Abstract
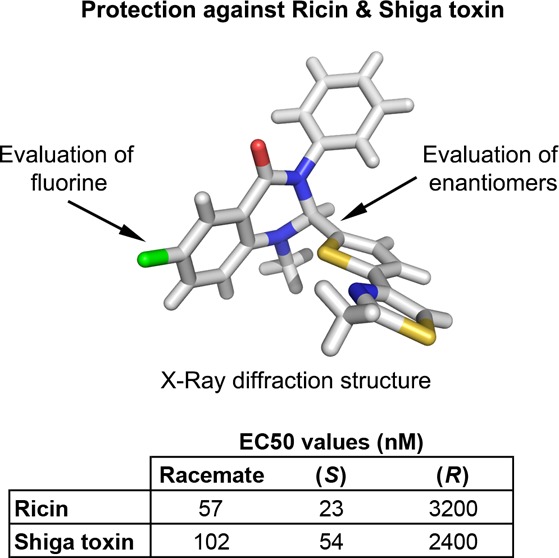
This study reports the synthesis, chromatographic separation, and pharmacological evaluation of the two enantiomers of a new compound, named Retro-2.1, active against toxins by inhibiting intracellular trafficking via the retrograde route. The absolute configuration of the bioactive enantiomer has been assigned from X-ray diffraction to the (S)-enantiomer. To date, (S)-Retro-2.1 is the most potent molecule to counteract the cytotoxic potential of ricin and Shiga toxin, with EC50 values of 23 and 54 nM, respectively.
Keywords: Retro-2.1, Shiga toxin, ricin, dihydroquinazolinones
Ricin is a highly toxic protein abundant in castor beans and classified as a potential bioweapon. After ingestion or inhalation, ricin causes major organ damages that can ultimately lead to the death of the exposed organism. To date no approved treatments or prophylaxis for human use is available. The Shiga toxin (Stx) family consists of related protein toxins that are produced by the bacteria Shigella dysenteriae and certain pathogenic strains of Escherichia coli.1,2 Bacteria producing these toxins are responsible for a number of diseases such as the life-threatening complication hemolytic uremic syndrome (HUS).3 In 2011, a major outbreak caused by E. coli O104:H4 infected about 4000 people in Europe, causing more than 900 cases of HUS resulting in 54 deaths.4 So far, no effective therapy exists for Stx intoxication.5,6 Ricin and Stx share several characteristics. They have one moiety (B-subunit) that binds to their respective cellular receptors (glycoproteins and glycolipids for ricin; the glycosphingolipid Gb3 for Stx), while another moiety (A-subunit) enters the cytosol and inactivates protein biosynthesis. Both toxins are transported in a retrograde manner from the plasma membrane via endosomes and the TGN to the endoplasmic reticulum (ER),7 before translocation to the cytosol, where they enzymatically inactivate the 28S RNA of the 60S ribosomal subunit (reviewed in refs (7−10)). Inhibitors acting on the intracellular routing of these toxins likely offer novel options for the development of therapeutic strategies.11
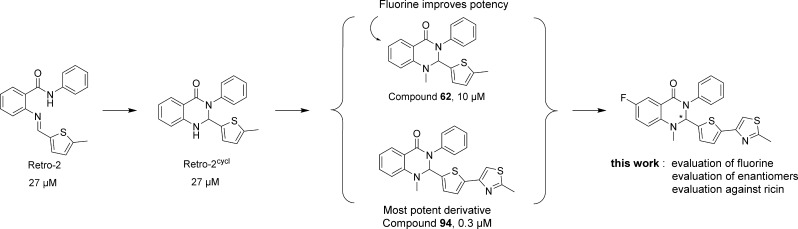
We have recently identified a compound named Retro-2 that protects human cells against ricin and Stx by blocking retrograde toxin transport at the early endosomes-TGN interface.12 Retro-2 also protects mice against lethal ricin challenge.12 A structure–activity relationship (SAR) study of Retro-2 led to an improved activity against Stx up to 100-fold for compounds sharing a dihydroquinazolinone scaffold (cyclized Retro-2).13,14 We also demonstrated that only one enantiomer (compound 62,13 Scheme 1) of dihydroquinazolinones was bioactive, but its absolute configuration was unsolved.
Scheme 1. Previous Studies on Retro-2 Optimization and Objectives of This Work (with EC50 against Shiga Toxin).
Here, we report the synthesis and evaluation of a new enantiopure dihydroquinazolinone compound, named Retro-2.1, acting against Stx cytotoxicity with improved potency (∼500-fold compared to Retro-2) and demonstrate that Retro-2.1 is also effective against ricin in vitro (∼1000-fold increased activity compared to Retro-2). We establish from crystal X-ray diffraction data that the antitoxin activity resides mainly in the S-enantiomer.
During the course of our previous SAR study starting from Retro-2, we obtained N-methylated cyclic analogues leading to a potent inhibitor of Stx action in cells (compound 94, EC50 = 0.3 μM,13 Scheme 1). In parallel, substitution of the phenyl ring of the dihydroquinazolinone scaffold of 62 was evaluated. Among all the substituents, only the 6-fluoro derivative showed a better activity compared to the parent Retro-2cycl (EC50 = 11 μM vs 27 μM, respectively)13 (Scheme 1). We describe here the synthesis and evaluation of a compound combining the best pharmacophores.
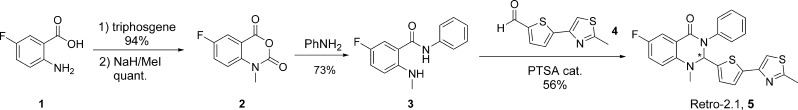
The synthesis of compound 5 was achieved according to our previously published procedure starting from the commercially available 4-fluoro anthranilic acid 1 (Scheme 2) in 39% yield over 4 steps.
Scheme 2. Synthesis of 6-Fluoro-1-methyl-2-(5-(2-methylthiazol-4-yl)thiophen-2-yl)-3-phenyl-2,3-dihydroquinazolin-4(1H)-one, Retro-2.1.
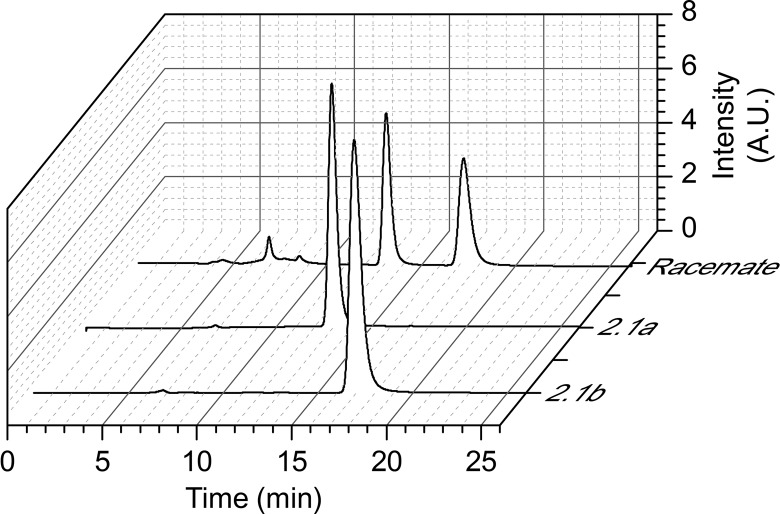
In addition, since only one enantiomer was active on previous dihydroquinazolinones synthesized as racemates,13 a chiral phase HPLC separation of Retro-2.1 was performed on a ChiralPak IB column (see Experimental Section and Figure 1). No contamination of Retro-2.1a by Retro-2.1b or vice versa could be identified on the chromatograms (Figure 1).
Figure 1.
Chiral HPLC chromatograms of rac-Retro-2.1 and purified enantiomers, Retro-2.1a and Retro-2.1b.
To unequivocally assign the absolute configuration of each enantiomer, crystal structure determinations were obtained on separate enantiomers. Among the different crystallization conditions that we screened, a mixture of CH2Cl2 and methanol (1:1) proved efficient to obtain monocrystals suitable for X-ray diffraction (slow evaporation method). These studies revealed that Retro-2.1a had a S-stereochemistry at the C-2 position of the dihydroquinazolinone core (Figure 2), while Retro-2.1b was the R-isomer.
Figure 2.
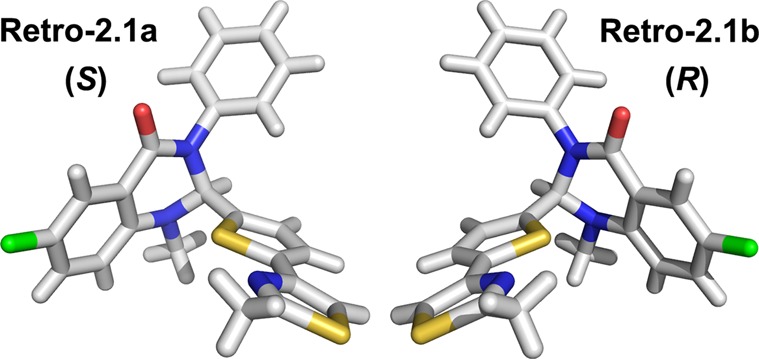
Structure determination of the Retro-2.1 enantiomers by X-ray crystallography.
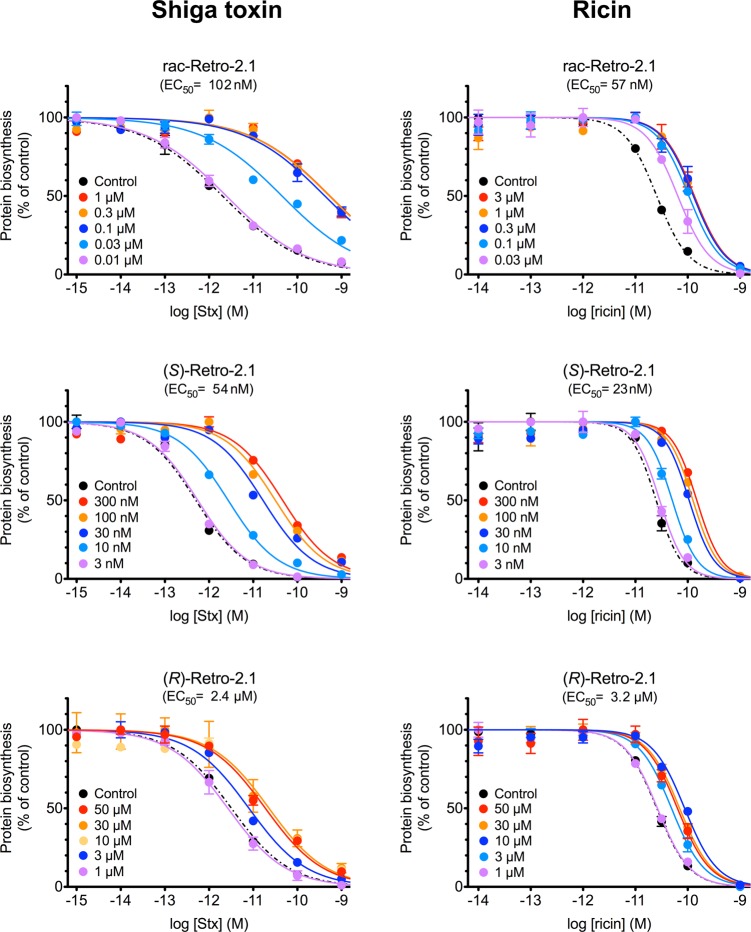
The biological activity of Retro-2.1 and each enantiomer was then evaluated against Stx and ricin (Figure 3). Retro-2.1 racemate inhibited Stx action on HeLa cells with an EC50 of 102 ± 15.0 nM (Figure 3, upper left panel; n = 9) more potently than the previously described best compound (compound 94,13 EC50 = 0.3 μM). It also inhibited ricin induced-toxicity on human pulmonary A549 cells with an EC50 of 57 ± 2.4 nM (Figure 3, upper right panel; n = 3).
Figure 3.
Retro-2.1 protects cells against Stx and ricin. Intoxication of HeLa cells by Stx (left) and intoxication of A549 cells by ricin (right) in the presence of inhibitors. Cells were incubated 1 h at 37 °C with DMSO (0.04%, black data points in all parts of the figure), rac-Retro-2.1 (various concentrations; upper panel), (S)-Retro-2.1 enantiomer (various concentrations; middle panel), or (R)-Retro-2.1 enantiomer (various concentrations; lower panel) before addition of toxins at the indicated concentrations. Each data point represents the mean of duplicate ± SEM of a representative experiment. EC50 values are the mean of n independent experiments (see text for more details). Note that the concentrations used for racemate and enantiomers are different.
When tested separately, Retro-2.1a ((S)-Retro-2.1) potently inhibited toxin cytotoxicity (eutomer), while the second eluted compound, Retro-2.1b ((R)-Retro-2.1), only showed moderate activity at micromolar concentrations (distomer). Indeed, (S)-Retro-2.1 inhibited both Stx cellular action (EC50 of 54 ± 16 nM; Figure 3, middle left panel; n = 7) and ricin (EC50 of 23 ± 4 nM; Figure 3, middle right panel; n = 4), whereas (R)-Retro-2.1 exhibited only an EC50 of 2.4 ± 1 μM against Stx (Figure 3, lower left panel; n = 4) and an EC50 of 3.2 ± 0.1 μM against ricin (Figure 3, lower right panel; n = 3). These results are in accordance with the EC50 values obtained from the racemate form with a 2-fold increase of potency of the eutomer. Eudismic ratios of 45 and 135, respectively for Stx and ricin, were calculated from the EC50 values of cytotoxicity assays reported in this paper, reflecting a high enantioselectivity. This eudismic ratio may also explain the absence of activity of the distomer of compound 62 reported previously13 on Stx, since the highest concentration tested for the distomer was 30 μM (due to low solubility) while the EC50 of the racemate was 10 μM.
It is worth noting that besides a lower potency, determined by a higher EC50, (R)-Retro-2.1 also displayed a reduced efficacy (lower maximal R-value) in comparison to (S)-Retro-2.1 against both Stx and ricin (for Stx, R(S) = 357 vs R(R) = 68, n = 6; for ricin, R(S) = 5 vs R(R) = 2.9, n = 3; R values are from experiments performed in parallel). These differential pharmacological activities proved that only the (S)-enantiomer fully retained the desired antitoxin activity.
Retro-2.1 has a stronger efficacy (R-values) against Stx than against ricin, as observed for Retro-2.12 Most likely, ricin can use several mechanisms to traffic into cells by binding to a variety of glycoreceptors. In contrast, Stx only binds to Gb3 and may be facing limited molecular redundancy for reaching TGN membranes. Ricin has also been proposed to cross endosomal membranes to reach the cytosol, as an alternative mechanism to retrotranslocation from the endoplasmic reticulum.15 This type of endosomal escape would allow bypassing a Retro-2.1-induced retrograde transport block.
We describe herein the synthesis and chromatographic separation of Retro-2.1 enantiomers. In vitro experiments proved the configuration of the stereocenter in position 2 is crucial for activity, since one enantiomer is active to protect cells against Stx and ricin. The absolute stereochemistry of the eutomer has been assigned by X-ray diffraction. The (S)-isomer proved to be the bioactive enantiomer, and (S)-Retro-2.1 is the most active compound against these toxins reported so far.
Since N-methyldihydroquinazolinones derived from Retro-2 block the retrograde pathway within cells, optimized (S)-Retro-2.1 has the potential to be developed as a broad-spectrum antidote to a wide array of pathogens, including toxins and parasites, as demonstrated previously in vitro and in vivo for Retro-2.12,16
Glossary
Abbreviations
- Stx
Shiga toxin
- E. coli
Escherichia coli
- HUS
hemolytic uremic syndrome
- Gb3
globotriaosylceramide
- ER
endoplasmic reticulum
- RNA
ribonucleic acid
- SAR
structure–activity relationship
- EC50
50% effective concentration
- HPLC
high performance liquid chromatography
- TGN
trans-Golgi network
- DMSO
dimethyl sulfoxide
- SEM
standard error of the mean
- RP-HPLC
reversed phase–high performance liquid chromatography
- THF
tetrahydrofuran
- DMF
dimethyl formamide
- PTSA
para-toluene sulfonic acid
- NMR
nuclear magnetic resonance
- MS
mass spectrometry
- HRMS
high resolution mass spectrometry
- LC/MS
liquid chromatography coupled to mass spectrometry
- IR
infrared
- UV
ultraviolet
- DAD
diode array detector
- DMEM
Dulbecco’s modified Eagle’s medium
- IC50
50% inhibition concentration
- TLC
thin-layer chromatography
Supporting Information Available
General procedures, characterization of new compounds, crystal structure determination of compound 5, chemicals for in vitro experiments, intoxication assays, and determination of EC50 values. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
∥ N.G. and V.P. contributed equally to this work.
This work (Project RetroScreen) has been funded by the French National Agency for Research (ANR) under Contract ANR-11-BSV2-0018, the Joint ministerial program of R&D against CBRNe risks and CEA. N.G. was funded by the CEA international PhD program.
The authors declare no competing financial interest.
Supplementary Material
References
- Acheson D. W. K.; Keusch G. T.. The family of Shiga toxins. In The comprehensive sourcebook of bacterial protein toxins, 2nd ed.; Alouf J. E.; Freer J. H., Eds.; Elsevier: Paris, 1999; pp 229–242. [Google Scholar]
- O’Brien A. D.; Holmes R. K. Shiga and Shiga-like toxins. Microbiol. Rev. 1987, 51, 206–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr P. I.; Gordon C. A.; Chandler W. L. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 2005, 365, 1073–1086. [DOI] [PubMed] [Google Scholar]
- Karch H.; Denamur E.; Dobrindt U.; Finlay B. B.; Hengge R.; Johannes L.; Ron E. Z.; Tønjum T.; Sansonetti P. J.; Vicente M. The enemy within us: lessons from the 2011 European Escherichia coli O104:H4 outbreak. EMBO Mol. Med. 2012, 4, 841–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr P. I.; Sadler J. E.; Chandler W. L.; George J. N.; Tsai H. M. Should all adult patients with diarrhoea-associated HUS receive plasma exchange?. Lancet 2012, 379, 516.(author reply pp 516–517). [DOI] [PubMed] [Google Scholar]
- Menne J.; Nitschke M.; Stingele R.; Abu-Tair M.; Beneke J.; Bramstedt J.; Bremer J. P.; Brunkhorst R.; Busch V.; Dengler R.; Deuschl G.; Fellermann K.; Fickenscher H.; Gerigk C.; Goettsche A.; Greeve J.; Hafer C.; Hagenmuller F.; Haller H.; Herget-Rosenthal S.; Hertenstein B.; Hofmann C.; Lang M.; Kielstein J. T.; Klostermeier U. C.; Knobloch J.; Kuehbacher M.; Kunzendorf U.; Lehnert H.; Manns M. P.; Menne T. F.; Meyer T. N.; Michael C.; Munte T.; Neumann-Grutzeck C.; Nuernberger J.; Pavenstaedt H.; Ramazan L.; Renders L.; Repenthin J.; Ries W.; Rohr A.; Rump L. C.; Samuelsson O.; Sayk F.; Schmidt B. M.; Schnatter S.; Schocklmann H.; Schreiber S.; von Seydewitz C. U.; Steinhoff J.; Stracke S.; Suerbaum S.; van de Loo A.; Vischedyk M.; Weissenborn K.; Wellhoner P.; Wiesner M.; Zeissig S.; Buning J.; Schiffer M.; Kuehbacher T. Validation of treatment strategies for enterohaemorrhagic Escherichia coli O104:H4 induced haemolytic uraemic syndrome: case-control study. BMJ [Br. Med. J.] 2012, 345, e4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes L.; Popoff V. Tracing the retrograde route in protein trafficking. Cell 2008, 135, 1175–1187. [DOI] [PubMed] [Google Scholar]
- Watson P.; Spooner R. A. Toxin entry and trafficking in mammalian cells. Adv. Drug. Delivery Rev. 2006, 58, 1581–1596. [DOI] [PubMed] [Google Scholar]
- Sandvig K.; Spilsberg B.; Lauvrak S. U.; Torgersen M. L.; Iversen T. G.; van Deurs B. Pathways followed by protein toxins into cells. Int. J. Med. Microbiol. 2004, 293, 483–490. [DOI] [PubMed] [Google Scholar]
- Lord J. M.; Roberts L. M.; Lencer W. I. Entry of protein toxins into mammalian cells by crossing the endoplasmic reticulum membrane: co-opting basic mechanisms of endoplasmic reticulum-associated degradation. Curr. Top. Microbiol. Immunol. 2005, 300, 149–168. [DOI] [PubMed] [Google Scholar]
- Barbier J.; Bouclier C.; Johannes L.; Gillet D. Inhibitors of the cellular trafficking of ricin. Toxins (Basel) 2012, 4, 15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stechmann B.; Bai S. K.; Gobbo E.; Lopez R.; Merer G.; Pinchard S.; Panigai L.; Tenza D.; Raposo G.; Beaumelle B.; Sauvaire D.; Gillet D.; Johannes L.; Barbier J. Inhibition of retrograde transport protects mice from lethal ricin challenge. Cell 2010, 141, 231–242. [DOI] [PubMed] [Google Scholar]
- Noel R.; Gupta N.; Pons V.; Goudet A.; Garcia-Castillo M. D.; Michau A.; Martinez J.; Buisson D. A.; Johannes L.; Gillet D.; Barbier J.; Cintrat J. C. N-Methyldihydroquinazolinone derivatives of Retro-2 with enhanced efficacy against Shiga Toxin. J. Med. Chem. 2013, 56, 3404–3413. [DOI] [PubMed] [Google Scholar]
- Park J. G.; Kahn J. N.; Tumer N. E.; Pang Y. P. Chemical structure of Retro-2, a compound that protects cells against ribosome-inactivating proteins. Sci. Rep. 2012, 2, 631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumelle B.; Alami M.; Hopkins C. R. ATP-dependent translocation of ricin across the membrane of purified endosomes. J. Biol. Chem. 1993, 268, 23661–23669. [PubMed] [Google Scholar]
- Canton J.; Kima P. E. Targeting host syntaxin-5 preferentially blocks Leishmania parasitophorous vacuole development in infected cells and limits experimental Leishmania infections. Am. J. Pathol. 2012, 181, 1348–1355. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.