Abstract
Isolated middle cerebral artery (MCA) stenosis in young patients with no other medical condition may be a unique pathologic entity with a benign long-term course. Generally, moyamoya disease shows a progression of stenosis from internal cerebral artery (ICA) to other intracranial vessel. A 26-year-old woman was admitted for choreic movements of the right arm and leg. Brain magnetic resonance imaging showed no stroke. Conventional angiography revealed 48% stenosis of the left M1 without ICA stenosis. Single photon emission computed tomography revealed perfusion asymmetry after acetazolamide injection, suggesting decreased uptake in the left basal ganglia and the cerebral cortex. Her hemichorea was mildly decreased with risperidone. One year later, follow-up angiography showed complete occlusion of the left M1 with neovascularization suggestive of moyamoya disease. The patient underwent bypass surgery and her hemichorea disappeared. This may be an atypical presentation of moyamoya disease. The bypass surgery was an effective measure for restoring the vascular insufficiency and, resultantly, controlling her hemichorea.
Keywords: Middle cerebral artery stenosis, Hemichorea, Moyamoya
Chorea is one of involuntary movements resulting from a continuous flow of random muscle contractions. The etiologies of chorea include stroke, infection, autoimmune disease, neurodegeneration, neoplasms, metabolic disease, genetic mutations, and drug exposure.
Hemichorea related to transient ischemic attack (TIA) or stroke has been reported that the majority of patients had an ischemic lesion in the basal ganglia, thalamic nuclei, subthalamic area,1 although some had no specific cerebral structural lesions but demonstrated either high-grade stenosis of the internal carotid artery (ICA) or Moyamoya disease (MMD).2,3
A prognosis of isolated middle cerebral artery (MCA) stenosis in young patients with no other medical conditions has not been documented well and may be a unique pathologic entity with a benign long-term course.4 There have been only a few young cases showing isolated MCA stenosis with no other medical condition.4–6 Its etiology and an association with MMD remain unclear. A few older patients with MCA stenosis with no ICA stenosis from a previous study showed hemichorea, but these patients had typical stroke risk factors.7,8 MCA stenosis associated with Moyamoya pattern collateralization were reported, but the progression of a stenosis from MCA to other intreacranial vessel was not documented.6,8
Here, we report a young patient presenting with hemichorea due to isolated MCA stenosis with no ICA stenosis and its progression into moyamoya collateralization.
Case
A 26-year-old woman visited our hospital with involuntary movements of the right arm and leg for ten days. The patient had no history of hypertension, diabetes mellitus, or other cardiac disease. She did not have a history of rheumatic fever or a history of exposure to relevant medications such as neuroleptics. She also had no family history of movement disorders. On admission, the patient showed slow choreic movements with a large amplitude in her right extremities. The hemichorea was precipitated by rising, exercise, or doing something with her right arm and leg and lasted for all day long. The neurological examination did not reveal any other neurological deficit except for this involuntary movement.
Laboratory tests for Wilson’s disease were in the normal range and slit lamp examination did not reveal Kayser-Fleischer rings. Genetic studies for Huntington’s disease and dentatorubral-pallidoluysian atrophy were negative. Other laboratory findings were also unremarkable: fasting glucose 95 mg/dL (74–110), blood urea nitrogen 8.8 mg/dL (8.0–20.0), creati-nine 0.61 mg/dL (0.40–1.00), erythrocyte sedimentation rate 7 mm/hr (0–20), negative autoimmune antibodies (Ab) (anti-nuclear Ab, anti-DNA Ab, anti-neutrophil cytoplasmic Ab, antiphospholipid Ab, lupus anticoagulant, anti-SSA/Ro Ab, anti-SSB/La Ab, anti-Smith Ab and anti-SCL-70 Ab), no evidence of protein S or C deficiency, homocysteine 10.2 umol/ L (7.6–18.2), cholesterol 150 mg/dL (0–240), low density lipoprotein-cholesterol 85 mg/dL (0–160), high density lipoprotein-cholesterol 36 mg/dL (29–71), and triglyceride 281 mg/ dL (0–190).
Brain magnetic resonance imaging showed bilateral stenosis of the MCAs, with greater severity on the left side. No concomitant ICA stenosis was seen (Figure 1A). There were no remarkable findings in the brain parenchyma. Transcranial Doppler also showed moderately to severely increased flow velocity with a turbulent waveform in both MCAs [mean velocity of 166 cm/sec (40–80) in the left MCA and 129 cm/sec (40–80) in the right MCA]. Single photon emission computed tomography (SPECT) revealed perfusion asymmetry after acetazolamide injection, suggesting decreased uptake in the left basal ganglia and the cerebral cortex. This finding suggests inadequate vascular reservoir function in the territory of the left MCA (Figure 2A and B). Conventional cerebral angiography was performed, and luminal irregularity with 48% stenosis of the left M1 segment and mild luminal irregularity of the right M1 segment were documented. The ICA and other intracranial arteries had no significant stenosis or luminal irregularity (Figure 1B). Other electrophysiological studies, including nerve conduction studies, somatosensory evoked potentials, and electroencephalography, showed normal findings.
Figure 1.
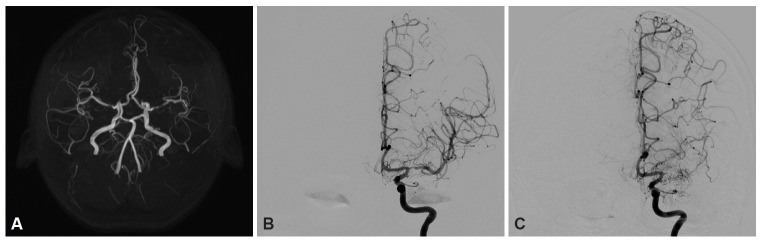
A: Brain MRI shows stenosis in the left MCA without concomitant ICA stenosis. B: Conventional cerebral angiography also reveals 48% stenosis of the left M1 segment without ICA stenosis. C: One year later, follow-up cerebral angiography shows complete occlusion of the left MCA with moyamoya pattern collateralization.
Figure 2.
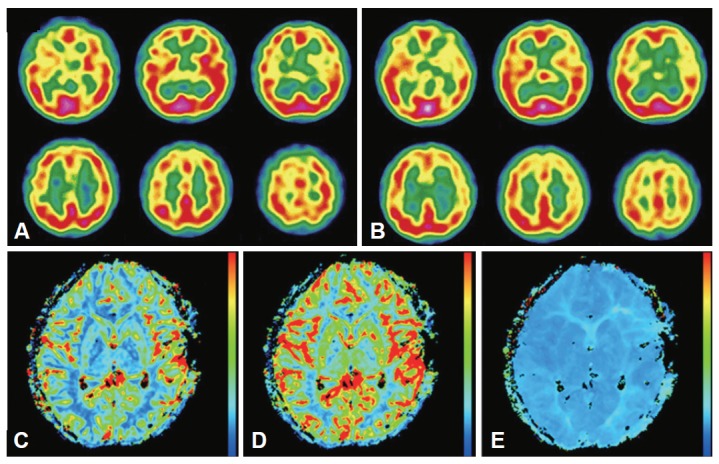
SPECT shows the inadequate vascular reservoir function in the territory of the left MCA, including the basal ganglia and cerebral cortex (A: resting state, B: after acetazolamide injection). After the extracranial-intracranial bypass surgery, brain MRI shows improvement in perfusion of the left MCA territory [C: cerebral blood volume (CBV), D: cerebral flood flow (CBF), E: time to peak (TTP)]. SPECT: Single photon emission computed tomography, MCA: middle cerebral artery.
Her hemichorea partly improved with risperidone and aspirin and hydration. On the day 19 after admission, the patient was discharged with reduced frequency (3 to 4 attacks per day) of hemichorea. She took aspirin and a lipid-lowering agent for stroke prevention.
Follow-up conventional cerebral angiography revealed occlusion of the left proximal MCA with MMD-like collateralization one year later. Although there was still no steno-occlusive lesion in the ICAs bilaterally, the stenosis of both MCAs had progressed. The ICAs still did not show significant stenosis bilaterally (Figure 1C). Vessel wall imaging showed no evidence of arteriosclerotic plaques or other vessel lesions such as vasculitis at the site of the left MCA occlusion, which suggests the possibility of MMD in the patient’s clinical course.
The patient underwent extracranial-intracranial bypass surgery with anastomosis of the superficial temporal artery and MCA. Brain MR perfusion imaging on the 7th day after the operation showed no definite hemodynamic compromise in the territory of the left MCA (Figure 2C–E). Her hemichorea disappeared after the bypass surgery.
Discussion
Hemichorea related to TIA is usually associated with either MMD or occlusion or high-grade stenosis of the ICA. The present case showed that initially, MCA stenosis alone with no significant stenosis of the ICAs caused hemichorea and then the MCA stenosis progressed to other intracranial vessels and moyamoya pattern collateralization came up with time. In the last vessel wall image, there was no evidence of arteriosclerotic plaques or other vessel lesions such as vasculitis at the site of the left MCA occlusion, which suggests a future possibility of progression to typical MMD rather than atherosclerosis or vasculitis.9
Isolated MCA stenosis in young patients with no other medical condition may be a unique pathologic entity with a benign long-term course, and most patients with this condition showed unchanged or decreased stenosis of MCA with time.4 However, there have been only a few report of isolated MCA stenosis in young patients with no other medical conditions, thus, it is not easy to predict the prognosis of these patients. A few older patients with MCA stenosis alone showing hemichorea had typical stroke risk factors in the previous study.7 Only one report has suggested that it might be an initial feature of MMD.5 Some cases with MCA stenosis associated with moyamoya pattern collateralization were reported, but the progression of a stenosis from MCA to other intreacranial vessel was not documented.6,8 MMD is a chronic, occlusive cerebrovascular disease with bilateral stenosis of the distal intracranial ICA, extending to the anterior and middle arteries with extensive collateral network.10 Six angiography stages of MMD have been suggested that usually progress over time from stenosis of the suprasellar ICAs to complete occlusion of the major cerebral arteries.11 To the best of our knowledge, this is the first case that presented hemichorea with progressive isolated MCA steno-occlusion. Previous MMD cases with chorea have been reported to have steno-occlusive lesions in the ICAs, which met the diagnostic criteria of MMD at the time of evaluation.2
In this case, SPECT showed decreased uptake in the left basal ganglia and the cerebral cortex, which was consistent findings with many reports of hemichorea associated with hypoperfusion of the basal ganglia or cerebral cortex.2 Such hemodynamic compromise might cause a functional imbalance in the striatum and cerebral cortex, which would result in decreased activity of the indirect pathway through disinhibition of the thalamic neurons in this case.
In conclusion, this case suggests that isolated MCA stenosis in a young patient can be related to neurologic symptoms such as hemichorea and evolve into MMD. A regular follow-up angiography may play an important role in young patients with isolated MCA stenosis for identification of its evolution into MMD.
REFERENCES
- 1.Ghika-Schmid F, Ghika J, Regli F, Bogousslavsky J. Hyperkinetic movement disorders during and after acute stroke: the Lausanne Stroke Registry. J Neurol Sci. 1997;146:109–116. doi: 10.1016/s0022-510x(96)00290-0. [DOI] [PubMed] [Google Scholar]
- 2.Lyoo CH, Oh SH, Joo JY, Chung TS, Lee MS. Hemidystonia and hemichoreoathetosis as an initial manifestation of moyamoya disease. Arch Neurol. 2000;57:1510–1512. doi: 10.1001/archneur.57.10.1510. [DOI] [PubMed] [Google Scholar]
- 3.Persoon S, Kappelle LJ, Klijn CJ. Limb-shaking transient ischaemic attacks in patients with internal carotid artery occlusion: a case-control study. Brain. 2010;133(Pt 3):915–922. doi: 10.1093/brain/awq009. [DOI] [PubMed] [Google Scholar]
- 4.Becker VU, Eckert B, Thie A. Isolated symptomatic stenosis of the middle cerebral artery in younger adults. A clinical and ultrasonic follow-up study of eight patients. Eur Neurol. 1996;36:65–70. doi: 10.1159/000117209. [DOI] [PubMed] [Google Scholar]
- 5.Choi HY, Lee JE, Jung YH, Cho HJ, Kim DJ, Heo JH. Progression of isolated middle cerebral artery stenosis into moyamoya disease. Neurology. 2007;68:954. doi: 10.1212/01.wnl.0000244412.18039.03. [DOI] [PubMed] [Google Scholar]
- 6.Edgell RC, Boulos AS, Borhani Haghighi A, Bernardini GL, Yavagal DR. Middle cerebral artery stenosis associated with moyamoya pattern collateralization. Front Neurol. 2010;1:119. doi: 10.3389/fneur.2010.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JM, Kim JS, Cho AH, Jeon SB, Lee DK, Suh DC, et al. Angioplasty of middle cerebral artery stenosis improves recurrent hemichorea caused by basal ganglia hypoperfusion. J Stroke Cerebrovasc Dis. 2006;15:69–71. doi: 10.1016/j.jstrokecerebrovasdis.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Seki Y, Fujita M, Mizutani N, Kimura M, Suzuki Y. Spontaneous middle cerebral artery occlusion leading to moyamoya phenomenon and aneurysm formation on collateral arteries. Surg Neurol. 2001;55:58–62. doi: 10.1016/s0090-3019(00)00339-6. discussion 62. [DOI] [PubMed] [Google Scholar]
- 9.Swartz RH, Bhuta SS, Farb RI, Agid R, Willinsky RA, Terbrugge KG, et al. Intracranial arterial wall imaging using high-resolution 3-tesla contrast-enhanced MRI. Neurology. 2009;72:627–634. doi: 10.1212/01.wnl.0000342470.69739.b3. [DOI] [PubMed] [Google Scholar]
- 10.Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med. 2009;360:1226–1237. doi: 10.1056/NEJMra0804622. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki J, Takaku A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969;20:288–299. doi: 10.1001/archneur.1969.00480090076012. [DOI] [PubMed] [Google Scholar]


