Abstract
Hemichorea-hemiballism (HC-HB) is a complication of non-ketotic hyperglycemia (NKH); in NKH patients, the frequency of occurrence of HC-HB is greater than that of bilateral chorea. We report the case of a hyperglycemic patient who showed chorea in both the lower limbs. Magnetic resonance imaging (MRI) of the brain revealed high signal intensity on T1-weighted images of the bilateral dorsolateral putamen. The abnormal involuntary movements disappeared after oral administration of haloperidol. Our case report that chorea associated with NKH is correlated with the topography of the basal ganglia.
Keywords: Chorea, Hyperglycemia, Basal ganglia
Chorea and ballismus can be caused by various conditions, including cerebrovascular disorders, infections, drugs, metabolic abnormalities, neurodegenerative diseases, and immunologic disorders. Non-ketotic hyperglycemia (NKH) is an unusual cause of chorea-ballismus, and chorea and ballismus is also a rare manifestation of primary diabetes mellitus.1 Clinically hemichorea-hemiballism (HC-HB) caused by NKH is often observed in elderly patients with a history of non-insulin dependent diabetes mellitus (NIDDM), and neuroimaging studies on these patients often reveal hyperintense regions in the contralateral putamen. In some reports, the occurrence of HC-HB in hyperglycemic patients was reported to be greater than that of bilateral chorea.2,3 In this paper, we have presented a unusual case of chorea associated with NKH that was confined to both the lower limbs and was correlated with the presence of hyperintense regions on both dorsolateral putamen in T1-weighted images.
Case Report
An 87-year-old woman with a 20-year history of NIDDM suddenly developed abnormal involuntary movements in both her lower limbs. 7 days after the onset of the hyperkinesias, the patient was referred to our department because the involuntary movements were sustained during metabolic control with insulin. The patient’s diabetes mellitus was poorly controlled; therefore, she had undergone glucose control in the department of endocrinology 3 weeks before she was referred to our department.
On examination, the patient was fully alert and well oriented. Her muscle strength, sensations and reflexes were symmetrically intact in all 4 extremities. There were no pathologic plantar responses. She had continuous, arrhythmic, and purposeless involuntary movements in both her lower limbs (Video, Segment 1). The choreic movements attenuated when she was relaxed, and it disappeared during her sleep. The initial reports obtained after admission to the endocrinology department showed significant evidence of poorly controlled diabetes mellitus, i.e., the fasting blood glucose level was 570 mg/dL; the serum osmolality was 313 mosmol/kg; and the glycosylated HbA1c level was 15.9%. The urine test was positive for glucose (4+) and negative for ketones. No abnormalities were observed in the electroencephalogram (EEG). Magnetic resonance imaging (MRI) of the brain revealed hyperintense regions on the bilateral dorsolateral putamen in the T1-weighted images (Figure 1). The patient’s blood sugar was controlled with insulin, but the chorea persisted. The choreic movements disappeared after oral administration of haloperidol (10 mg/day) for 10 days (Video, Segment 2).
Figure 1.
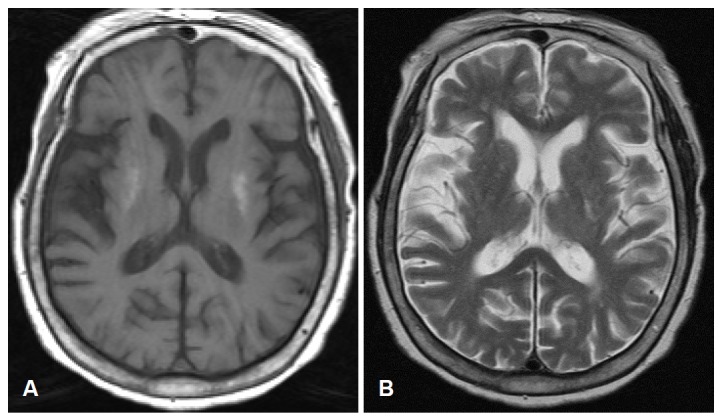
Magnetic resonance imaging of the brain at the level of basal ganglia. The bilateral dorsolateral putamen had high signal intense regions in the T1-weighted images (A) and iso signal intense regions in the T2-weighted images (B).
Discussion
NKH has been occasionally associated with various neurological abnormalities, including movement disorders. NKH is the second most common cause of HC-HB. Several studies on patients with HC-HB caused by NKH have reported that computed tomography (CT) and T1-weighted MR images of the brain show hyperintense regions in the contralateral striatum.1–4
Hyperglycemia produces a global decrease in the regional cerebral blood flow with the maximal reduction in the basal ganglia. Consequently, cerebral metabolism shifts to anaerobic pathways, causing inhibition of the tricarboxylic acid (Kreb’s) cycle. As an alternative source of energy, the brain may be forced to metabolized gamma-aminobutyrate acid (GABA) into succinic acid via the GABA shunt, thereby inducing metabolic acidosis. In contrast to the ketotic hyperglycemia, where can be resynthesized, GABA and acetate are depleted rapidly in NKH. The depletion of GABA and acetate in the basal ganglia in combination with energy depletion and metabolic acidosis may then lead to chorea.5–7 However, this hypothesis does not explain the persistence of chorea after the normalization of the blood sugar levels. Moreover, most patients with chorea associated with NKH develop hemichorea. This is unusual for the dyskinesia to be caused by systemic metabolic disorders. Overall, these findings suggest that NKH itself may not be the only mechanism responsible for developing chorea.
Dopaminergic neurons are direct targets for estrogen and that estrogen stimulates neurite extension/branching and the expression of tyrosine hydroxylase, the key enzyme in dopamine synthesis.8 Moreover, estrogen can change the sensitivity of dopamine autoreceptors.9 Acute estradiol studies come to the conclusion that physiological doses of estrogen not only increase dopamine activity but also affect dopamine-mediated behaviors such as head deviation and dyskinesia.10 Dopamine concentration of estrogen decrease in women after menopause, which contributes to the development of super-sensitivity in the striatal dopamine receptor.8–10 Dopamine receptor hypersensitivity in postmenopausal women is the reason for their predisposition to the development of chorea in NKH. Therefore, chorea caused by NKH have been mostly reported in older women2; even in our case, the patient was also an old woman.
In HC-HB that accompanies hyperglycemia, the T1-weighted images show high-signal intensity in the striatum contralateral to the involuntary movement. The T2-weighted images of the corresponding area are more variable.1,2,5 In this case, bilateral dorsolateral putamen were high signal intense on T1-weighted images. Several reports support the notion that putamen is characterized by a dorsoventral topography of somato-topic representations with leg dorsal, arm in the center and face ventral, based on observation of movements evoked by microstimulation, the neuronal activity related to movements, and the mapping of corticostriatal projections.11–13 In this case, the lesion in the bilateral dorsolateral putamen was somatotopically correlated with the chorea in both the lower limbs.
Legends of the Video
Segment 1
The pattern of involuntary movements was mainly continuous, arrhythmic, purposeless, and dancing like movements that were confined to both the lower limbs. The involuntary movements attenuated when she was relaxed, and it disappeared during her sleep.
Segment 2
The involuntary movements in both the lower limbs disappeared after oral administration of haloperidol for 10 days.
REFERENCES
- 1.Lai PH, Tien RD, Chang MH, Teng MM, Yang CF, Pan HB, et al. Chorea-ballismus with nonketotic hyperglycemia in primary diabetes mellitus. Am J Neuroradiol. 1996;17:1057–1064. [PMC free article] [PubMed] [Google Scholar]
- 2.Lee BC, Hwang SH, Chang GY. Hemiballismus-hemichorea in older diabetic women: a clinical syndrome with MRI correlation. Neurology. 1999;52:646–648. doi: 10.1212/wnl.52.3.646. [DOI] [PubMed] [Google Scholar]
- 3.Lin JJ, Lin GY, Shin C, Shen WC. Presentation of striatal hyperintensity on T1-weighted MRI in patients with hemiballism-hemichorea caused by non-ketotic hyperglycemia: report of seven new cases and a review of literature. J Neurol. 2001;248:750–755. doi: 10.1007/s004150170089. [DOI] [PubMed] [Google Scholar]
- 4.Yahikozawa H, Hanyu N, Yamamoto K, Hashimoto T, Shimozono K, Nakagawa S, et al. Hemiballism with striatal hyperintensity on T1-weighted MRI in diabetes patients: a unique syndrome. J Neurol Sci. 1994;124:208–214. doi: 10.1016/0022-510x(94)90328-x. [DOI] [PubMed] [Google Scholar]
- 5.Lee EJ, Choi JY, Lee SH, Song SY, Lee YS. Hemichorea-hemiballism in primary diabetic patients: MR correlation. J Comput Assist Tomogr. 2002;26:905–911. doi: 10.1097/00004728-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Duckrow RB, Beard DC, Brennan RW. Regional cerebral blood flow decreases during hyperglycemia. Ann Neurol. 1985;17:267–272. doi: 10.1002/ana.410170308. [DOI] [PubMed] [Google Scholar]
- 7.Ziemann U, Koc J, Reimers CD, Finkenstaedt M, Paulus W. Exploration of motor cortex excitability in a diabetic patient with hemiballismhemichorea. Mov Disord. 2000;15:1000–1005. doi: 10.1002/1531-8257(200009)15:5<1000::aid-mds1037>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 8.Küppers E, Beyer C. Expression of estrogen receptor-alpha and beta mRNA in the developing and adult mouse striatum. Neurosci Lett. 1999;276:95–98. doi: 10.1016/s0304-3940(99)00815-0. [DOI] [PubMed] [Google Scholar]
- 9.Piccardi P, Bernardi F, Rossetti Z, Corsini G. Effect of estrogens on dopamine autoreceptors in male rats. Eur Col J Pharmacol. 1983;91:1–9. doi: 10.1016/0014-2999(83)90355-2. [DOI] [PubMed] [Google Scholar]
- 10.Di Paolo T. Modulation of brain dopamine transmission by sex steroids. Rev Neurosci. 1994;5:27–41. doi: 10.1515/revneuro.1994.5.1.27. [DOI] [PubMed] [Google Scholar]
- 11.Nambu A, Kaneda K, Tokuno H, Takada M. Organization of corticostriatal motor inputs in monkey putamen. J Neurophysiol. 2002;88:1830–1842. doi: 10.1152/jn.2002.88.4.1830. [DOI] [PubMed] [Google Scholar]
- 12.Takada M, Tokuno H, Nambu A, Inase M. Corticostriatal input zones from the supplementary motor area overlap those from the contra-rather than ipsilateral primary motor cortex. Brain Res. 1998;791:335–340. doi: 10.1016/s0006-8993(98)00198-x. [DOI] [PubMed] [Google Scholar]
- 13.Miyachi S, Lu X, Imanishi M, Sawada K, Nambu A, Takada M. Somatotopically arranged inputs from putamen and subthalamic nucleus to primary motor cortex. Neurosci Res. 2006;56:300–308. doi: 10.1016/j.neures.2006.07.012. [DOI] [PubMed] [Google Scholar]


