Abstract
In the course of myosin-catalyzed ATP hydrolysis, certain amino acid residues in myosin interact with counterparts in actin to produce the relational changes that underlie muscle contraction; some of these interactions are ionic, but the stronger interactions are hydrophobic. In an effort to identify myosin residues participating in hydrophobic interactions, myosin (from smooth muscle) fragments with mutations at suspected sites were engineered and compared with wild-type fragments. It was found that the ATPase of doubly mutated (Trp546Ser and Phe547His) fragments was minimally activated by actin and did not decorate actin well to form the regular arrowhead pattern characteristic of myosin binding to actin filaments. Thus, we suggest that Trp546 and Phe547 are important participants in the hydrophobic actin-myosin interaction.
Full text
PDF

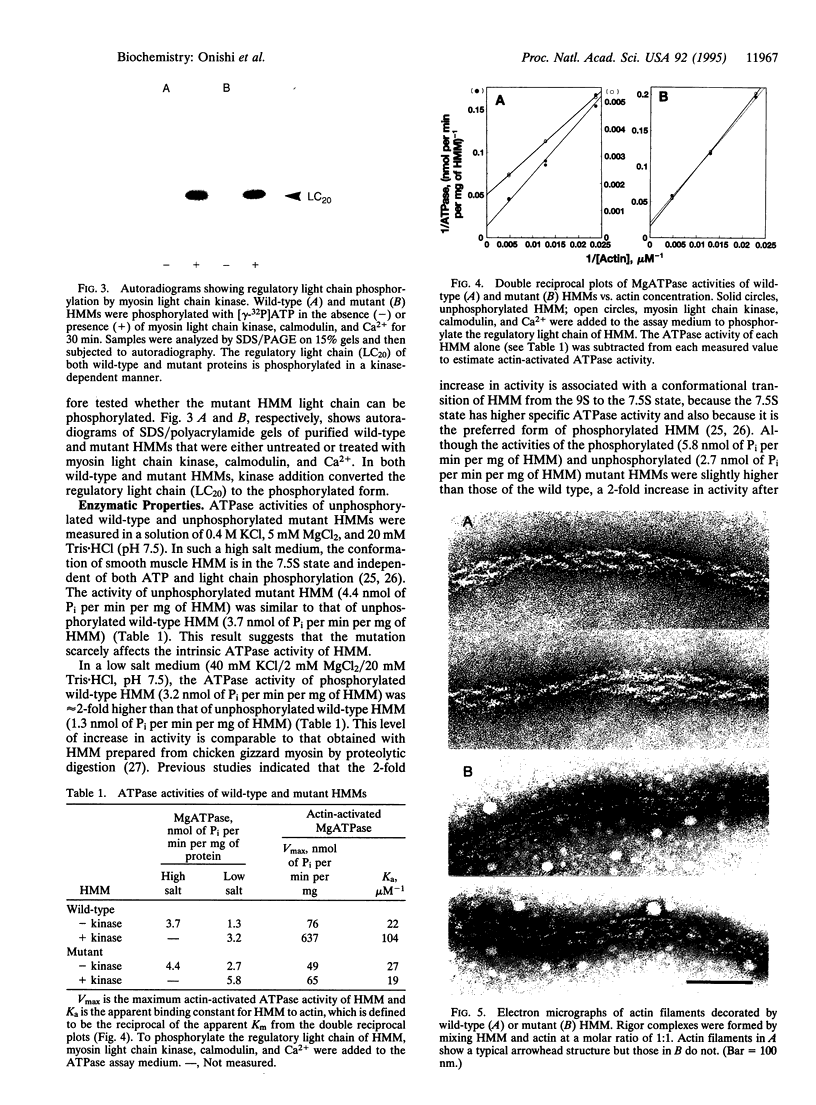


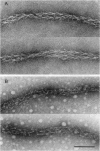
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Eisenberg E. Regulation and kinetics of the actin-myosin-ATP interaction. Annu Rev Biochem. 1980;49:921–956. doi: 10.1146/annurev.bi.49.070180.004421. [DOI] [PubMed] [Google Scholar]
- Adelstein R. S., Klee C. B. Purification and characterization of smooth muscle myosin light chain kinase. J Biol Chem. 1981 Jul 25;256(14):7501–7509. [PubMed] [Google Scholar]
- Botts J., Thomason J. F., Morales M. F. On the origin and transmission of force in actomyosin subfragment 1. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2204–2208. doi: 10.1073/pnas.86.7.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaussepied P., Morales M. F., Kassab R. The myosin SH2-50-kilodalton fragment cross-link: location and consequences. Biochemistry. 1988 Mar 8;27(5):1778–1785. doi: 10.1021/bi00405a059. [DOI] [PubMed] [Google Scholar]
- Chaussepied P., Morales M. F. Modifying preselected sites on proteins: the stretch of residues 633-642 of the myosin heavy chain is part of the actin-binding site. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7471–7475. doi: 10.1073/pnas.85.20.7471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeves M. A., Conibear P. B. The role of three-state docking of myosin S1 with actin in force generation. Biophys J. 1995 Apr;68(4 Suppl):194S–201S. [PMC free article] [PubMed] [Google Scholar]
- Highsmith S. The effects of temperature and salts on myosin subfragment-1 and F-actin association. Arch Biochem Biophys. 1977 Apr 30;180(2):404–408. doi: 10.1016/0003-9861(77)90054-6. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Mannherz H. G., Suck D., Pai E. F., Holmes K. C. Atomic structure of the actin:DNase I complex. Nature. 1990 Sep 6;347(6288):37–44. doi: 10.1038/347037a0. [DOI] [PubMed] [Google Scholar]
- Kodama T., Fukui K., Kometani K. The initial phosphate burst in ATP hydrolysis by myosin and subfragment-1 as studied by a modified malachite green method for determination of inorganic phosphate. J Biochem. 1986 May;99(5):1465–1472. doi: 10.1093/oxfordjournals.jbchem.a135616. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Onishi H., Maéda K., Maéda Y., Inoue A., Fujiwara K. Functional chicken gizzard heavy meromyosin expression in and purification from baculovirus-infected insect cells. Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):704–708. doi: 10.1073/pnas.92.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi H., Watanabe S. Chicken gizzard heavy meromyosin that retains the two light-chain components, including a phosphorylatable one. J Biochem. 1979 Feb;85(2):457–472. doi: 10.1093/oxfordjournals.jbchem.a132352. [DOI] [PubMed] [Google Scholar]
- Rayment I., Holden H. M., Whittaker M., Yohn C. B., Lorenz M., Holmes K. C., Milligan R. A. Structure of the actin-myosin complex and its implications for muscle contraction. Science. 1993 Jul 2;261(5117):58–65. doi: 10.1126/science.8316858. [DOI] [PubMed] [Google Scholar]
- Rayment I., Rypniewski W. R., Schmidt-Bäse K., Smith R., Tomchick D. R., Benning M. M., Winkelmann D. A., Wesenberg G., Holden H. M. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993 Jul 2;261(5117):50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Sutoh K. Identification of myosin-binding sites on the actin sequence. Biochemistry. 1982 Jul 20;21(15):3654–3661. doi: 10.1021/bi00258a020. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kamata T., Onishi H., Watanabe S. Adenosine triphosphate-induced reversible change in the conformation of chicken gizzard myosin and heavy meromyosin. J Biochem. 1982 May;91(5):1699–1705. doi: 10.1093/oxfordjournals.jbchem.a133861. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Stafford W. F., 3rd, Slayter H. S., Seidel J. C. A conformational transition in gizzard heavy meromyosin involving the head-tail junction, resulting in changes in sedimentation coefficient, ATPase activity, and orientation of heads. J Biol Chem. 1985 Nov 25;260(27):14810–14817. [PubMed] [Google Scholar]
- Sweeney H. L., Straceski A. J., Leinwand L. A., Tikunov B. A., Faust L. Heterologous expression of a cardiomyopathic myosin that is defective in its actin interaction. J Biol Chem. 1994 Jan 21;269(3):1603–1605. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trybus K. M. Regulation of expressed truncated smooth muscle myosins. Role of the essential light chain and tail length. J Biol Chem. 1994 Aug 19;269(33):20819–20822. [PubMed] [Google Scholar]
- Yanagisawa M., Hamada Y., Katsuragawa Y., Imamura M., Mikawa T., Masaki T. Complete primary structure of vertebrate smooth muscle myosin heavy chain deduced from its complementary DNA sequence. Implications on topography and function of myosin. J Mol Biol. 1987 Nov 20;198(2):143–157. doi: 10.1016/0022-2836(87)90302-0. [DOI] [PubMed] [Google Scholar]
- Yazawa M., Sakuma M., Yagi K. Calmodulins from muscles of marine invertebrates, scallop and sea anemone. J Biochem. 1980 May;87(5):1313–1320. doi: 10.1093/oxfordjournals.jbchem.a132869. [DOI] [PubMed] [Google Scholar]