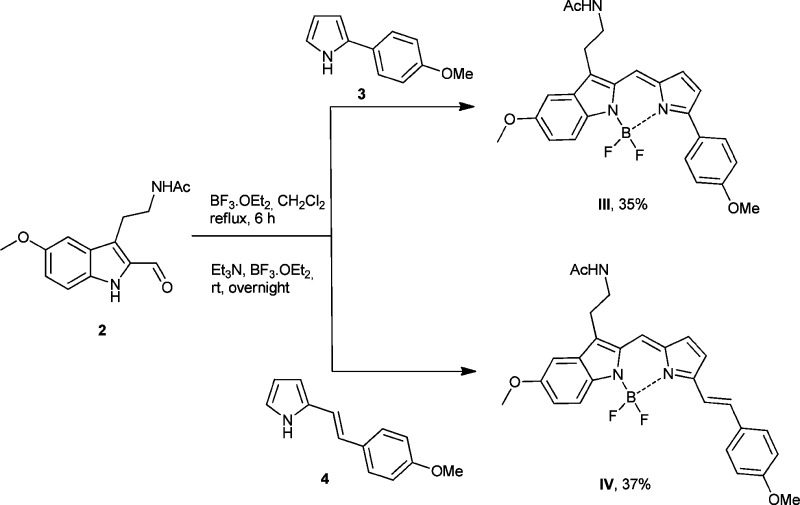
Accentuation of the conjugation in BODIPY is known to induce a red-shift in the excitation and emission wavelengths, which is more compatible for biological observations.17−19,27,28 Such a π extension can be performed by introducing an aryl or a hetaryl group at the 3 and/or 5 positions of the pyrrole moiety using metal catalyzed reactions,29−32 the Knoevenagel reaction,33,34 vicarious nucleophilic substitution (VNS),35 or by modifying the substituents linked to the boron atom.36 For our purpose, aryl and styryl groups were envisaged and pyrroles 3 and 4 were first synthesized. Condensation with 2-formylmelatonin according to the previous protocol finally afforded the desired boron complexes III and IV with extended π-delocalization in 35 and 37% yield, respectively (Scheme 2).
Scheme 2. Synthetic Pathway to Molecules III and IV.
The photophysical properties of these new dyes were measured in dimethylsulfoxide. Absorption (Figure 2) and molar extinction coefficients of compounds I–IV are reported in Table 1.
Figure 2.
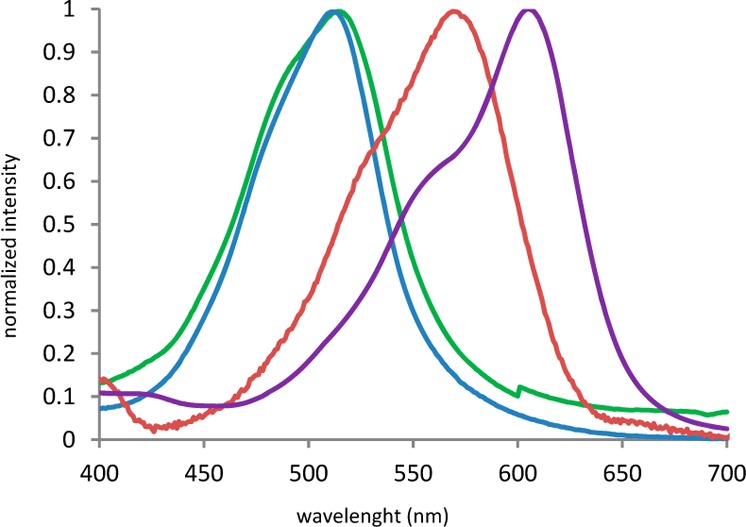
Normalized absorption of compounds I (green), II (blue), III (red), and IV (purple) in DMSO.
Table 1. Absorption Wavelengths and Molar Extinction Coefficients of Compounds I–IV in DMSO.
| compd | I | II | III | IV |
|---|---|---|---|---|
| absmax (nm) | 515 | 512 | 569 | 604 |
| ελmax (L·mol–1·cm–1) | 31094 | 32970 | 29544 | 31230 |
All four boron complexes show fluorescent properties. The emission spectra (Figure 3) for I and II are around 490–540 nm. Compounds III and IV, with extra π-conjugation, show excitation and emission bands at lower energy as expected. These new indole-based BODIPYs present Stokes shifts between 21 and 111 nm comparable with standard BODIPY dye values.17−19
Figure 3.
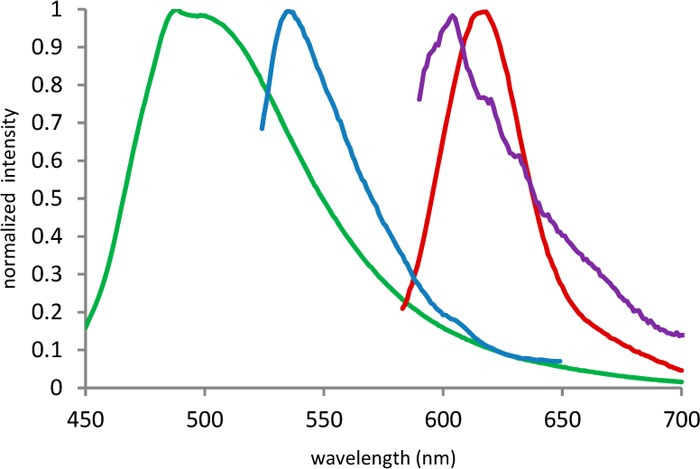
Normalized fluorescence emission spectra properties of compounds I (green), II (blue), III (red), and IV (purple) in DMSO (for λexc, see Table 2).
Table 2. Photophysical Properties of Compounds I–IV in DMSO.
| compd | I | II | III | IV |
|---|---|---|---|---|
| λexc (nm) | 426 | 424 | 573 | 583 |
| λem (nm) | 493 | 535 | 616 | 604 |
| Stokes shift (nm) | 67 | 111 | 43 | 21 |
The binding affinities of the four fluorescent derivatives I–IV were evaluated (Table 3) on human MT1 and MT2 receptors. They all show good affinities: ligands I and IV present affinities in the range of tens of nanomolar concentrations for the two receptors, while ligand II is more selective for the MT2 receptor, and ligand III displays a larger MT1 receptor selectivity.
Table 3. Binding Affinity of Compounds I–IV on Human MT1 and MT2 Receptors.
| compd | MT1Ki ± SEM (nM) | MT2Ki ± SEM (nM) |
|---|---|---|
| I | 32 ± 5 | 10 ± 0.7 |
| II | 256 ± 40 | 96 ± 20 |
| III | 49 ± 15 | 315 ± 9 |
| IV | 71 ± 15 | 26 ± 1 |
In conclusion, by fusing the endogenous ligand of melatonin receptors with the well-known and efficient fluorescent BODIPY core, we have been able to design and isolate four new condensed fluorescent probes with good melatonin receptor affinities. Extension of the π-conjugation of ligands I and II by coupling with an aryl (ligand III) or a styryl group (ligand IV) induces a bathochromic shift with slight impact on the affinity. Cellular imaging studies are currently under way.
Acknowledgments
The authors would like to thank Dr. S. Poupart for her advice.
Supporting Information Available
Experimental details for the synthesis and the characterization of ligand I–IV and intermediates, spectroscopic data, and pharmacology. This material is available free of charge via the Internet at http://pubs.acs.org.
This work was supported by La Région Centre (APR2009-LOIREMEL). During this study, J.T. and J.M. were supported (Ph.D grants) by the Conseil Général du Loiret (J.T.) and La Région Centre (J.M., APR2012-LIFERMEL)
The authors declare no competing financial interest.
Supplementary Material
References
- Kuder K.; Kieć-Kononowicz K. Fluorescent GPCR Ligands as New Tools in Pharmacology. Curr. Med. Chem. 2008, 15, 2132–2143. [DOI] [PubMed] [Google Scholar]
- Böhme I.; Beck-Sickinger A. G. Illuminating the Life of GPCRs. Cell Commun. Signal 2009, 7, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard A. D.; Watts A. Contributions of Fluorescence Techniques to Understanding G Protein-Coupled Receptor Dimerisation. Biophys. Rev. 2012, 4, 291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobs D.; Sorkalla T.; Häberlein H. Ligands for Fluorescence Correlation Spectroscopy on g Protein-Coupled Receptors. Curr. Med. Chem. 2012, 19, 4722–4730. [DOI] [PubMed] [Google Scholar]
- Genovese T.; Mazzon E.; Muià C.; Bramanti P.; De Sarro A.; Cuzzocrea S. Attenuation in the Evolution of Experimental Spinal Cord Trauma by Treatment with Melatonin. J. Pineal Res. 2005, 38, 198–208. [DOI] [PubMed] [Google Scholar]
- Delagrange P.; Atkinson J.; Boutin J. A.; Casteilla L.; Lesieur D.; Misslin R.; Pellissier S.; Pénicaud L.; Renard P. Therapeutic Perspectives for Melatonin Agonists and Antagonists. J. Neuroendocrinol. 2003, 15, 442–448. [DOI] [PubMed] [Google Scholar]
- De Bodinat C.; Guardiola-Lemaitre B.; Mocaër E.; Renard P.; Muñoz C.; Millan M. J. Agomelatine, the First Melatonergic Antidepressant: Discovery, Characterization and Development. Nature Rev. Drug Discovery 2010, 9, 628–642. [DOI] [PubMed] [Google Scholar]
- Spadoni G.; Bedini A.; Rivara S.; Mor M. Melatonin Receptor Agonists: New Options for Insomnia and Depression Treatment. CNS Neurosci. Ther. 2011, 17, 733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakkuri O.; Lämsä E.; Rahkamaa E.; Ruotsalainen H.; Leppäluoto J. Iodinated Melatonin: Preparation and Characterization of the Molecular Structure by Mass and 1H NMR Spectroscopy. Anal. Biochem. 1984, 142, 284–289. [DOI] [PubMed] [Google Scholar]
- Wu P.-W.; Hsieh W.-T.; Cheng Y.-M.; Wei C.-Y.; Chou P.-T. Synthesis of 7-Azaserotonin: Its Photophysical Properties Associated with Excited State Proton Transfer Reaction. J. Am. Chem. Soc. 2006, 128, 14426–144327. [DOI] [PubMed] [Google Scholar]
- Wu P.-W.; Cheng Y.-M.; Hsieh W.-T.; Wang Y.-H.; Wei C.-Y.; Chou P.-T. 7-Azamelatonin: Efficient Synthetic Routes, Excited-state Double Proton Transfer Properties and Biomedical Implications. ChemMedChem 2007, 2, 1071–1075. [DOI] [PubMed] [Google Scholar]
- Legros C.; Matthey U.; Grelak T.; Pedragona-Moreau S.; Hassler W.; Yous S.; Thomas E.; Suzenet F.; Folleas B.; Lefoulon F.; Berthelot P.; Caignard D.-H.; Guillaumet G.; Delagrange P.; Brayer J.-L.; Nosjean O.; Boutin J. A. New Radioligands for Describing the Molecular Pharmacology of MT1 and MT2 Melatonin Receptors. Int. J. Mol. Sci. 2013, 14, 8948–8962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanty M.; Suzenet F.; Delagrange P.; Nosjean O.; Boutin J. A.; Caignard D. H.; Guillaumet G. Design and Synthesis of 1-(2-Alkanamidoethyl)-6-methoxy-7-azaindole Derivatives as Potent Melatonin Agonists. Bioorg. Med. Chem. Lett. 2011, 21, 2316–2319. [DOI] [PubMed] [Google Scholar]
- El Kazzouli S.; Griffon du Bellay A.; Berteina-Raboin S.; Delagrange P.; Caignard D.-H.; Guillaumet G. Design and Synthesis of 2-Phenylimidazo[1,2-a]pyridines as a Novel Class of Melatonin Receptor Ligands. Eur. J. Med. Chem. 2011, 46, 4252–4257. [DOI] [PubMed] [Google Scholar]
- Suzenet F.; Guillaumet G.; Jeanty M.; Delagrange P.; Caignard D.-H.; Spedding M. Derives Indoles, leur Procede de Preparation et les Compositions Pharamaceutiques qui les Contiennent. WO2010061074A1, 2010.
- Jeanty M.; Blu J.; Suzenet F.; Guillaumet G. Synthesis of 4- and 6-Azaindoles via the Fischer Reaction. Org. Lett. 2009, 11, 5142–5145. [DOI] [PubMed] [Google Scholar]
- Loudet A.; Burgess K. BODIPY Dyes and Their Derivatives: Syntheses and Spectroscopic Properties. Chem. Rev. 2007, 107, 4891–4932. [DOI] [PubMed] [Google Scholar]
- Ulrich G.; Ziessel R.; Harriman A. The Chemistry of Fluorescent BODIPY Dyes: Versatility Unsurpassed. Angew. Chem., Int. Ed. 2008, 47, 1184–1201. [DOI] [PubMed] [Google Scholar]
- Boens N.; Leen V.; Dehaen W. Fluorescent Indicators Based on BODIPY. Chem. Soc. Rev. 2012, 41, 1130–1172. [DOI] [PubMed] [Google Scholar]
- Zhao C.; Zhou Y.; Lin Q.; Zhu L.; Feng P.; Zhang Y.; Cao J. Development of an Indole-Based Boron-Dipyrromethene Fluorescent Probe for Benzenethiols. J. Phys. Chem. B 2011, 115, 642–647. [DOI] [PubMed] [Google Scholar]
- Zhao C.; Feng P.; Cao J.; Zhang Y.; Wang X.; Yang Y.; Zhang Y.; Zhang J. 6-Hydroxyindole-based Borondipyrromethene: Synthesis and Spectroscopic Studies. Org. Biomol. Chem. 2012, 10, 267–272. [DOI] [PubMed] [Google Scholar]
- Franschini F.; Stankov B.; Di Bella L.; Duranti E.; Lagguzzi A.. Contraceptive And Menstrual Cycle Controlling Drug Having Oncostatic Properties. EP0483077A2, 1992.
- Leclerc V.; Yous S.; Delagrange P.; Boutin J. A.; Renard P.; Lesieur D. Synthesis of Nitroindole Derivatives with High Affinity and Selectivity for Melatoninergic Binding Sites MT3. J. Med. Chem. 2002, 45, 1853–1859. [DOI] [PubMed] [Google Scholar]
- Baran P. S.; Shenvi R. A. Total Synthesis of (±)-Chartelline C. J. Am. Chem. Soc. 2006, 128, 14028–14029. [DOI] [PubMed] [Google Scholar]
- Tokuyama H.; Kaburagi Y.; Chen X.; Fukuyama T. Synthesis of 2,3-Disubstituted Indoles by Palladium-Mediated Coupling of 2-Iodoindoles. Synthesis 2000, 429–434. [Google Scholar]
- Li Z.; Mintzer E.; Bittman R. First Synthesis of Free Cholesterol-BODIPY Conjugates. J. Org. Chem. 2006, 71, 1718–1721. [DOI] [PubMed] [Google Scholar]
- Dost Z.; Atilgan S.; Akkaya E. U. Distyryl-Boradiazaindacenes: Facile Synthesis of Novel Near IR Emitting Fluorophores. Tetrahedron 2006, 62, 8484–8488. [Google Scholar]
- Baruah M.; Qin W.; Flors C.; Hofkens J.; Vallée R. A. L.; Beljonne D.; Van der Auweraer M.; De Borggraeve W. M.; Boens N. Solvent and pH Dependent Fluorescent Properties of a Dimethylaminostyryl Borondipyrromethene Dye in Solution. J. Phys. Chem. A 2006, 110, 5998–6009. [DOI] [PubMed] [Google Scholar]
- Rohand T.; Qin W.; Boens N.; Dehaen W. Palladium-Catalyzed Coupling Reactions for the Functionalization of BODIPY Dyes with Fluorescence Spanning the Visible Spectrum. Eur. J. Org. Chem. 2006, 20, 4658–4663. [Google Scholar]
- Cho D. W.; Fujitsuka M.; Ryu J. H.; Lee M. H.; Kim H. K.; Majima T.; Im C. S2 Emission from Chemically Modified BODIPYs. Chem. Commun. 2012, 48, 3424–3426. [DOI] [PubMed] [Google Scholar]
- Chen J.; Mizumura M.; Shinokubo H.; Osuka A. Functionalization of Boron Dipyrrin (BODIPY) Dyes Through Iridium and Rhodium Catalysis: a Complementary Approach to Alpha- and Beta-substituted BODIPYs. Chem.—Eur. J. 2009, 15, 5942–5949. [DOI] [PubMed] [Google Scholar]
- Han J.; Gonzalez O.; Aguilar-Aguilar A.; Peña-Cabrera E.; Burgess K. 3- and 5-Functionalized BODIPYs via the Liebeskind–Srogl Reaction. Org. Biomol. Chem. 2009, 7, 34–36. [DOI] [PubMed] [Google Scholar]
- Bura T.; Hablot D.; Ziessel R. Fluorescent Boron Dipyrromethene (BODIPY) Dyes Having Two and Four Vinyl Residues. Tetrahedron Lett. 2011, 52, 2370–2374. [Google Scholar]
- Niu S. L.; Massif C.; Ulrich G.; Ziessel R.; Renard P.-Y.; Romieu A. Water-Solubilisation and Bio-Conjugation of a Red-Emitting BODIPY Marker. Org. Biomol. Chem. 2011, 9, 66–69. [DOI] [PubMed] [Google Scholar]
- Leen V.; Van der Auweraer M.; Boens N.; Dehaen W. Vicarious Nucleophilic Substitution of A-hydrogen of BODIPY and Its Extension to Direct Ethenylation. Org. Lett. 2011, 13, 1470–1473. [DOI] [PubMed] [Google Scholar]
- Goze C.; Ulrich G.; Mallon L. J.; Allen B. D.; Harriman A.; Ziessel R. Synthesis and Photophysical Properties of Borondipyrromethene Dyes Bearing Aryl Substituents at the Boron Center. J. Am. Chem. Soc. 2006, 128, 10231–10239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.