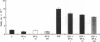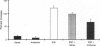Abstract
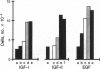
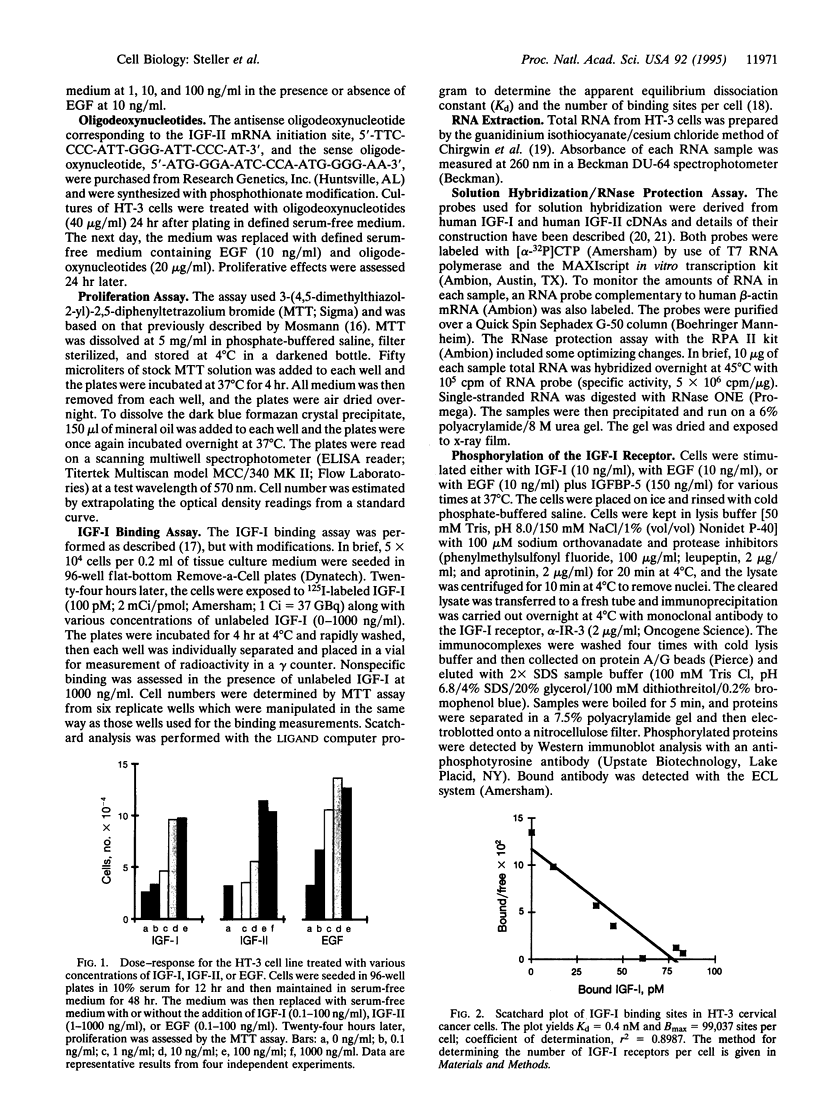
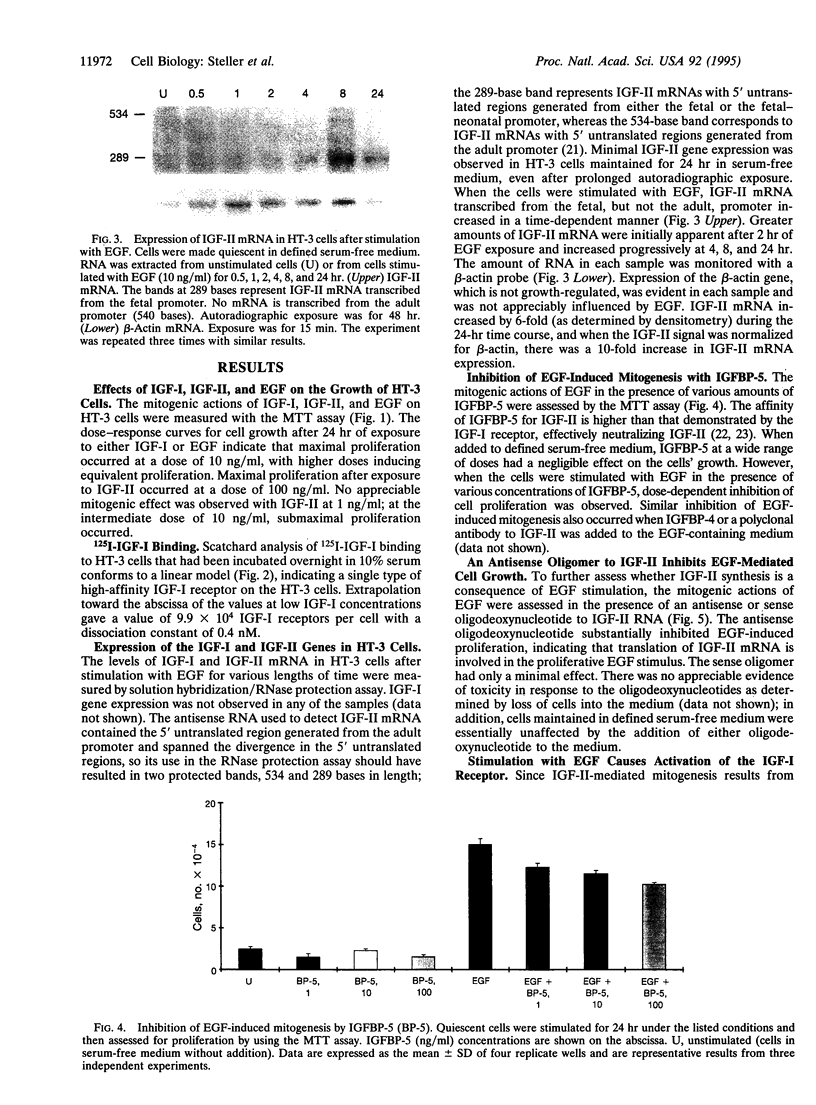
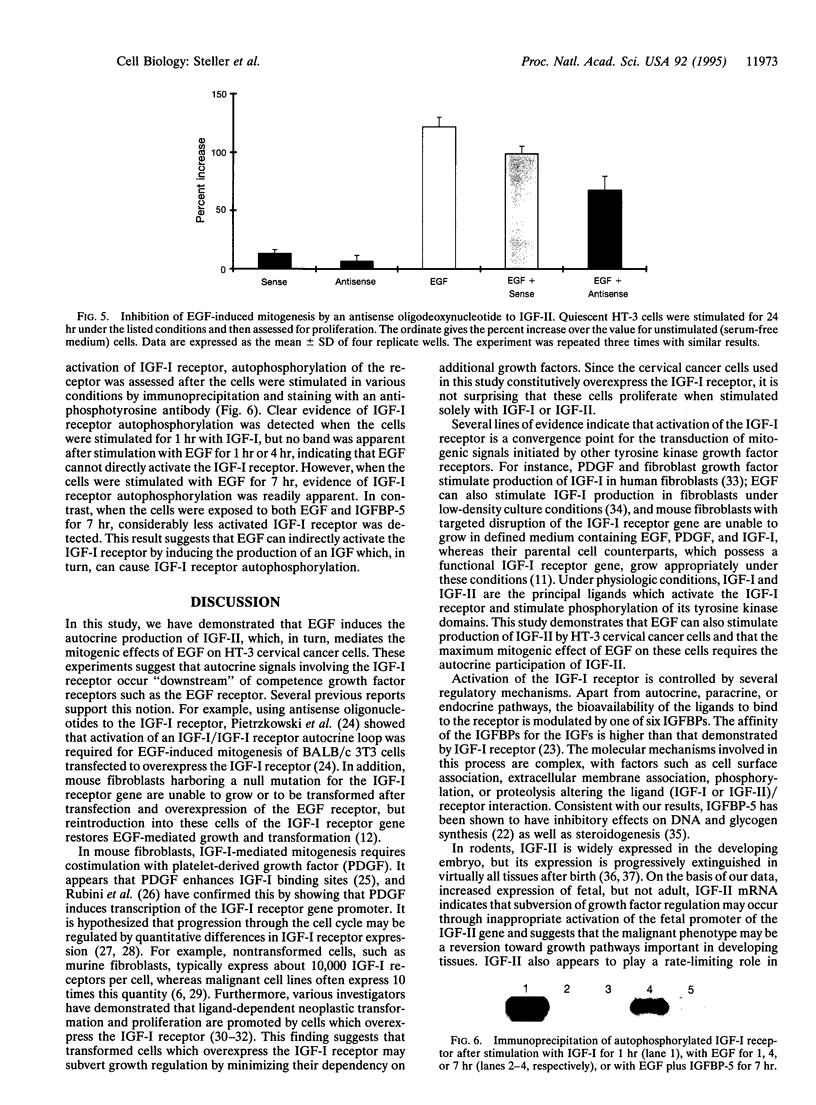
There is increasing evidence that activation of the insulin-like growth factor I (IGF-I) receptor plays a major role in the control of cellular proliferation of many cell types. We studied the mitogenic effects of IGF-I, IGF-II, and epidermal growth factor (EGF) on growth-arrested HT-3 cells, a human cervical cancer cell line. All three growth factors promoted dose-dependent increases in cell proliferation. In untransformed cells, EGF usually requires stimulation by a "progression" factor such as IGF-I, IGF-II, or insulin (in supraphysiologic concentrations) in order to exert a mitogenic effect. Accordingly, we investigated whether an autocrine pathway involving IGF-I or IGF-II participated in the EGF-induced mitogenesis of HT-3 cells. With the RNase protection assay, IGF-I mRNA was not detected. However, IGF-II mRNA increased in a time-dependent manner following EGF stimulation. The EGF-induced mitogenesis was abrogated in a dose-dependent manner by IGF-binding protein 5 (IGFBP-5), which binds to IGF-II and neutralizes it. An antisense oligonucleotide to IGF-II also inhibited the proliferative response to EGF. In addition, prolonged, but not short-term, stimulation with EGF resulted in autophosphorylation of the IGF-I receptor, and coincubations with both EGF and IGFBP-5 attenuated this effect. These data demonstrate that autocrine secretion of IGF-II in HT-3 cervical cancer cells can participate in EGF-induced mitogenesis and suggest that autocrine signals involving the IGF-I receptor occur "downstream" of competence growth factor receptors such as the EGF receptor.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker J., Liu J. P., Robertson E. J., Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993 Oct 8;75(1):73–82. [PubMed] [Google Scholar]
- Baserga R. IGF-1 receptor as the restriction point of the cell cycle. Ann N Y Acad Sci. 1992 Nov 21;663:154–157. doi: 10.1111/j.1749-6632.1992.tb38658.x. [DOI] [PubMed] [Google Scholar]
- Baserga R., Porcu P., Rubini M., Sell C. Cell cycle control by the IGF-1 receptor and its ligands. Adv Exp Med Biol. 1993;343:105–112. doi: 10.1007/978-1-4615-2988-0_11. [DOI] [PubMed] [Google Scholar]
- Baserga R., Rubin R. Cell cycle and growth control. Crit Rev Eukaryot Gene Expr. 1993;3(1):47–61. [PubMed] [Google Scholar]
- Chen S. C., Chou C. K., Wong F. H., Chang C. M., Hu C. P. Overexpression of epidermal growth factor and insulin-like growth factor-I receptors and autocrine stimulation in human esophageal carcinoma cells. Cancer Res. 1991 Apr 1;51(7):1898–1903. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Christofori G., Naik P., Hanahan D. A second signal supplied by insulin-like growth factor II in oncogene-induced tumorigenesis. Nature. 1994 Jun 2;369(6479):414–418. doi: 10.1038/369414a0. [DOI] [PubMed] [Google Scholar]
- Clemmons D. R. Multiple hormones stimulate the production of somatomedin by cultured human fibroblasts. J Clin Endocrinol Metab. 1984 May;58(5):850–856. doi: 10.1210/jcem-58-5-850. [DOI] [PubMed] [Google Scholar]
- Clemmons D. R., Shaw D. S. Variables controlling somatomedin production by cultured human fibroblasts. J Cell Physiol. 1983 May;115(2):137–142. doi: 10.1002/jcp.1041150206. [DOI] [PubMed] [Google Scholar]
- Clemmons D. R., Van Wyk J. J., Pledger W. J. Sequential addition of platelet factor and plasma to BALB/c 3T3 fibroblast cultures stimulates somatomedin-C binding early in cell cycle. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6644–6648. doi: 10.1073/pnas.77.11.6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola D., Ferber A., Miura M., Sell C., D'Ambrosio C., Rubin R., Baserga R. A functional insulin-like growth factor I receptor is required for the mitogenic and transforming activities of the epidermal growth factor receptor. Mol Cell Biol. 1994 Jul;14(7):4588–4595. doi: 10.1128/mcb.14.7.4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez E. R., Hurwitz A., Vera A., Pellicer A., Adashi E. Y., LeRoith D., Roberts C. T., Jr Expression of the genes encoding the insulin-like growth factors and their receptors in the human ovary. J Clin Endocrinol Metab. 1992 Feb;74(2):419–425. doi: 10.1210/jcem.74.2.1309838. [DOI] [PubMed] [Google Scholar]
- Jones J. I., Clemmons D. R. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995 Feb;16(1):3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- Kaleko M., Rutter W. J., Miller A. D. Overexpression of the human insulinlike growth factor I receptor promotes ligand-dependent neoplastic transformation. Mol Cell Biol. 1990 Feb;10(2):464–473. doi: 10.1128/mcb.10.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer M. C., Schmid C., Waldvogel M., Schläpfer I., Futo E., Masiarz F. R., Green K., Barr P. J., Zapf J. Characterization of recombinant human insulin-like growth factor binding proteins 4, 5, and 6 produced in yeast. J Biol Chem. 1992 Jun 25;267(18):12692–12699. [PubMed] [Google Scholar]
- Kleinman D., Roberts C. T., Jr, LeRoith D., Schally A. V., Levy J., Sharoni Y. Regulation of endometrial cancer cell growth by insulin-like growth factors and the luteinizing hormone-releasing hormone antagonist SB-75. Regul Pept. 1993 Oct 20;48(1-2):91–98. doi: 10.1016/0167-0115(93)90338-9. [DOI] [PubMed] [Google Scholar]
- Leof E. B., Wharton W., van Wyk J. J., Pledger W. J. Epidermal growth factor (EGF) and somatomedin C regulate G1 progression in competent BALB/c-3T3 cells. Exp Cell Res. 1982 Sep;141(1):107–115. doi: 10.1016/0014-4827(82)90073-8. [DOI] [PubMed] [Google Scholar]
- Ling N. C., Liu X. J., Malkowski M., Guo Y. L., Erickson G. F., Shimasaki S. Structural and functional studies of insulin-like growth factor binding proteins in the ovary. Growth Regul. 1993 Mar;3(1):70–74. [PubMed] [Google Scholar]
- Liu J. P., Baker J., Perkins A. S., Robertson E. J., Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell. 1993 Oct 8;75(1):59–72. [PubMed] [Google Scholar]
- Lowe W. L., Jr, Roberts C. T., Jr, LeRoith D., Rojeski M. T., Merimee T. J., Fui S. T., Keen H., Arnold D., Mersey J., Gluzman S. Insulin-like growth factor-II in nonislet cell tumors associated with hypoglycemia: increased levels of messenger ribonucleic acid. J Clin Endocrinol Metab. 1989 Dec;69(6):1153–1159. doi: 10.1210/jcem-69-6-1153. [DOI] [PubMed] [Google Scholar]
- Macaulay V. M. Insulin-like growth factors and cancer. Br J Cancer. 1992 Mar;65(3):311–320. doi: 10.1038/bjc.1992.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubrey J. A., Steelman L. S., Mayo M. W., Algate P. A., Dellow R. A., Kaleko M. Growth-promoting effects of insulin-like growth factor-1 (IGF-1) on hematopoietic cells: overexpression of introduced IGF-1 receptor abrogates interleukin-3 dependency of murine factor-dependent cells by a ligand-dependent mechanism. Blood. 1991 Aug 15;78(4):921–929. [PubMed] [Google Scholar]
- Miura M., Li S., Baserga R. Effect of a mutation at tyrosine 950 of the insulin-like growth factor I receptor on the growth and transformation of cells. Cancer Res. 1995 Feb 1;55(3):663–667. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Nagamani M., Stuart C. A., Dunhardt P. A., Doherty M. G. Specific binding sites for insulin and insulin-like growth factor I in human endometrial cancer. Am J Obstet Gynecol. 1991 Dec;165(6 Pt 1):1865–1871. doi: 10.1016/0002-9378(91)90047-u. [DOI] [PubMed] [Google Scholar]
- Pietrzkowski Z., Lammers R., Carpenter G., Soderquist A. M., Limardo M., Phillips P. D., Ullrich A., Baserga R. Constitutive expression of insulin-like growth factor 1 and insulin-like growth factor 1 receptor abrogates all requirements for exogenous growth factors. Cell Growth Differ. 1992 Apr;3(4):199–205. [PubMed] [Google Scholar]
- Pietrzkowski Z., Sell C., Lammers R., Ullrich A., Baserga R. Roles of insulinlike growth factor 1 (IGF-1) and the IGF-1 receptor in epidermal growth factor-stimulated growth of 3T3 cells. Mol Cell Biol. 1992 Sep;12(9):3883–3889. doi: 10.1128/mcb.12.9.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzkowski Z., Wernicke D., Porcu P., Jameson B. A., Baserga R. Inhibition of cellular proliferation by peptide analogues of insulin-like growth factor 1. Cancer Res. 1992 Dec 1;52(23):6447–6451. [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. An ordered sequence of events is required before BALB/c-3T3 cells become committed to DNA synthesis. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2839–2843. doi: 10.1073/pnas.75.6.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. Induction of DNA synthesis in BALB/c 3T3 cells by serum components: reevaluation of the commitment process. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4481–4485. doi: 10.1073/pnas.74.10.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnicoff M., Ambrose D., Coppola D., Rubin R. Insulin-like growth factor-1 and its receptor mediate the autocrine proliferation of human ovarian carcinoma cell lines. Lab Invest. 1993 Dec;69(6):756–760. [PubMed] [Google Scholar]
- Rubini M., Werner H., Gandini E., Roberts C. T., Jr, LeRoith D., Baserga R. Platelet-derived growth factor increases the activity of the promoter of the insulin-like growth factor-1 (IGF-1) receptor gene. Exp Cell Res. 1994 Apr;211(2):374–379. doi: 10.1006/excr.1994.1101. [DOI] [PubMed] [Google Scholar]
- Sell C., Baserga R., Rubin R. Insulin-like growth factor I (IGF-I) and the IGF-I receptor prevent etoposide-induced apoptosis. Cancer Res. 1995 Jan 15;55(2):303–306. [PubMed] [Google Scholar]
- Sell C., Dumenil G., Deveaud C., Miura M., Coppola D., DeAngelis T., Rubin R., Efstratiadis A., Baserga R. Effect of a null mutation of the insulin-like growth factor I receptor gene on growth and transformation of mouse embryo fibroblasts. Mol Cell Biol. 1994 Jun;14(6):3604–3612. doi: 10.1128/mcb.14.6.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele-Perkins G., Turner J., Edman J. C., Hari J., Pierce S. B., Stover C., Rutter W. J., Roth R. A. Expression and characterization of a functional human insulin-like growth factor I receptor. J Biol Chem. 1988 Aug 15;263(23):11486–11492. [PubMed] [Google Scholar]
- Stiles C. D., Capone G. T., Scher C. D., Antoniades H. N., Van Wyk J. J., Pledger W. J. Dual control of cell growth by somatomedins and platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1279–1283. doi: 10.1073/pnas.76.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee D., Morales F. R., Hamilton T. C., Von Hoff D. D. Expression of insulin-like growth factor I, its binding proteins, and its receptor in ovarian cancer. Cancer Res. 1991 Oct 1;51(19):5107–5112. [PubMed] [Google Scholar]