Abstract

A series of novel antifungal carboline derivatives was designed and synthesized, which showed broad-spectrum antifungal activity. Particularly, compound C38 showed comparable in vitro antifungal activity to fluconazole without toxicity to human embryonic lung cells. It also exhibited good fungicidal activity against both fluconazole-sensitive and -resistant Candida albicans cells and had potent inhibition activity against Candida albicans biofilm formation and hyphal growth. Moreover, C38 showed good synergistic antifungal activity in combination with fluconazole (FLC) against FLC-resistant Candida species. Preliminary mechanism studies revealed that C38 might act by inhibiting the synthesis of fungal cell wall.
Keywords: Antifungal lead compounds, carboline derivatives, fungicidal activity
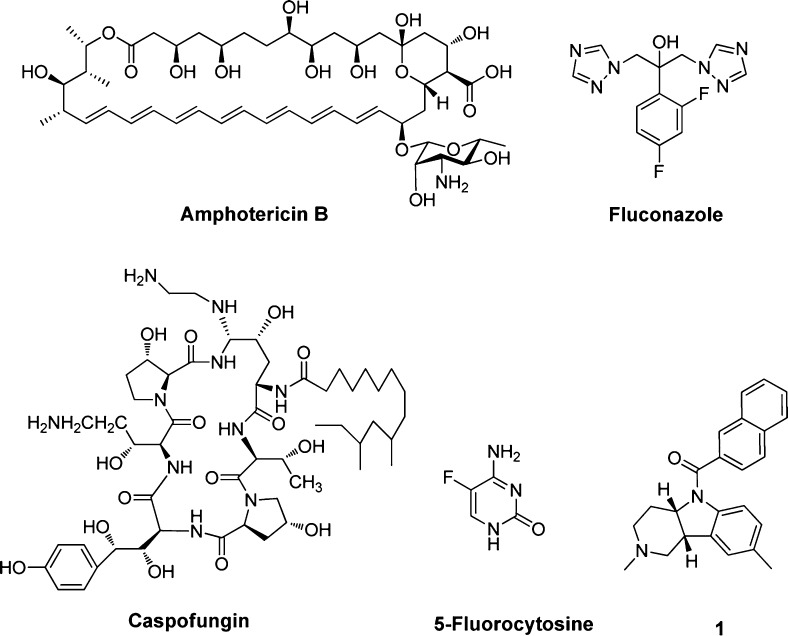
In recent years, the incidence and associated mortality of invasive fungal infections (IFIs) has been increasing dramatically.1,2 However, effective and safe antifungal agents are very limited. Clinically available antifungals (Figure 1) for IFIs mainly include: polyenes (e.g., amphotericin B), fluorinated pyrimidines (e.g., 5-fluorocytosine), azoles (e.g., fluconazole and voriconazole), and echinocandins (e.g., caspofungin, micafungin, and anidulafungin).3,4 Among them, amphotericin B has been used as the gold standard for almost all IFIs for more than 40 years, but severe nephrotoxicity has been associated with this drug.5,6 5-Fluorocytosine is usually used as adjunctive therapy. In combination with amphotericin B, it is effective against a number of mycoses, including some caused by the cryptococcus spp., dematiaceous fungi, and some Candida spp. The azole antifungals have emerged as front-line drugs in antifungal chemotherapy.7,8 Among the azoles, fluconazole plays an important role in treating both superficial and invasive yeast fungal infections due to its favorable pharmacokinetics, excellent activity against Candida spp., and safety profile.7,8 However, it is not effective against invasive aspergillosis and has suffered severe drug resistance. Echinocandin antifungals show potent activity against Candida and Aspergillus spp. but are not active in other common and emerging pathogens, such as Fusarium, Scedosporium, and Zygomycetes.9 Moreover, resistance to echinocandins has also been reported.10 Therefore, there is a need to discover and develop antifungal agents with novel chemotype and fungal-specific mode of action.
Figure 1.
Chemical structures of representative antifungal agents for the treatment of invasive fungal infections and hit compound 1.
Continuing our efforts on antifungal drug discovery,11−15 we performed a cell-based antifungal screen of an in-house library using the standard NCCLS (National Committee for Clinical Laboratory Standards) protocol. Compound 1 with a β-carboline scaffold was identified as a modest fungal inhibitor, with an MIC80 value of 32 and 8 μg/mL against Candida albicans and Cryptococcus neoformans, respectively. Because the chemical scaffold of compound 1 differs from that of all reported antifungal agents, it is interesting to investigate its structure–activity relationships (SARs) and discover the novel antifungal lead compound.
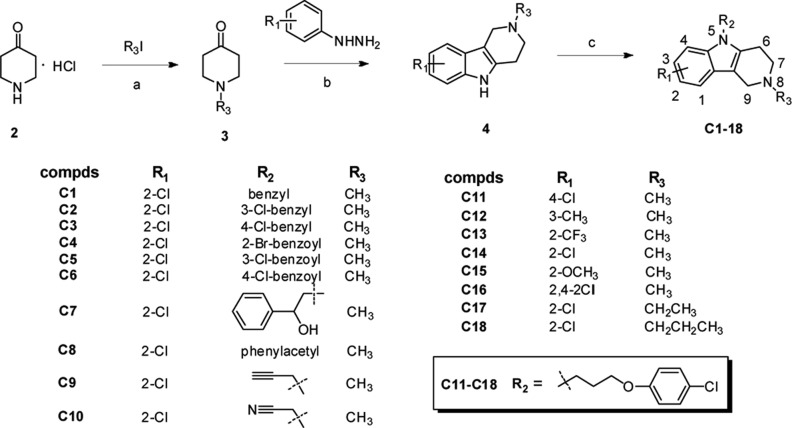
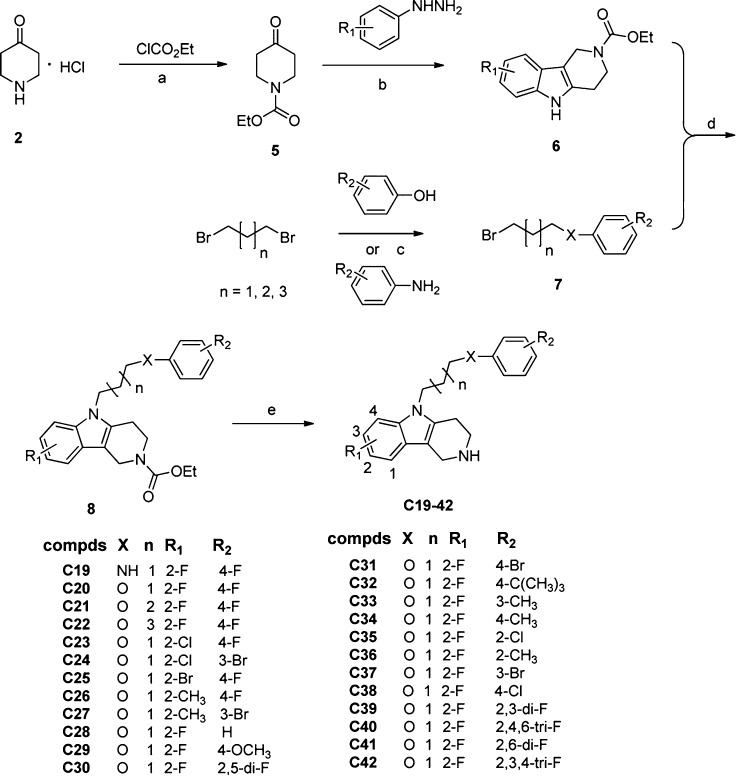
Thus, a series of novel carboline derivatives was designed and synthesized (Schemes 1 and 2). The in vitro antifungal activities are summarized in Tables 1 and 2. Most of the target compounds showed moderate to good inhibitory activity with broad spectrum. Among them, compound C38 showed potent inhibitory activity against all the tested fungal pathogens (MIC80 range: 1 to 4 μg/mL). Its activity against C. albicans (MIC80 = 2 μg/mL), C. neoformans (MIC80 = 1 μg/mL), M. gypseum (MIC80 = 1 μg/mL), and C. krusei (MIC80 = 4 μg/mL) was comparable or superior to FLC. Moreover, the cytotoxicity of C38 against WI38 cell line (human embryonic lung cell) was determined using the standard MTT method. The IC50 value of C38 was larger than 40 μg/mL, indicating that it had selective antifungal activity.
Scheme 1. Chemical Synthesis of Compounds C1–C18.
Reagents and conditions: (a) K2CO3, EtOH, reflux, 2 h; (b) 1,4-dioxane, conc. H2SO4, 80 °C, 3 h; (c) base (NaH or KOH), solvent (DMF or DMSO), rt or 45 °C.
Scheme 2. Chemical Synthesis of Compounds C19–C42.
Reagents and conditions: (a) K2CO3, CH2Cl2, rt, 6 h; (b) EtOH, reflux, 2 h; (c) K2CO3, EtOH, reflux, 4 h; (d) DMSO, KOH, rt, 12 h; (e) EtOH, Claisen hydrolysate, reflux, 3 h.
Table 1. In Vitro Antifungal Activity of Compounds C1–C18 (MIC80, μg·mL–1)a.
| compd | C. alb. | C. par. | C. neo. | C. gla. | A. fum. | T. rub. | M. gyp. |
|---|---|---|---|---|---|---|---|
| 1 | 32 | 16 | 8 | >64 | >64 | 16 | 32 |
| C1 | 64 | 64 | 32 | 32 | 64 | 16 | 16 |
| C2 | 64 | 32 | 16 | 16 | 32 | 8 | 16 |
| C3 | 64 | 64 | 16 | 16 | 64 | 64 | 16 |
| C4 | >64 | 64 | >64 | >64 | >64 | >64 | >64 |
| C5 | >64 | 64 | 32 | 32 | >64 | >64 | >64 |
| C6 | >64 | 64 | 32 | 32 | >64 | 32 | >64 |
| C7 | >64 | 64 | 32 | 32 | >64 | >64 | 32 |
| C8 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| C9 | >64 | 64 | 32 | 64 | >64 | >64 | 32 |
| C10 | >64 | >64 | 64 | >64 | >64 | >64 | 64 |
| C11 | 32 | 32 | 16 | 32 | >64 | >64 | 16 |
| C12 | 32 | 16 | 8 | 16 | 16 | 8 | 8 |
| C13 | >64 | >64 | 64 | 64 | >64 | 64 | 64 |
| C14 | 64 | 16 | 8 | 16 | >64 | >64 | 16 |
| C15 | 32 | 32 | 8 | >64 | 32 | 8 | 8 |
| C16 | >64 | >64 | 16 | >64 | >64 | >64 | 16 |
| C17 | 64 | 64 | 16 | 32 | >64 | >64 | 32 |
| C18 | >64 | 64 | 16 | 16 | >64 | >64 | 16 |
| FLC | 0.5 | 2 | 2 | 0.5 | >64 | 4 | 1 |
Abbreviations and strain numbers: C. alb., Candida albicans (SC5314); C. par., Candida parapsilosis (ATCC 22019); C. neo., Cryptococcus neoformans (32609); C. gla., Candida glabrata (537); A. fum., Aspergillus fumigatus (07544); T. rub., Trichophyton rubrum (Cmccftla); M. gyp., Microsporum gypseum (Cmccfmza); FLC, fluconazole.
Table 2. In Vitro Antifungal Activity of Compounds C19–C42 (MIC80, μg·mL–1)a.
| compd | C. neo. | C. alb. | C. par. | C. tro. | C. krusei | T. rub. | M. gyp. |
|---|---|---|---|---|---|---|---|
| C19 | 32 | 8 | 16 | 64 | 16 | 32 | 16 |
| C20 | 8 | 8 | 16 | 16 | 4 | 8 | 8 |
| C21 | 16 | 16 | 16 | 16 | 4 | 16 | 8 |
| C22 | 16 | 16 | 16 | 4 | 4 | 32 | 8 |
| C23 | 4 | 4 | 4 | 4 | 4 | 8 | 4 |
| C24 | 4 | 8 | 16 | 16 | 4 | 16 | 16 |
| C25 | 4 | 4 | 4 | 16 | 4 | 8 | 4 |
| C26 | 16 | 4 | 16 | 64 | 16 | 4 | 4 |
| C27 | 16 | 16 | 16 | 16 | 4 | 16 | 16 |
| C28 | 8 | 8 | >64 | 64 | 16 | 8 | 4 |
| C29 | 8 | 8 | >64 | >64 | 16 | 8 | 4 |
| C30 | 16 | 4 | 64 | 16 | 4 | 8 | 2 |
| C31 | 4 | 8 | 64 | 16 | 4 | 8 | 2 |
| C32 | 8 | 8 | 4 | 4 | 16 | 4 | 4 |
| C33 | 8 | 8 | 4 | 16 | 16 | 4 | 4 |
| C34 | 4 | 8 | 4 | 16 | 4 | 2 | 4 |
| C35 | 16 | 32 | 64 | 64 | 64 | 32 | 16 |
| C36 | 4 | 4 | 16 | 64 | 16 | 4 | 4 |
| C37 | 4 | 2 | 4 | 16 | 4 | 4 | 2 |
| C38 | 1 | 2 | 4 | 4 | 4 | 2 | 1 |
| C39 | 32 | 32 | 4 | 16 | 4 | 32 | 16 |
| C40 | 4 | 8 | 4 | 16 | 4 | 4 | 4 |
| C41 | 8 | 4 | 64 | 16 | 4 | 2 | 1 |
| C42 | 4 | 8 | 64 | 4 | 4 | 8 | 4 |
| FLC | 2 | 0.5 | 2 | 1 | 4 | 4 | 1 |
Abbreviations and strain numbers: C. alb., Candida albicans (SC5314); C. par., Candida parapsilosis (ATCC 22019); C. neo., Cryptococcus neoformans (32609); C. tro., Candida tropicalis; C. krusei, Candida krusei (0710397); T. rub., Trichophyton rubrum (Cmccftla); M. gyp., Microsporum gypseum (Cmccfmza); FLC, fluconazole.
Time-kill testing assay was used to test whether this new type of antifungal compound had fungicidal activity. The blank control and FLC at 32 μg/mL were used as controls. As shown in Figure 2A, compound 1 showed no significant difference compared with FLC against C. albicans SC5314 (FLC sensitive, MIC80 = 0.5 μg/mL), indicating that it may only had fungistatic activity, which was similar to that of FLC. The fungicidal activity of C38 was also investigated at the concentration range of 2–32 μg/mL. As shown in Figure 2B, C38 at 2 μg/mL showed no significant difference compared with FLC at 32 μg/mL against the C. albicans SC5314 strain. Interestingly, C38 showed fungicidal activity at the concentration of 4 μg/mL. When the concentration was increased to 8 and 16 μg/mL, the fungicidal activity was enhanced. To our delight, under the treatment of C38 at 32 μg/mL for 24 h, density of fungi cells was reduced to zero, whereas FLC had no significant effect at the same concentration. The results confirmed that carboline derivative C38 had excellent fungicidal activity against FLC-sensitive C. albicans in a dose-dependent manner.
Figure 2.
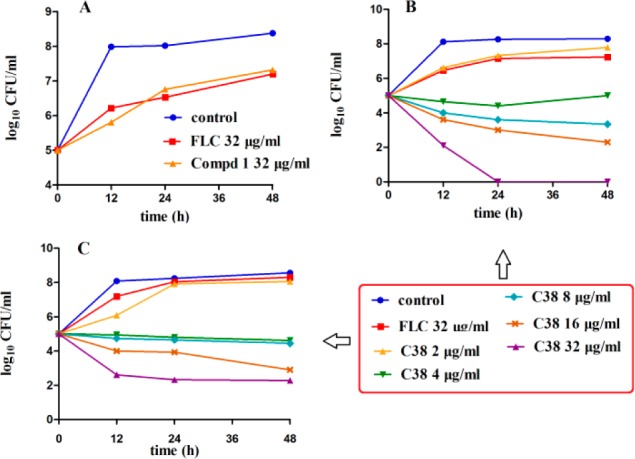
Time-kill curves of C. albicans obtained by using an initial inoculums of 105 CFU/mL. CFUs/mL were determined after 0, 12, 24, and 48 h of incubation. (A) C. albicans SC5314 (FLC-sensitive, MIC80 = 0.5 μg/mL) was treated/untreated with FLC(32 μg/mL) or lead compound 1 (32 μg/mL). (B) C. albicans SC5314 was treated/untreated with FLC (32 μg/mL) or different concentrations of compound C38. (C) C. albicans 103 (FLC-resistant, MIC80 > 64 μg/mL) was treated/untreated with FLC (32 μg/mL) or different concentrations of compound C38.
Importantly, fungal resistance caused by broad use of azoles is becoming a serious problem in recent years.16 It is highly desirable to find a new type of inhibitors that could be effective against azole-resistant fungal cells. C. albicans 103 (FLC resistant, MIC80 > 64 μg/mL) was used to test whether C38 could be effective against FLC-resistant fungi. As shown in Figure 2C, C38 obviously inhibited the growth of C. albicans 103 at the concentrations of 4 and 8 μg/mL. When the concentration was increased to 16 and 32 μg/mL, C. albicans cells were decreased to a larger extent. The results confirmed that compound C38 had potent fungicidal activity against FLC-resistant C. albicans.
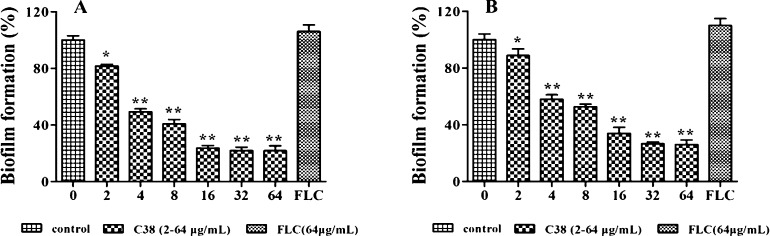
An important reason for the severe drug resistance is the strong ability of Candida cells to form biofilms.17,18 More over, biofilms could shield Candida cells from attack by the immune system and also block antifungal agents from reaching infected cells.19,20 The ability of C38 to inhibit biofilm formation of C. albicans SC5314 (FLC sensitive) and C. albicans 103 (FLC resistant) was investigated. FLC at 64 μg/mL and the blank control were used as controls. For the strains of C. albicans SC5314 and C. albicans 103 (Figure 3), the fungi cells treated with 64 μg/mL of FLC showed no inhibitory effect of biofilm formation and had no significant difference compared with the blank control, which was consistent with the previous reports.21−23 Interestingly, C38 showed a potent inhibitory effect on biofilm formation. Moreover, the inhibitory effect appeared to be dose-dependent. As shown in Figure 3A, C38 could inhibit >50% of biofilm formation at the concentration of 4 μg/mL. When the concentration was increased to 64 μg/mL, biofilm formation was significantly inhibited (>80%). More importantly, C38 also showed potent inhibitory effect on biofilm formation of the FLC-resistant C. albicans 103 strain (Figure 3B). It inhibited biofilm formation by >75% at the concentration of 32 μg/mL. The biofilm inhibition assay confirmed that C38 showed dose-related inhibitory activity on biofilm formation and that it was effective against both FLC-sensitive and FLC-resistant fungi.
Figure 3.
Effects of different concentrations of compound C38 on biofilm formation. C. albicans SC5314 (A) and C. albicans 103 (B) were used in this assay. Cells were treated with compound C38 (2–64 μg/mL) or FLC (64 μg/mL). Biofilm formation (as measured by XTT reduction) is expressed as a percentage of that of blank control. Results represent the mean ± standard deviations for three independent experiments. Statistical significance among the groups was determined by the analyses of variance (ANOVA). For comparison between two groups, the Student t test was used. A p-value of less than 0.05 was considered significant. *p < 0.05 compared to the control group; **p < 0.01 compared to the control group.
A striking feature of C. albicans biology is its ability to grow in a variety of morphological forms, such as yeast, pseudohyphae, and hyphae.24 In particular, the hyphae form could promote fungi cells to penetrate human epithelial and endothelial cells during the early stages of infection and cause damage.25,26 Moreover, hyphae is essential for pathogenicity by forming biofilms,27 which are highly resistant to standard antifungal treatments. Therefore, the inhibitory ability of C38 on the growth of C. albicans hyphae was determined (Figure 4). In the blank control group (Figure 4A), most of the C. albicans cells showed elongated hyphae forms. After treated with FLC at 8 μg/mL (Figure 4B), the hyphal growth was not inhibited, and the morphology was similar to that of the blank control. The result was consistent with the fact that FLC was not effective in C. albicans hyphal growth.28,29 After treated with C38 at 2 μg/mL (Figure 4C), most of the C. albicans cells appeared as pseudohyphae form, and the cells were much shorter than the control group. More interestingly, only unicellular budding yeast form was observed after exposure with C38 at 4 μg/mL or higher concentration (Figures 4D–F). In this assay, C38 showed potent inhibitory effect on the C. albicans hyphal growth, whereas FLC was ineffective.
Figure 4.
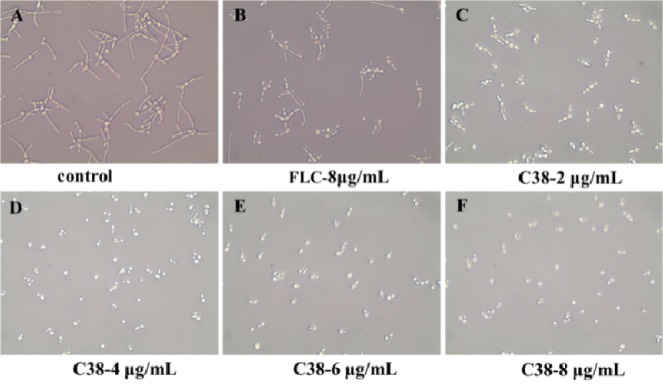
Effects of different concentrations of compound C38 or FLC on C. albicans SC5314 hyphal growth. The fungi cells were incubated in RPMI 1640 liquid medium at 37 °C for 16 h in the absence or presence of different concentrations of compound C38 (2 μg/mL to 8 μg/mL) or FLC (8 μg/mL). The hyphal development was viewed by light microscopy at ×400 and photographed.
The in vitro synergistic antifungal activity of compound C38 in combination with FLC was also conducted (Table 3). Four FLC-resistant C. albicans spp. (MIC80 > 1024 μg/mL) were used in this testing. To our delight, C38 alone showed good inhibitory activities against all tested Candida species with MIC80 values of 4 μg/mL. More interesting, C38 showed good synergistic activities in combination with FLC. The fractional inhibitory concentration index (FICI) value of C38 was 0.252, indicating C38 exhibited good synergistic activities against tested Candida species in combination with FLC. In this assay, C38 not only showed good antifungal activities against FLC-resistant Candida species but also showed potent synergistic activities in combination with FLC.
Table 3. In Vitro Synergistic Antifungal Activities of Compound C38 and FLC (MIC80, μg·mL–1).
| MIC80 alone |
MIC80 in combination |
|||||
|---|---|---|---|---|---|---|
| clinical isolates | FLC | C38 | FLC | C38 | FICIa | mode of interaction |
| 103 | >1024 | 4 | 2 | 1 | 0.252 | syn |
| 100 | >1024 | 4 | 2 | 1 | 0.252 | syn |
| 07109 | >1024 | 4 | 2 | 1 | 0.252 | syn |
| 805 | >1024 | 4 | 2 | 1 | 0.252 | syn |
Synergy and antagonism were defined by FICI ≤ 0.5 and >4, respectively. Indifferent was defined by 0.5 < FICI ≤ 4.
In order to reveal the antifungal mechanism of compound C38, its influence on the morphology of C. albicans was monitored by transmission electron microscopy (TEM). The normal C. albicans cells and cells treated with FLC at 8 μg/mL for 8 h were used as controls. As shown in Figure 5A, the normal C. albicans cell has a uniform central density and an intact cell wall. For the cells treated with FLC (Figure 5B), the cell membrane was damaged obviously, and the shape of the cells became abnormal. These alterations caused by FLC were consistent with the reported results that FLC acts on fungal cell membrane.30 After exposure to C38 (Figure 5C), the outer sphere of the cell wall became thinner, and the middle sphere became thicker. No obvious damage was observed on cell membrane. Some abnormal shapes of the vacuoles were also observed. In addition, reduced cytoplasmic density and necrosis could be observed. The TEM results indicated that C38 caused significant damage on C. albicans cells. Obvious cell wall change suggests that this new type of antifungal compound may have an effect on some synthetic routes of fungal cell wall.
Figure 5.

Transmission electron micrographs of C. albicans cells under different conditions. The organelles indicated by the arrows are as follows: 1, cell wall; 2, cell membrane; 3, the outer sphere of cell wall; 4, the middle sphere of cell wall; 5, vacuole; 6, reduced cytoplasmic density. The normal C. albicans cell (A) shows a uniform central density and a regularly outlined cell wall. The cell treated with FLC at 8 μg/mL for 8 h (B) shows damaged cell membrane and abnormal shape. Picture C shows the cell treated with compound C38 at 8 μg/mL for 8 h. It shows that the outer sphere of cell wall has become thinner and that the middle sphere has become thicker. Moreover, the reduced cytoplasmic density, necrosis, and some abnormal shape of vacuoles could also be observed.
GC-MS method was used to analyze the change of sterol composition in C. albicans cells, which could reflect the effect on C. albicans membrane. The assay has been successfully used in studying the mechanism of fungal resistance31,32 and the influence of inhibitors on sterol biosynthesis pathway.33 Cholesterol was added as internal standard and the results were shown in Table 4. In the blank control group, ergosterol and lanosterol comprised 90.0% and 1.5% of the total sterol fraction, respectively. Other 14-methylated sterols such as obtusifoliol and eburicol were not observed. When the cells were treated with FLC at 8 μg/mL for 24 h, the content of eburicol was increased up to 82.2% of the total sterol fraction. Other 14-methylated sterols (lanosterol and obtusifoliol) also increased obviously. In contrast, ergosterol content was reduced to lower than 1% in FLC treated group. These alternations were caused by competitive inhibition of CYP51 by FLC and consistent with the reported results.31,32 Interestingly, treatment with C38 also resulted in a significant reduction of ergosterol content (reduced to 40%) and an obvious accumulation of eburicol and lanosterol precursors (Table 4). However, the sterol composition change was not as obvious as FLC induced effects. The level of ergosterol (about 40% of the total sterol fraction) may not induce obvious change of fungal membrane structure, which was consistent with the results that no obvious damage was observed on cell membrane in the TEM testing. The GC-MS results infer that this new type of inhibitor might have a different mode of action with FLC.
Table 4. Relative Sterol Composition of C. albicans SC5314 after 24 h of Incubation.
| % of total
sterols (Candida albicans SC5314)a |
|||
|---|---|---|---|
| sterol | controlb | C38c | FLCd |
| ergosterol | 90.0 | 40.2 | 0.6 |
| obtusifoliol | 0.0 | 3.7 | 6.5 |
| lanosterol | 1.5 | 12.2 | 5.9 |
| eburicol | 0.0 | 12.4 | 82.2 |
| unidentified | 8.5 | 31.5 | 4.8 |
Sterol proportions varied by less than 10% in three experiments.
Control (no drug).
Treated with compound C38 at 8 μg/mL.
Treated with FLC at 8 μg/mL. The data was the average of three independent experiments.
Cytochrome P450 (CYP) enzymes play an important role in the metabolic clearance of the vast majority of prescribed drugs. The assessment of CYP enzymes inhibition or inactivation remains an important role in overall drugs’ safety assessment. Clinical drug–drug interactions (DDI) caused by inhibiting these CYP enzymes can result in dangerous side effects, caused by reduced clearance/increased exposure of a second drug.34 Thus, the DDI potential for compound C38 was investigated in vitro using four major human CYP isoforms (2C19, 2C9, 3A4-M, and 3A4-T). As shown in Table 5, compound C38 had weak inhibitory activity against 2C19, 2C9, 3A4-M, and 3A4-T with IC50 values of ≥25 μM, indicating it had low potential to cause DDI35 (see Supporting Information for data of positive drugs).
Table 5. In Vitro CYP Inhibition Assessment of Compound C38.
| % inhibition |
||||||
|---|---|---|---|---|---|---|
| compd | isoenzyme | 25 μM | 2.5 μM | 0.25 μM | IC50 (μM) | potential inhibitiona |
| C38 | 2C19 | 33 | 5 | 4 | >25 | low |
| C38 | 2C9 | 36 | 8 | –2 | >25 | low |
| C38 | 3A4-M | –35 | –10 | 1 | 50 | low |
| C38 | 3A4-T | 50 | 10 | 5 | 25 | low |
IC50 > 10 μM, CYP inhibition low; 10 μM > IC50 > 3 μM, CYP inhibition moderate; 3 μM > IC50, CYP inhibition high.35
In summary, a series of novel antifungal carboline derivatives was designed and synthesized. The most active compound C38 showed several advantages as a promising antifungal lead: (1) it showed broad-spectrum antifungal activity, comparable to FLC; (2) it revealed potent fungicidal activity against both FLC-sensitive and FLC-resistant C. albicans cells, whereas FLC only showed fungistatic activity; (3) it was a good inhibitor of C. albicans biofilm formation and hyphal growth, suggesting its potential to overcome FLC-related drug resistance; (4) it showed good synergistic antifungal activities in combination with FLC against FLC-resistant Candida species; (5) it might have a new mode of action resulting from inhibiting the synthetic route of fungal cell wall; (6) Preliminary CYP enzymes inhibition assay showed C38 had weak inhibition against tested human CYP isoforms, indicating it had low potential to cause DDI. Further structural optimization and antifungal mechanism of the carboline derivatives are in progress.
Glossary
ABBREVIATIONS
- IFI
Invasive fungal infection
- AmB
amphotericin B
- FLC
fluconazole
- SAR
structure–activity relationship
- FICI
fractional inhibitory concentration index
- CYP
cytochrome P450
- DDI
drug–drug interactions
- TEM
transmission electron microscopy
Supporting Information Available
Structure–activity relationships; chemical synthesis and structural characterization of the target compounds; protocols of biological assays. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
∥ S.W. and Y.W. contributed equally to this work.
This work was supported in part by National Natural Science Foundation of China (grants 81222044 and 81273558), Key Project of Science and Technology of Shanghai (grant 13431900301), the 863 Hi-Tech Program of China (grant 2012AA020302), the National Basic Research Program of China (grant 2014CB541800), Shanghai Rising-Star Program (grant 12QH1402600), and Shanghai Municipal Health Bureau (grant XYQ2011038) for financial support.
The authors declare no competing financial interest.
Supplementary Material
References
- Lai C. C.; Tan C. K.; Huang Y. T.; Shao P. L.; Hsueh P. R. Current challenges in the management of invasive fungal infections. J. Infect. Chemother. 2008, 14, 77–85. [DOI] [PubMed] [Google Scholar]
- Park B. J.; Wannemuehler K. A.; Marston B. J.; Govender N.; Pappas P. G.; Chiller T. M. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. Aids 2009, 23, 525–530. [DOI] [PubMed] [Google Scholar]
- Odds F. C.; Brown A. J.; Gow N. A. Antifungal agents: mechanisms of action. Trends Microbiol. 2003, 11, 272–279. [DOI] [PubMed] [Google Scholar]
- Odds F. C. Genomics, molecular targets and the discovery of antifungal drugs. Rev. Iberoam. Micol. 2005, 22, 229–237. [DOI] [PubMed] [Google Scholar]
- Gallis H. A.; Drew R. H.; Pickard W. W. Amphotericin B: 30 years of clinical experience. Rev. Infect. Dis. 1990, 12, 308–329. [DOI] [PubMed] [Google Scholar]
- Aperis G.; Alivanis P. Posaconazole: a new antifungal weapon. Rev. Recent Clin. Trials 2011, 6, 204–219. [DOI] [PubMed] [Google Scholar]
- Lass-Florl C. Triazole antifungal agents in invasive fungal infections: a comparative review. Drugs 2011, 71, 2405–2419. [DOI] [PubMed] [Google Scholar]
- Watt K.; Manzoni P.; Cohen-Wolkowiez M.; Rizzollo S.; Boano E.; Jacqz-Aigrain E.; Benjamin D. K. Triazole use in the nursery: fluconazole, voriconazole, posaconazole, and ravuconazole. Curr. Drug Metab. 2013, 14, 193–202. [PMC free article] [PubMed] [Google Scholar]
- Bal A. M. The echinocandins: three useful choices or three too many?. Int. J. Antimicrob. Agents 2010, 35, 13–18. [DOI] [PubMed] [Google Scholar]
- Baixench M. T.; Aoun N.; Desnos-Ollivier M.; Garcia-Hermoso D.; Bretagne S.; Ramires S.; Piketty C.; Dannaoui E. Acquired resistance to echinocandins in Candida albicans: case report and review. J. Antimicrob. Chemother. 2007, 59, 1076–1083. [DOI] [PubMed] [Google Scholar]
- Sheng C.; Zhang W.; Ji H.; Zhang M.; Song Y.; Xu H.; Zhu J.; Miao Z.; Jiang Q.; Yao J.; Zhou Y.; Lu J. Structure-based optimization of azole antifungal agents by CoMFA, CoMSIA, and molecular docking. J. Med. Chem. 2006, 49, 2512–2525. [DOI] [PubMed] [Google Scholar]
- Sheng C.; Chen S.; Ji H.; Dong G.; Che X.; Wang W.; Miao Z.; Yao J.; Lu J.; Guo W.; Zhang W. Evolutionary trace analysis of CYP51 family: implication for site-directed mutagenesis and novel antifungal drug design. J. Mol. Model. 2010, 16, 279–284. [DOI] [PubMed] [Google Scholar]
- Wang W.; Wang S.; Liu Y.; Dong G.; Cao Y.; Miao Z.; Yao J.; Zhang W.; Sheng C. Novel conformationally restricted triazole derivatives with potent antifungal activity. Eur. J. Med. Chem. 2010, 45, 6020–6026. [DOI] [PubMed] [Google Scholar]
- Yao J.; Liu H.; Zhou T.; Chen H.; Miao Z.; Sheng C.; Zhang W. Total synthesis and structure–activity relationships of new echinocandin-like antifungal cyclolipohexapeptides. Eur. J. Med. Chem. 2012, 50, 196–208. [DOI] [PubMed] [Google Scholar]
- Jiang Z.; Wang Y.; Wang W.; Wang S.; Xu B.; Fan G.; Dong G.; Liu Y.; Yao J.; Miao Z.; Zhang W.; Sheng C. Discovery of highly potent triazole antifungal derivatives by heterocycle-benzene bioisosteric replacement. Eur. J. Med. Chem. 2013, 64C, 16–22. [DOI] [PubMed] [Google Scholar]
- Pfaller M. A. Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. Am. J. Med. 2012, 125, S3–13. [DOI] [PubMed] [Google Scholar]
- Tumbarello M.; Fiori B.; Trecarichi E. M.; Posteraro P.; Losito A. R.; De Luca A.; Sanguinetti M.; Fadda G.; Cauda R.; Posteraro B. Risk factors and outcomes of candidemia caused by biofilm-forming isolates in a tertiary care hospital. PLoS One 2012, 7, e33705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhomme J.; d’Enfert C. Candida albicans biofilms: building a heterogeneous, drug-tolerant environment. Curr. Opin. Microbiol. 2013, 16, 398–403. [DOI] [PubMed] [Google Scholar]
- Nett J. E.; Sanchez H.; Cain M. T.; Andes D. R. Genetic basis of Candida biofilm resistance due to drug-sequestering matrix glucan. J. Infect. Dis. 2010, 202, 171–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nett J. E.; Sanchez H.; Cain M. T.; Ross K. M.; Andes D. R. Interface of Candida albicans biofilm matrix-associated drug resistance and cell wall integrity regulation. Eukaryot. Cell 2011, 10, 1660–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn D. M.; George T.; Chandra J.; Mukherjee P. K.; Ghannoum M. A. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob. Agents Chemother. 2002, 46, 1773–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falagas M. E.; Kapaskelis A. M.; Kouranos V. D.; Kakisi O. K.; Athanassa Z.; Karageorgopoulos D. E. Outcome of antimicrobial therapy in documented biofilm-associated infections: a review of the available clinical evidence. Drugs 2009, 69, 1351–1361. [DOI] [PubMed] [Google Scholar]
- Vandeputte P.; Ferrari S.; Coste A. T. Antifungal resistance and new strategies to control fungal infections. Int. J. Microbiol. 2012, 2012, 713687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudbery P.; Gow N.; Berman J. The distinct morphogenic states of Candida albicans. Trends Microbiol. 2004, 12, 317–324. [DOI] [PubMed] [Google Scholar]
- Filler S. G.; Sheppard D. C. Fungal invasion of normally non-phagocytic host cells. PLoS Pathog. 2006, 2, e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalle F.; Wachtler B.; L’Ollivier C.; Holland G.; Bannert N.; Wilson D.; Labruere C.; Bonnin A.; Hube B. Cellular interactions of Candida albicans with human oral epithelial cells and enterocytes. Cell Microbiol. 2010, 12, 248–271. [DOI] [PubMed] [Google Scholar]
- ten Cate J. M.; Klis F. M.; Pereira-Cenci T.; Crielaard W.; de Groot P. W. Molecular and cellular mechanisms that lead to Candida biofilm formation. J. Dent. Res. 2009, 88, 105–115. [DOI] [PubMed] [Google Scholar]
- Brayman T. G.; Wilks J. W. Sensitive assay for antifungal activity of glucan synthase inhibitors that uses germ tube formation in Candida albicans as an end point. Antimicrob. Agents Chemother. 2003, 47, 3305–3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N. A.; Miyazaki M.; Horii T.; Sagane K.; Tsukahara K.; Hata K. E1210, a new broad-spectrum antifungal, suppresses Candida albicans hyphal growth through inhibition of glycosylphosphatidylinositol biosynthesis. Antimicrob. Agents Chemother. 2012, 56, 960–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montes B.; Mallie M.; Jouvert S.; Bastide J. M. Morphological changes of Candida albicans induced by saperconazole. Mycoses 1991, 34, 287–292. [DOI] [PubMed] [Google Scholar]
- Jia X. M.; Wang Y.; Jia Y.; Gao P. H.; Xu Y. G.; Wang L.; Cao Y. Y.; Cao Y. B.; Zhang L. X.; Jiang Y. Y. RTA2 is involved in calcineurin-mediated azole resistance and sphingoid long-chain base release in Candida albicans. Cell. Mol. Life Sci. 2009, 66, 122–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel C. M.; Parker J. E.; Bader O.; Weig M.; Gross U.; Warrilow A. G.; Rolley N.; Kelly D. E.; Kelly S. L. Identification and characterization of four azole-resistant erg3 mutants of Candida albicans. Antimicrob. Agents Chemother. 2010, 54, 4527–4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle P. S.; Chen C. K.; Johnston J. B.; Hopkins S. D.; Leung S. S.; Jacobson M. P.; Engel J. C.; McKerrow J. H.; Podust L. M. A nonazole CYP51 inhibitor cures Chagas’ disease in a mouse model of acute infection. Antimicrob. Agents Chemother. 2010, 54, 2480–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettleton D. O.; Einolf H. J. Assessment of cytochrome p450 enzyme inhibition and inactivation in drug discovery and development. Curr. Top. Med. Chem. 2011, 11, 382–403. [DOI] [PubMed] [Google Scholar]
- Kerns E. H.; Di L.. Cytochrome P450 Inhibition. In Drug-like Properties: Concepts, Structure Design and Methods; Kerns E. H., Di L., Eds.; Academic Press: San Diego, CA, 2008; Chapter 15, pp 197–208. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.