Abstract
Background:
Plasma homocysteine (Hcy) levels are increased in patients with Parkinson’s disease (PD) undergoing levodopa treatment. We measured the Hcy levels in PD patients and assessed the relationship between Hcy level and features of PD, cognitive function and vitamin B status.
Methods:
Concentrations of Hcy, vitamin B12 and folate were measured in 33 PD patients and 41 normal control individuals. Mini-mental Status Examination (MMSE) was assessed in all subjects. In PD patients, Hoehn & Yahr stage and Unified Parkinson Disease Rating Scale (UPDRS) motor scores were also examined.
Results:
Plasma Hcy levels were lower in PD patients than in control individuals. Hcy level was inversely correlated with vitamin B12 and folate levels in the PD group but not in control individuals. Age, symptom duration, UPDRS motor scores, MMSE score, levodopa dose and duration of treatment did not differ between patients with Hcy >14 μmol/L and those with Hcy <14 μmol/L.
Conclusions:
Plasma Hcy levels were increased in PD patients with levodopa treatment and were related to vitamin B level. These results indicate that vitamin supplementation may be beneficial in levodopa-treated PD patients, although hyperhomocysteinemia did not affect the motor and cognitive status of PD patients.
Keywords: Parkinson’s disease, Homocysteine, Vitamin B
Introduction
Plasma homocysteine (Hcy) levels are increased in Parkinson’s disease (PD) patients with levodopa treatment compared with control individuals.1–9 Increased Hcy levels in PD patients result from the metabolism of levodopa. S-adenosylmethionine (SAM), which is the methyl donor of catechol-O-methyltransferase (COMT), forms S-adenosylhomocysteine (SAH), which is converted to Hcy.9
Hcy has various neuronal and endothelial cellular toxicities,10–12 and hyperhomocysteinemia is related to cardiovascular, cerebrovascular,13,14 and neurodegenerative disease.1,2,15–17 Therefore, increased Hcy levels have been suggested to have a role in progression of disease or development of cognitive impairment in PD patients. Several previous studies have investigated the relationship between hyperhomocysteinemia and vitamin B status in PD patients and have proposed that vitamin supplementation might prevent the possible harmful effects of Hcy in PD patients.18–21 We compared Hcy and vitamin B levels in PD patients with those in normal control individuals and evaluated the relationship between Hcy level and clinical features in PD patients.
Subjects and Methods
Subjects
Thirty-three patients with PD who were treated with levodopa for at least for 3 years and 41 age-matched control individuals with no history of neurological disease were included in this study. All PD patients were diagnosed according to UK Brain Bank criteria and showed a good response to levodopa treatment.
Assessment of subjects
Concentrations of Hcy, vitamin B12 and folate were measured by competitive immunoassays assessing direct chemiluminescence in the plasma of the subjects; plasma was obtained from the centrifuged blood. Mini-mental status examination (MMSE) was evaluated in all subjects. In the PD group, symptom duration, treatment duration, Hoehn & Yahr stage, Unified Parkinson Disease Rating Scale (UPDRS) motor score and levodopa dose were also assessed.
Data analysis
Laboratory measures including Hcy, vitamin B12 and folate concentrations and MMSE scores were compared between patients and control individuals using t-tests. The difference of sex ratio between the groups was analyzed using Chi-square tests. In addition, clinical characteristics were also compared between PD patients with Hcy level greater than 14 μmol/L and those with Hcy level less than 14 μmol/L using a t-test. Linear regression analysis was used to evaluate the correlations between Hcy concentration and vitamin B12 concentration, folate concentration and clinical parameters, both in PD patients and control individuals. p value <0.05 was considered statistically significant.
Results
Hcy level in PD patients and controls
The demographic characteristics of the PD patients and control individuals are listed in Table 1. The mean age of the PD patients and control individuals was similar (63.6±8.0 years, range 47–80 years, vs. 65.4±7.8 years, range 48–77 years). PD patients had a higher Hcy level than the control individuals (p<0.05), although there were no differences in MMSE score or folate level between the groups. Vitamin B12 level was lower in the PD patients compared with the control group, but the difference was not significant (p=0.06). In the control group, Hcy level was not correlated with vitamin B12 or folate levels; however, it was inversely correlated with both vitamin B12 and folate levels in PD patients (F=6.670, r2=0.177, p<0.05, F=11.341, r2=0.268, p<0.05, for vitamin B12 and folate levels, respectively) (Figure 1).
Table 1.
Demographic characteristics, and Hcy, vitamin B12 and folate levels in PD patients and control individuals
| PD patients | Control individuals | p value | |
|---|---|---|---|
| Age (years) | 63.5±7.8 | 65.4±7.8 | 0.30 |
| Gender (M/F) | 10/23 | 12/29 | 0.92 |
| MMSE | 23.5±4.7 | 24.8±3.9 | 0.17 |
| Hcy (μmol/L) | 13.6±7.3 | 11.0±2.9 | <0.05 |
| Vitamin B12 (pg/mL) | 716.6±406.2 | 883.6±320.2 | 0.06 |
| Folate (ng/mL) | 8.5±4.0 | 10.2±4.8 | 0.12 |
Hcy: homocysteine, MMSE: Mini-mental status examination, PD: Parkinson’s disease.
Figure 1.
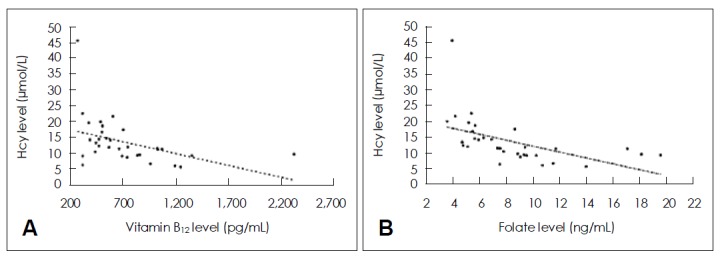
Hcy level was inversely correlated with vitamin B12 (A) and folate levels (B) in PD patients (F=6.670, r2= 0.177, p<0.05, F=11.341, r2=0.268, p< 0.05, respectively). Hcy: homocysteine, PD: Parkinson’s disease.
Clinical characteristics and vitamin status in PD patients with Hcy >14 μmol/L and Hcy <14 μmol/L
In the PD group, there were no differences in age, symptom duration, UPDRS motor score, MMSE score, levodopa dose and duration of levodopa treatment between patients with Hcy >14 μmol/L and those with Hcy <14 μmol/L. Vitamin B12 and folate levels were decreased in patients with Hcy >14 μmol/L compared with those with Hcy <14 μmol/L (Table 2).
Table 2.
Clinical characteristics, demographic features and vitamin B12 and folate levels in PD patients with Hcy level >14 and those with Hcy <14 μmol/L
| Hcy >14 μmol/L (n=12) | Hcy <14 μmol/L (n=21) | p value | |
|---|---|---|---|
| Age (years) | 63.3±6.7 | 63.7±8.6 | 0.90 |
| Symptom duration (years) | 11.9±6.7 | 11.7±6.5 | 0.93 |
| Homocysteine (μmol/L) | 20.0±8.6 | 9.9±2.2 | <0.05 |
| Vitamin B12 (pg/mL) | 481.3±124.1 | 851.0±450.9 | <0.05 |
| Folate (ng/mL) | 5.6±1.4 | 10.1±4.1 | <0.005 |
| Stage | 2.7±0.5 | 2.2±0.4 | 0.13 |
| UPDRS motor score | 21.9±5.2 | 21.1±11.1 | 0.90 |
| MMSE | 21.9±5.2 | 24.3±4.2 | 0.16 |
| Dose of levodopa (mg) | 666.7±154.2 | 777.4±372.5 | 0.34 |
| Treatment duration (years) | 8.8±4.8 | 8.2±4.0 | 0.72 |
PD: Parkinson’s disease, Hcy: homocysteine, UPDRS: Unified Parkinson Disease Rating Scale, MMSE: Mini-mental status examination.
Discussion
Our study showed that Hcy levels are increased in levodopa-treated PD patients compared with normal control individuals. This is in agreement with findings from previous studies of hyperhomocysteinemia in PD patients. In a previous prospective study, levodopa was shown to increase Hcy levels in PD patients,5 whereas Hcy levels in untreated PD patients were similar to those of normal control individuals.22 Therefore, increased Hcy levels in levodopa-treated PD patients might be caused by levodopa treatment rather than by the disease itself.
There were no differences in age, symptom duration, UPDRS score, duration of levodopa treatment and levodopa dose between PD patients with Hcy level >14 μmol/L and those with Hcy <14 μmol/L. Experimental studies found that hyperhomocysteinemia was associated with neurotoxicity in neurodegenerative disorders.12,23,24 However, most previous clinical studies of hyperhomocysteinemia in PD patients failed to show a relationship between increased Hcy levels with severity or duration of the disease,1–7 similar to our study. In addition, a 2-year follow-up study demonstrated similar clinical deterioration in PD patients with increased Hcy levels and those with normal Hcy levels.10 These results indicate that increased Hcy levels in PD patients are not enough toxic to affect the disease severity of such patients. A long-term prospective study may determine the effects of increased Hcy levels on progression of neurodegeneration in PD patients.
Epidemiological studies have shown that plasma Hcy level is correlated with cognitive function in normal elderly individuals and that plasma Hcy level is increased in patients with Alzheimer’s disease (AD). PD patients with increased Hcy level showed worse cognitive performance compared with those with normal Hcy levels.6,8,22,25 Our data showed no significant difference in MMSE score between patients with Hcy >14 μmol/L and those with Hcy <14 μmol/L. However, mean MMSE scores tend to be lower in patients with Hcy >14 μmol/L than in those with Hcy <14 μmol/L (21.9 vs. 24.3, respectively, p=0.17). PD patients in the present study were not compatible with overt dementia. This is in agreement with the results of a recent study that showed that Hcy is not associated with cognitive function in non-demented PD patients.26 Although MMSE score is a useful tool for the screening of dementia patients, it cannot evaluate the various cognitive domains in detail, particularly those of frontal-executive function. Frontal-executive dysfunction is a prominent finding of dementia in PD patients, and can sometimes be seen in PD patients without dementia. There-fore, detailed neuropsychological tests, including frontal-executive function tests, will be needed to evaluate significant differences in cognitive performance between patients with hyperhomocysteinemia and those with a normal Hcy level.
The vitamin B12 level was decreased in PD patients compared with control individuals, although the difference was not significant. In addition, vitamin B12 and folate levels were lower in PD patients with hyperhomocysteinemia compared with those without hyperhomocysteinemia. Re-methylation of Hcy involves vitamin B12- and folate-dependent pathways;9 hence, hyperhomocysteinemia might reduce the vitamin B12 level. The inverse correlation between Hcy level and vitamin status supports the hypothesis that Hcy levels are affected by the vitamin status in PD patients receiving levodopa treatment. Hcy levels in normal control individuals were not correlated with vitamin B12 or folate levels, indicating that Hcy levels are dependent on the vitamin status in patients with hyperhomocysteinemia, including PD patients undergoing levodopa treatment. Although this study failed to show a relationship between hyperhomocysteinemia and motor and cognitive status of PD patients, the differences in vitamin status between PD patients and control individuals suggest a need for vitamin supplementation in levodopa-treated PD patients, in agreement with the results of previous studies.18–21
REFERENCES
- 1.Kuhn W, Roebroek R, Blom H, van Oppenraaij D, Müller T. Hyperhomocysteinaemia in Parkinson’s disease. J Neurol. 1998;245:811–812. doi: 10.1007/s004150050292. [DOI] [PubMed] [Google Scholar]
- 2.Kuhn W, Roebroek R, Blom H, van Oppenraaij D, Przuntek H, Kretschmer A, et al. Elevated plasma levels of homocysteine in Parkinson’s disease. Eur Neurol. 1998;40:225–227. doi: 10.1159/000007984. [DOI] [PubMed] [Google Scholar]
- 3.Müller T, Woitalla D, Hauptmann B, Fowler B, Kuhn W. Decrease of methionine and S-adenosylmethionine and increase of homocysteine in treated patients with Parkinson’s disease. Neurosci Lett. 2001;308:54–56. doi: 10.1016/s0304-3940(01)01972-3. [DOI] [PubMed] [Google Scholar]
- 4.Yasui K, Nakaso K, Kowa H, Takeshima T, Nakashima K. Levodopa-induced hyperhomocysteinaemia in Parkinson’s disease. Acta Neurol Scand. 2003;108:66–67. doi: 10.1034/j.1600-0404.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- 5.O’Suilleabhain PE, Bottiglieri T, Dewey RB, Jr, Sharma S, Diaz-Arrastia R. Modest increase in plasma homocysteine follows levodopa initiation in Parkinson’s disease. Mov Disord. 2004;19:1403–1408. doi: 10.1002/mds.20253. [DOI] [PubMed] [Google Scholar]
- 6.O’Suilleabhain PE, Sung V, Hernandez C, Lacritz L, Dewey RB, Jr, Bottiglieri T, et al. Elevated plasma homocysteine level in patients with Parkinson disease: motor, affective, and cognitive associations. Arch Neurol. 2004;61:865–868. doi: 10.1001/archneur.61.6.865. [DOI] [PubMed] [Google Scholar]
- 7.Zoccolella S, Lamberti P, Armenise E, de Mari M, Lamberti SV, Mastronardi R, et al. Plasma homocysteine levels in Parkinson’s disease: role of antiparkinsonian medications. Parkinsonism Relat Disord. 2005;11:131–133. doi: 10.1016/j.parkreldis.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Ozer F, Meral H, Hanoglu L, Aydemir T, Yilsen M, Cetin S, et al. Plasma homocysteine levels in patients treated with levodopa: motor and cognitive associations. Neurol Res. 2006;28:853–858. doi: 10.1179/016164106X110445. [DOI] [PubMed] [Google Scholar]
- 9.Martignoni E, Tassorelli C, Nappi G, Zangaglia R, Pacchetti C, Blandini F. Homocysteine and Parkinson’s disease: a dangerous liaison? J Neurol Sci. 2007;257:31–37. doi: 10.1016/j.jns.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 10.Blundell G, Rose FA, Tudball N. Homocysteine induced endothelial cell toxicity and its protection. Biochem Soc Trans. 1994;22:341S. doi: 10.1042/bst022341s. [DOI] [PubMed] [Google Scholar]
- 11.Blundell G, Jones BG, Rose FA, Tudball N. Homocysteine mediated endothelial cell toxicity and its amelioration. Atherosclerosis. 1996;122:163–172. doi: 10.1016/0021-9150(95)05730-7. [DOI] [PubMed] [Google Scholar]
- 12.Lipton SA, Kim WK, Choi YB, Kumar S, D’Emilia DM, Rayudu PV, et al. Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proc Natl Acad Sci U S A. 1997;94:5923–5928. doi: 10.1073/pnas.94.11.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bots ML, Launer LJ, Lindemans J, Hofman A, Grobbee DE. Homocysteine, atherosclerosis and prevalent cardiovascular disease in the elderly: The Rotterdam Study. J Intern Med. 1997;242:339–347. doi: 10.1046/j.1365-2796.1997.00239.x. [DOI] [PubMed] [Google Scholar]
- 14.Bostom AG, Rosenberg IH, Silbershatz H, Jacques PF, Selhub J, D’Agostino RB, et al. Nonfasting plasma total homocysteine levels and stroke incidence in elderly persons: the Framingham Study. Ann Intern Med. 1999;131:352–355. doi: 10.7326/0003-4819-131-5-199909070-00006. [DOI] [PubMed] [Google Scholar]
- 15.Clarke R, Smith AD, Jobst KA, Refsum H, Sutton L, Ueland PM. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol. 1998;55:1449–1455. doi: 10.1001/archneur.55.11.1449. [DOI] [PubMed] [Google Scholar]
- 16.Müller T, Werne B, Fowler B, Kuhn W. Nigral endothelial dysfunction, homocysteine, and Parkinson’s disease. Lancet. 1999;354:126–127. doi: 10.1016/s0140-6736(99)01660-8. [DOI] [PubMed] [Google Scholar]
- 17.Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 18.Miller JW, Selhub J, Nadeau MR, Thomas CA, Feldman RG, Wolf PA. Effect of L-dopa on plasma homocysteine in PD patients: relationship to B-vitamin status. Neurology. 2003;60:1125–1129. doi: 10.1212/01.wnl.0000055899.24594.8e. [DOI] [PubMed] [Google Scholar]
- 19.Lamberti P, Zoccolella S, Armenise E, Lamberti SV, Fraddosio A, de Mari M, et al. Hyperhomocysteinemia in L-dopa treated Parkinson’s disease patients: effect of cobalamin and folate administration. Eur J Neurol. 2005;12:365–368. doi: 10.1111/j.1468-1331.2004.00973.x. [DOI] [PubMed] [Google Scholar]
- 20.de Lau LM, Koudstaal PJ, Witteman JC, Hofman A, Breteler MM. Dietary folate, vitamin B12, and vitamin B6 and the risk of Parkinson disease. Neurology. 2006;67:315–318. doi: 10.1212/01.wnl.0000225050.57553.6d. [DOI] [PubMed] [Google Scholar]
- 21.Postuma RB, Espay AJ, Zadikoff C, Suchowersky O, Martin WR, Lafontaine AL, et al. Vitamins and entacapone in levodopa-induced hyperhomocysteinemia: a randomized controlled study. Neurology. 2006;66:1941–1943. doi: 10.1212/01.wnl.0000219815.83681.f7. [DOI] [PubMed] [Google Scholar]
- 22.Religa D, Czyzewski K, Styczynska M, Peplonska B, Lokk J, Chodakowska-Zebrowska M, et al. Hyperhomocysteinemia and methylenetetrahydrofolate reductase polymorphism in patients with Parkinson’s disease. Neurosci Lett. 2006;404:56–60. doi: 10.1016/j.neulet.2006.05.040. [DOI] [PubMed] [Google Scholar]
- 23.Duan W, Ladenheim B, Cutler RG, Kruman II, Cadet JL, Mattson MP. Dietary folate deficiency and elevated homocysteine levels endanger dopaminergic neurons in models of Parkinson’s disease. J Neurochem. 2002;80:101–110. doi: 10.1046/j.0022-3042.2001.00676.x. [DOI] [PubMed] [Google Scholar]
- 24.Kruman II, Kumaravel TS, Lohani A, Pedersen WA, Cutler RG, Kruman Y, et al. Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer’s disease. J Neurosci. 2002;22:1752–1762. doi: 10.1523/JNEUROSCI.22-05-01752.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irizarry MC, Gurol ME, Raju S, Diaz-Arrastia R, Locascio JJ, Tennis M, et al. Association of homocysteine with plasma amyloid beta protein in aging and neurodegenerative disease. Neurology. 2005;65:1402–1408. doi: 10.1212/01.wnl.0000183063.99107.5c. [DOI] [PubMed] [Google Scholar]
- 26.Camicioli RM, Bouchard TP, Somerville MJ. Homocysteine is not associated with global motor or cognitive measures in nondemented older Parkinson’s disease patients. Mov Disord. 2009;24:176–182. doi: 10.1002/mds.22227. [DOI] [PubMed] [Google Scholar]


