Abstract
Chorea and ballism are movement disorders that result from a variety of conditions. They are an uncommon manifestation of diabetes mellitus. We report a 52-year-old diabetic man who presented with acute onset chorea-ballism with a putaminal high-signal-intensity lesion on T1-weighted magnetic resonance imaging (MRI).
Keywords: Chorea, Ballism, Hyperglycemia
Chorea-ballism is a hyperkinetic disorder characterized by continuous non-rhythmic, rapid, purposeless involuntary movements involving both sides of the body. Chorea-ballism may be associated with a variety of factors such as cerebrovascular and inflammatory disease, drugs, metabolic derangement, neurodegenerative disorders, and tumors.1–5 Hyperglycemia is an unusual cause of chorea-ballism.
Case
A 52-year-old man presented at the movement disorder clinic with a 10-day history of involuntary movements involving both sides of the body. The abnormal involuntary movements (AIM) began in his left leg and spread to all of his extremities, trunk, and face. Gradually his AIM became more violent, flinging movements. He had no history of fever. He had a 2-month history of hypertension and noninsulin-dependent diabetes mellitus (NIDDM) and was taking insulin and an oral hypoglycemic agent. The family history and general clinical examination were unremarkable. He worked as a welder. On neurologic examination, the patient had generalized choreic movements involving all four limbs, with left-side dominance. At the time, his blood glucose was 79 mg/dL, and the glycosylated hemoglobin A1c was 9.7%. Serial blood glucose levels ranged from 63 to 167 mg/dL. Urinalysis was negative for ketones. Thyroid and liver function tests were normal. The serum sodium was 143 mmol/L, serum osmolality was 328 mOsm/L, and creatine kinase (CK) was 1,489 IU/dL. The serum concentrations of heavy metals (copper, manganese, iron) were within the reference ranges. No definite abnormality was observed in the waking electroencephalogram, nerve conduction study, median nerve somatosensory evoked potential, and posterior tibial somatosensory evoked potential. Brain magnetic resonance imaging (MRI) was performed 15 days after onset and showed an irregular area of high signal intensity in T1-weighted images in the striatum bilaterally (Figure 1). On T2-weighted and fluid attenuated inversion recovery imaging (FLAIR) MRI, the striatum appeared normal. His AIM disappeared almost completely after 4 days with haloperidol treatment (10 mg/day) and glucose control.
Figure 1.
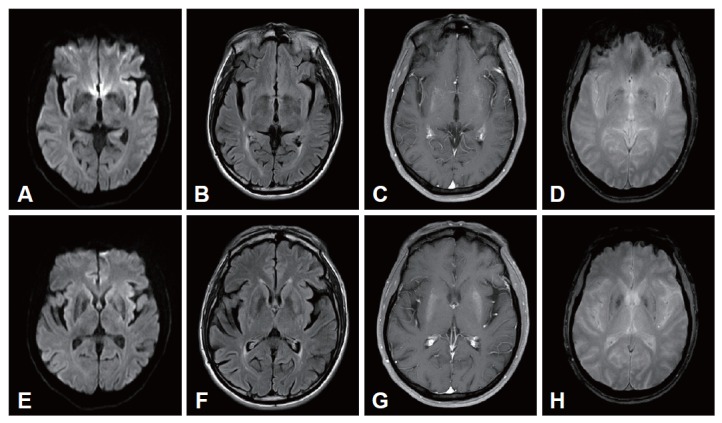
Diffusion-weighted (A and E), fluid attenuated inversion recovery (B and F), T1-weighted (C and G), and gradient echo (D and H) MRI. The T1-weighted brain MRI shows increased signal intensity in the putamen bilaterally.
Discussion
Hemichorea-hemiballism (HCHB) is associated with a variety of diseases such as cerebrovascular and inflammatory diseases, drugs, metabolic derangement, neurodegenerative disorders, and tumors. It has been reported as an unusual manifestation of type 2 diabetes mellitus, usually occurring in Korean, Japanese, and Chinese women.6 HCHB may be a complication in patients already known to be diabetics or at the first manifestation of the syndrome. Our patient had been diagnosed with diabetes 2 months earlier.
The exact pathophysiology of HCHB is unknown, but focal microhemorrhage, ischemic injury, hyperglycemia, hyperviscosity, and mineralization have been suggested in radiological and pathological studies.7 Most cases were associated with hyperglycemia and improved rapidly after correcting the hyperglycemia. In this case, considering the patient’s high HbA1c (9.7%) on a high-dose oral hypoglycemic agent and insulin for 2 months, it is possible that his blood glucose had been higher before the onset of the chorea and was on the way to being corrected rapidly. In our case, we thought that the HCHB was induced not only by hyperglycemia, but also by the rapid fluctuation in the blood glucose itself.
Footnotes
The authors have no financial conflicts of interest.
REFERENCES
- 1.Mizushima N, Park-Matsumoto YC, Amakawa T, Hayashi H. A case of hemichorea-hemiballism associated with parietal lobe infarction. Eur Neurol. 1997;37:65–66. doi: 10.1159/000117408. [DOI] [PubMed] [Google Scholar]
- 2.Lin JJ, Lin GY, Shih C, Shen WC. Presentation of striatal hyperintensity on T1-weighted MRI in patients with hemiballism-hemichorea caused by non-ketotic hyperglycemia: report of seven new cases and a review of literature. J Neurol. 2001;248:750–755. doi: 10.1007/s004150170089. [DOI] [PubMed] [Google Scholar]
- 3.Gelosa G, Tremolizzo L, Galbussera A, Perego R, Capra M, Frigo M, et al. Narrowing the window for ‘senile chorea’: a case with primary antiphospholipid syndrome. J Neurol Sci. 2009;284:211–213. doi: 10.1016/j.jns.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Kumar H, Masiowski P, Jog M. Chorea in the elderly with mutation positive polycythemia vera: a case report. Can J Neurol Sci. 2009;36:370–372. doi: 10.1017/s0317167100007149. [DOI] [PubMed] [Google Scholar]
- 5.Ozben S, Erol C, Ozer F, Tiras R. Chorea as the presenting feature of neurosyphilis. Neurol India. 2009;57:347–349. doi: 10.4103/0028-3886.53277. [DOI] [PubMed] [Google Scholar]
- 6.Lee EJ, Choi JY, Lee SH, Song SY, Lee YS. Hemichorea-hemiballism in primary diabetic patients: MR correlation. J Comput Assist Tomogr. 2002;26:905–911. doi: 10.1097/00004728-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Battisti C, Forte F, Rubenni E, Dotti MT, Bartali A, Gennari P, et al. Two cases of hemichorea-hemiballism with nonketotic hyperglycemia: a new point of view. Neurol Sci. 2009;30:179–183. doi: 10.1007/s10072-009-0039-5. [DOI] [PubMed] [Google Scholar]


