Abstract
Background and Purpose
We investigated the cognitive profiles in a large sample of patients with multiple system atrophy-cerebellar ataxia (MSA-C) and compared directly them in patients with clinical diagnosis of probable MSA-C without dementia and control subjects with intact cognition.
Methods
We prospectively enrolled 26 patients with clinical diagnosis of probable MSA-C. All patients underwent a standardized neuropsychological test of the Seoul Neuropsychological Screening Battery.
Results
The score of Korean version of the Mini- Mental State Examination was significantly lower in patients with MSA-C (27.2 ± 2.5) than in control subjects (28.9 ± 1.0, p = 0.003). Patients with MSA-C showed a significantly worse performance in visuospatial function, 3 words recall, verbal immediate, delayed and recognition memory, visual delayed memory, phonemic and sementic Controlled Oral Word Association Test, and ideomotor praxis (p < 0.05).
Conclusions
Patients with MSA-C show more severe and more widespread cognitive dysfunctions than controls. Our results also indicate that cognitive dysfunction in patients with MCA-C is suggestive of disruption of the cerebellocortical circuits.
Keywords: Multiple system atrophy
Multiple system atrophy (MSA) is an adult-onset, sporadic, progressive neurodegenerative disease that presents with parkinsonism, cerebellar ataxia, autonomic failure, and corticospinal disorders in any combination.1–3 MSA was classified into two categories, by a consensus conference in 1998: MSA with predominant parkinsonism (MSA-P) and MSA with predominant cerebellar ataxia (MSA-C).1 Although dementia as defined Diagnostic and Statistical Manual of Mental Disorders is a nonsupporting feature for cases with diagnosing case of possible MSA, some studies have demonstrated that patients with MSA are cognitively impaired on neuropsychological tests, particularly with regard to frontal function and some memory dysfunction(retrieval and recognition and a verbal list-learning task).1,4–8 These studies mainly considered patients with the MSA-P type and only a few studies have reported on patients with MSA-C.9 In this study, we investigated the cognitive profiles of a large sample of patients with MSA-C and compared them directly to patients with a clinical diagnosis of probable MSA-C without dementia and control subjects with intact cognition.
Methods
Subjects
Participants were recruited consecutively from a university hospital. We prospectively enrolled 26 patients with a clinical diagnosis of probable MSA-C. The diagnosis of MSA-C was made according to the consensus criteria for probable MSA-C.2 Exclusion criteria included symptomatic causes of ataxia, most frequently spinocerebellar ataxia (SCA1,2,3,6,7) or evidence of focal brain lesions by magnetic resonance imaging (MRI). We examined all patients with MSA-C by brain MRI and brain [18F]-deoxyglucose positron emission tomography (FDG PET) and other neurological diseases were excluded by these images. All patients were evaluated by UMSARS (Unified Multiple System Atrophy Rating Scale)10 and interviewed and examined by the authors (Drs. Lee and Song) to assess of the severity of parkinsonism and ataxia. The clinical severity of MSA was expressed in terms of the total score on the unified MSA rating scale (UMSARS) (UMSARS-I, historical; UMSARS-II, motor examination; UMSARS-IV, global disability scales; sum of UMSARS-I, II and IV)10. Elderly volunteers were used as controls to compare cognitive profiles (n = 26; age, 60.4 ± 7.7 yr). The control subjects had no active neurological disorders, no cognitive disorders, and a minimum score of 28 on the Korean version of the Mini-Mental State Examination (K-MMSE). Informed consent was obtained from all patients and control subjects, and this study was approved by the institutional review board of our hospital.
Neuropsychological tests
All patients underwent a standardized neuropsychological test of the Seoul Neuropsychological Screening Battery (SNSB).11,12 The SNSB covers the following cognitive subsets: attention (forward and backward digit span and letter-cancellation tests); language and related functions (reading, writing, comprehension, repetition, confrontational naming using the Korean version of the Boston Naming Test (K-BNT);13 finger naming, right-left orientation, body-part identification, calculation, ideomotor and buccofacial praxis); visuospatial function [drawing an interlocking pentagon and the Rey Complex Figure Test (RCFT)]; verbal memory [three-word registration and recall, and the Seoul Verbal Learning Test (SVLT)]; visual memory (the RCFT, immediate recall, 20- min delayed recall, and recognition); and frontal executive function (motor impersistence, contrasting program, go-no-go test, fist-edge-palm, alternating hand movement, alternating square and triangle, Luria loop, phonemic and semantic Controlled Oral Word Association Test (COWAT), and Stroop test). We considered attention function to be abnormal if at least two of the three items. Abnormal memory function was defined as a score below the 16th percentile of the norm for the delayed recall on the SVLT or RCFT. Language was considered abnormal if the score on the K-BNT was below the 16th percentile of the norm, and abnormal visuospatial function was defined as a RCFT copying score below the 16th percentile of the norm. The frontal/executive function tests were classified into three groups: motor executive function, COWAT, and the Stroop test. Frontal/executive function was considered to be abnormal when at least two of three tests were abnormal. Age-, sex-, and education- specific norms for each test based on 447 normal subjects were available. The scores of these items were classified as abnormal if they were below the 16th percentiles of the scores from the age-, sex-, and education-matched normal controls.
MCI classification
Based on the above-mentioned criteria, mild cognitive impairment (MCI) was classified into four clinical subtypes: single- domain amnestic MCI, multiple-domain amnestic MCI, single-domain nonamnestic MCI, and multiple-domain nonamnestic MCI.14–16 We classified patients with MCI and MSA-C (MSA-MCI) based on five cognitive domains: attention, visuospatial, language, memory (verbal or visual), and frontal functions.14–16
Statistical analysis
Independent t-test and pearson Chi-square tests were used compare subscores of the SNSB between patients with MSA-C and control subjects when the variables were categorical and continuous, respectively. A two-sided significance level of p < 0.05 was deemed to be statistically significant. Statistical analyses were performed using commercially available software (SPSS, Version 13.0, SPSS Inc, Chicago, IL, USA).
Results
Demographic characteristics
The demographic characteristics of the patients with MSA-C and the control are shown in Table 1. No significant differences in age, education level, or Clinical Dementia Rating (CDR) were found between patients with MSA-C and control subjects. The K-MMSE scores were significantly lower in patients with MSA-C (27.2 ± 2.5) than in control subjects (28.9 ± 1.0, p = 0.003). The sum of the CDR box score was higher in patients with MSA-C (0.8 ± 0.6) than in control subjects (1.3 ± 0.8, p = 0.03).
Table 1.
Demographic characteristics between patients with MSA-C and control subjects
| MSA-C (n = 26) | Controls (n = 26) | p-value | |
|---|---|---|---|
| Age at examination (yr) | 57.3 (7.5) | 60.4 (7.7) | NS |
| Gender (number of men) | 20 | 11 | 0.01 |
| Education durations (yrs) | 12.1 (4.4) | 12.1 (4.4) | NS |
| K-MMSE | 27.2 (2.5) | 28.9 (1.0) | 0.003 |
| CDR | 0.5 (0.1) | 0.4 (0.2) | NS |
| SOB | 1.3 (0.8) | 0.8 (0.6) | 0.03 |
| Age at onset (yr) | 54.1 (7.9) | ||
| Disease duration (months) | 34.4 (15.7) | ||
| Cognitive impairment duration (months) | 7.7 (11.3) | ||
| UMSARS total | 39.4 (6.6) | ||
| UMSARS I | 19.2 (4.3) | ||
| UMSARS II | 18.0 (4.2) | ||
| UMSARS IV | 2.1 (0.7) |
MSA-C: multiple system atrophy if cerebellar features are predominant, UMSARS: unified multiple system atrophy rating scale, Values are expressed as mean (standard deviation), NS: not significant. K-MMSE: Korea version-Mini mental state examination, CDR: clinical dementia rating scale, SOB: the sum of boxes score of the CDR
MCI subtypes and frequency
According to these MCI classifications, there were 15 MSA- MCI patients and 11 MSA patients with intact cognition. One of the 26 patients with MSA-C (3.8%) was classified as single- domain amnestic MCI (verbal memory type). Of the four patients (15.4%) with single-domain nonamnestic MCI, the frontal domain was affected in one, and the attention domain was affected in three. Ten of 26 patients with MSA-C (38.5%) were classified as multiple-domain amnestic MCI, and no multiple-domain nonamnestic MCI was observed. Of 10 patients with multiple-domain amnestic MCI, one additional domain was impaired in three patients, two additional domains were impaired in four patients, and three additional domains were impaired in two patients.
The percentage of affected dominant was as follows: attention, 50%; visuospatial function, 90%; verbal memory, 70%; visual memory, 60%; frontal function, 60% (Figure 1).
Figure 1.
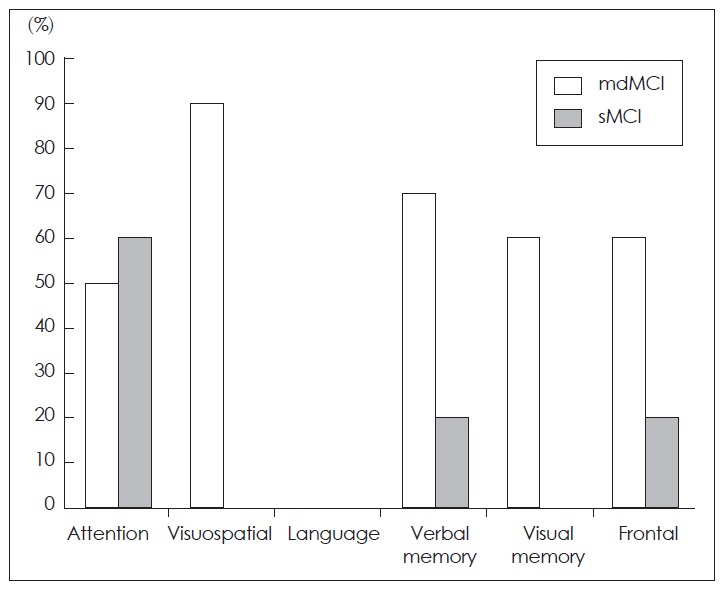
The percentage of patients with multiple domain MCI and single domain MCI according to the involved domain. MCI: mild cognitive impairment.
Neuropsycholgical findings
A comparison of the neuropsychological characteristics in control subjects and patients with MSA-C is summarized in Tables 2 and 3. Compared with control subjects, patients with MSA-C showed a significantly worse performance on measures of repetition, visuospatial function, three-word recall, verbal immediacy, delayed and recognition memory, visual delayed memory, sementic and phonemic generative naming on the COWAT, and ideomotor praxis (p < 0.05).
Table 2.
Neuropsychological data in patients with MSA-C and control subjects
| Test | MSA-C (n = 26) | Controls (n = 26) | p-value |
|---|---|---|---|
| Attention | |||
| Digit span (forward, 9) | 6.9 (1.5) | 6.7 (1.3) | NS |
| Digit span (backward, 8) | 4.0 (1.6) | 4.8 (1.7) | 0.09 |
| Digit span total (17) | 10.9 (2.6) | 11.5 (2.8) | NS |
| Language and related function | |||
| K-BNT (60) | 52.2 (5.5) | 51.3 (6.0) | NS |
| Repetition (15) | 14.7 (0.6) | 15.0 (0.2) | 0.01 |
| Calculation (12) | 11.3 (1.1) | 11.4 (1.1) | NS |
| Visuospatial function | |||
| RCFT (36) | 30.6 (7.0) | 35.4 (0.9) | 0.001 |
| Verbal memory function | |||
| 3 words registration (3) | 3.0 (0.2) | 3.0 (0.0) | NS |
| 3 words recall (3) | 1.8 (2.0) | 2.7 (0.5) | <0.0001 |
| SVLT | |||
| Immediate recall (36) | 18.9 (4.5) | 23.7 (4.0) | <0.0001 |
| Delayed recall (12) | 5.2 (2.6) | 8.4 (1.7) | <0.0001 |
| Recognition (24) | 20.5 (2.4) | 22.3 (1.3) | 0.002 |
| Visual memory function (RCFT) | |||
| Immediate recall (36) | 17.0 (9.4) | 21.3 (5.1) | 0.05 |
| Delayed recall (36) | 16.4 (8.9) | 21.1 (4.7) | 0.02 |
| Recognition (24) | 18.8 (2.2) | 19.7 (1.2) | 0.09 |
| Frontal executive function | |||
| Contrasting program (20) | 20.3 (1.8) | 19.9 (0.5) | NS |
| Go-no-go test (20) | 19.6 (3.3) | 19.9 (0.4) | NS |
| COWAT (Animal) | 13.9 (3.6) | 17.0 (3.4) | 0.002 |
| COWAT (Supermarket) | 15.6 (4.2) | 20.5 (4.0) | <0.001 |
| Phonemic generative naming | 25.4 (7.3) | 30.9 (8.0) | 0.01 |
| Word stroop test (112) | 104.0 (20.9) | 111.6 (1.4) | 0.07 |
| Color stroop test (112) | 82.5 (23.7) | 93.0 (15.7) | 0.07 |
MSA-C: multiple system atrophy if cerebellar features are predominant, K-BNT: the Korean version of Boston Naming Test, RCFT: rey complex figure test, SVLT: seoul verbal learning test, COWAT: the controlled oral word association Test. Values are expressed as mean (standard deviation), NS: not significant. Number in parenthesis of test column indicates possible maximal performance score
Table 3.
Neuropsychological data in control subjects and patients with MSA-C
| Test | MSA-C (n = 26) | Controls (n = 26) | p-value |
|---|---|---|---|
| Attention | |||
| Letter cancellation | 3 | 1 | NS |
| Language and related function | |||
| Comprehension | 2 | 1 | NS |
| Reading | 1 | 0 | NS |
| Writing | 0 | 0 | NS |
| Finger naming | 2 | 2 | NS |
| Right-left orientation | 2 | 7 | 0.07 |
| Body part identification | 0 | 0 | NS |
| Ideomotor praxis | 4 | 0 | 0.04 |
| Buccofacial praxis | 0 | 0 | NS |
| Visuospatial function | |||
| Interlocking pentagon | 2 | 1 | NS |
| Frontal executive function | |||
| Motor impersistence | 0 | 0 | NS |
| Fist-edge-palm test | 1 | 1 | NS |
| Alternating hand movement | 3 | 1 | NS |
| Alternating square and triangle | 5 | 0 | NS |
| Luria loop | 2 | 1 | NS |
All data were represented by the number of patients with abnormal score. MSA-C: multiple system atrophy if cerebellar features are predominant
Additionally, patients with MSA-C tended to have lower scores on backward digit span (p = 0.09), visual immediate memory (p = 0.05), recognition memory (p = 0.09), word-reading and color-reading tasks of the Stroop test (p = 0.07), and right-left disorientation (p = 0.07).
Discussion
Fifteen of the 26 MSA-C patients showed mild cognitive impairment in attention, visuospatial function, verbal memory, visual memory, and frontal function domains; of these, 38.5% (10 of 26 patients) had multiple-domain amnestic MCI, 15.4% (4 of 26 patients) had single-domain non-amnestic MCI, and 3.8% (1 of 26) had single-domain amnestic MCI. Furthermore, patients with MSA-C were impaired in repetition, visuospatial function, verbal immediacy, delayed and recognition memory, visual delayed memory, semantic and phonemic generative naming COWAT, and ideomotor praxis. In 2006, a pathologically confirmed case of MSA presenting with frontal executive and semantic language deficits was reported, and the pathological background of this cognitive impairment was shown to be widespread cortical involvement.17 Actually, several neurophysiological reports have shown significant cognitive decline in patients with MSA, suggesting frontal lobe impairment. These studies were concerned mainly with patients of the MSA-P type, and only a few studies considered patients with MSA-C.9 Patients with MSA-P showed wide-ranging cognitive dysfunctions such as visuospatial and constructive dysfunction, impaired verbal fluency, and dysexecutive syndrome, whereas patients with MSA-C had impaired visuospatial and constructional function, verbal memory, and executive function.8,9 The patients with MSA-P had significant hypoperfusion in the medial frontal cortices and dorsolateral prefrontal cortex, and the severity of cognitive impairment was significantly correlated with hypoperfusion in the dorsolateral prefrontal cortex. However, brain perfusion was decreased significantly in the cerebellum of patients with MSA-C.8
One possible explanation for the cognitive deficit in patients with MSA-C is to assume that parts of the cerebral cortex undergo degeneration in parallel with the pontocerebellar system. Another explanation for the cognitive deficits in cerebellar disease is based on a disruption in cerebrocerebellar connections. Previous studies have shown that impaired spatial cognition (visuospatial organization and memory), executive function (planning, set shifting, verbal fluency, abstract reasoning, and working memory), personality changes, and language deficits (agrammatism and dysprosodia) in patients with pure cerebellar involvement are related to corticocerebellar circuits, particularly the prefrontal cortex and cerebellum.8,17–20 Anatomical and physiological studies have revealed that cerebral association areas subserving higher order behavior are linked preferentially with the lateral hemispheres of the cerebellar posterior lobe. The output of the cerebellum is directed back to regions of the prefrontal cortex; particularly, the dorsolateral prefrontal cortex is a cortical target for the cerebello-thalamocortical pathway from the dentate, and this pathway is distinct from that innervating motor areas of the cerebral cortex. These connections may provide part of the anatomical substrate for the involvement of these subcortical nuclei in cognitive processing. 9,18–20
Our results also demonstrated that the extent of cognitive involvement was variable among individual patients with MSA-C. More patients were included in our study than in previous studies. Factors determining the evolution of cognitive involvement in MSA-C that are associated with cerebellar modulation of neural circuits and that link lateral prefrontal and other cortical areas (e.g., posterior parietal, superior temporal, and limbic cortices) need to be identified. Neuropsychological tests are needed for patients with MSA-C, Parkinson’s disease, and MSA-P. Furthermore, we must pay close attention to the cognitive impairment in patients with MSA-C.
Footnotes
The authors have no financial conflicts of interest.
REFERENCES
- 1.Gilman S, Low PA, Quinn N, Albanese A, Ben-Shlomo Y, Fowler CJ, et al. Consensus statement on the diagnosis of multiple system atrophy. J Neurol Sci. 1999;163:94–98. doi: 10.1016/s0022-510x(98)00304-9. [DOI] [PubMed] [Google Scholar]
- 2.Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim TS, Lee PH, Kim HS, Yong SW. White matter hyperintensities in patients with multiple system atrophy. J Neurol. 2009;256:1663–1670. doi: 10.1007/s00415-009-5176-5. [DOI] [PubMed] [Google Scholar]
- 4.Robbins TW, James M, Owen AM, Lange KW, Lees AJ, Leigh PN, et al. Cognitive deficits in progressive supranuclear palsy, Parkinson’s disease, and multiple system atrophy in tests sensitive to frontal lobe dysfunction. J Neurol Neurosurg Psychiatry. 1994;57:79–88. doi: 10.1136/jnnp.57.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berent S, Giordani B, Gilman S, Trask CL, Little RJ, Johanns JR, et al. Patterns of neuropsychological performance in multiple system atrophy compared to sporadic and hereditary olivopontocerebellar atrophy. Brain Cogn. 2002;50:194–206. doi: 10.1016/s0278-2626(02)00503-1. [DOI] [PubMed] [Google Scholar]
- 6.Soliveri P, Monza D, Paridi D, Carella F, Genitrini S, Testa D, et al. Neuropsychological follow up in patients with Parkinson’s disease, striatonigral degeneration-type multisystem atrophy, and progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 2000;69:313–318. doi: 10.1136/jnnp.69.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lange KW, Tucha O, Alders GL, Preier M, Csoti I, Merz B, et al. Differentiation of parkinsonian syndromes according to differences in executive functions. J Neural Transm. 2003;110:983–995. doi: 10.1007/s00702-003-0011-0. [DOI] [PubMed] [Google Scholar]
- 8.Kawai Y, Suenaga M, Takeda A, Ito M, Watanabe H, Tanaka F, et al. Cognitive impairments in multiple system atrophy: MSA-C vs MSA-P. Neurology. 2008;70:1390–1396. doi: 10.1212/01.wnl.0000310413.04462.6a. [DOI] [PubMed] [Google Scholar]
- 9.Bürk K, Daum I, Rüb U. Cognitive function in multiple system atrophy of the cerebellar type. Mov Disord. 2006;21:772–776. doi: 10.1002/mds.20802. [DOI] [PubMed] [Google Scholar]
- 10.Wenning GK, Tison F, Seppi K, Sampaio C, Diem A, Yekhlef F, et al. Development and validation of the Unified Multiple System Atrophy Rating Scale (UMSARS) Mov Disord. 2004;19:1391–1402. doi: 10.1002/mds.20255. [DOI] [PubMed] [Google Scholar]
- 11.Kang YND. Seoul Neuropsychological Screening Battery. Incheon: Human Brain Research & Consulting Co; 2003. [Google Scholar]
- 12.Lee JE, Park HJ, Park B, Song SK, Sohn YH, Lee JD, et al. A comparative analysis of cognitive profiles and white-matter alterations using voxel-based diffusion tensor imaging between patients with Parkinson’s disease dementia and dementia with Lewy bodies. J Neurol, Neurosurg Psychiatry. 2010;81:320–326. doi: 10.1136/jnnp.2009.184747. [DOI] [PubMed] [Google Scholar]
- 13.Kim H, Na DL. Normative data on the Korean version of the Boston Naming Test. J Clin Exp Neuropsychol. 1999;21:127–133. doi: 10.1076/jcen.21.1.127.942. [DOI] [PubMed] [Google Scholar]
- 14.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 15.Seo SW, Im K, Lee JM, Kim YH, Kim ST, Kim SY, et al. Cortical thickness in single- versus multiple-domain amnestic mild cognitive impairment. Neuroimage. 2007;36:289–297. doi: 10.1016/j.neuroimage.2007.02.042. [DOI] [PubMed] [Google Scholar]
- 16.Kim JW, Cheon SM, Park MJ, Kim SY, Jo HY. Cognitive Impairment in Parkinson’s Disease without Dementia: Subtypes and Influences of Age. J Clin Neurol. 2009;5:133–138. doi: 10.3988/jcn.2009.5.3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Apostolova LG, Klement I, Bronstein Y, Vinters HV, Cummings JL. Multiple system atrophy presenting with language impairment. Neurology. 2006;67:726–727. doi: 10.1212/01.wnl.0000230136.82118.16. [DOI] [PubMed] [Google Scholar]
- 18.Schmahmann JD, Caplan D. Cognition, emotion and the cerebellum. Brain. 2006;129:290–292. doi: 10.1093/brain/awh729. [DOI] [PubMed] [Google Scholar]
- 19.Middleton FA, Strick PL. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994;266:458–461. doi: 10.1126/science.7939688. [DOI] [PubMed] [Google Scholar]
- 20.Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]


