Abstract
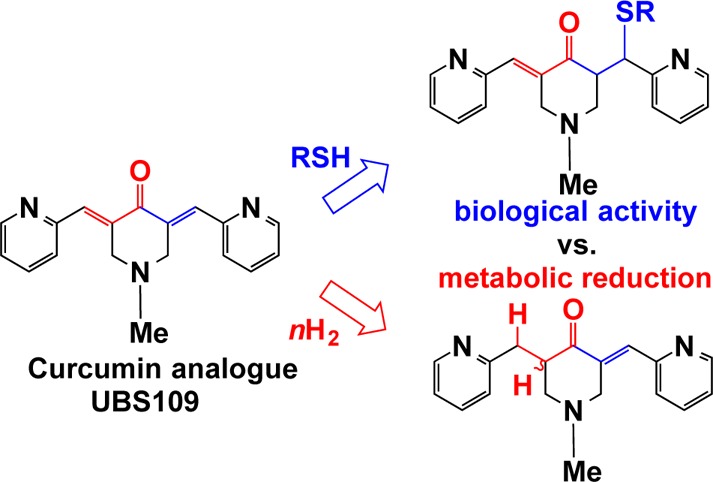
To address the shortcomings of the natural product curcumin, many groups have created analogues that share similar structural features while displaying superior properties, particularly in anticancer drug discovery. Relatively unexplored have been the mechanisms by which such compounds are metabolized. A comprehensive in vitro study of a curcumin analogue (UBS109) in liver S9 fractions from five different species is presented. Further, we examine the cell-based bioactivity of the major metabolites. In spite of the fact that UBS109 reduces tumor growth in mice, it is quickly metabolized in vitro and 94% protein bound in mouse plasma. The primary monounsaturated metabolite is only modestly bioactive against MDA-MB-231 breast cancer cells. These observations suggest that while the α,β-unsaturated ketone common to curcumin analogues is important for bioactivity, protein binding and tissue distribution may serve to protect UBS109 from full metabolism in vivo while allowing it to exert a pharmacological effect by means of slow drug release.
Keywords: Curcumin analogue, curcumin, UBS109, liver S9 fraction, metabolism
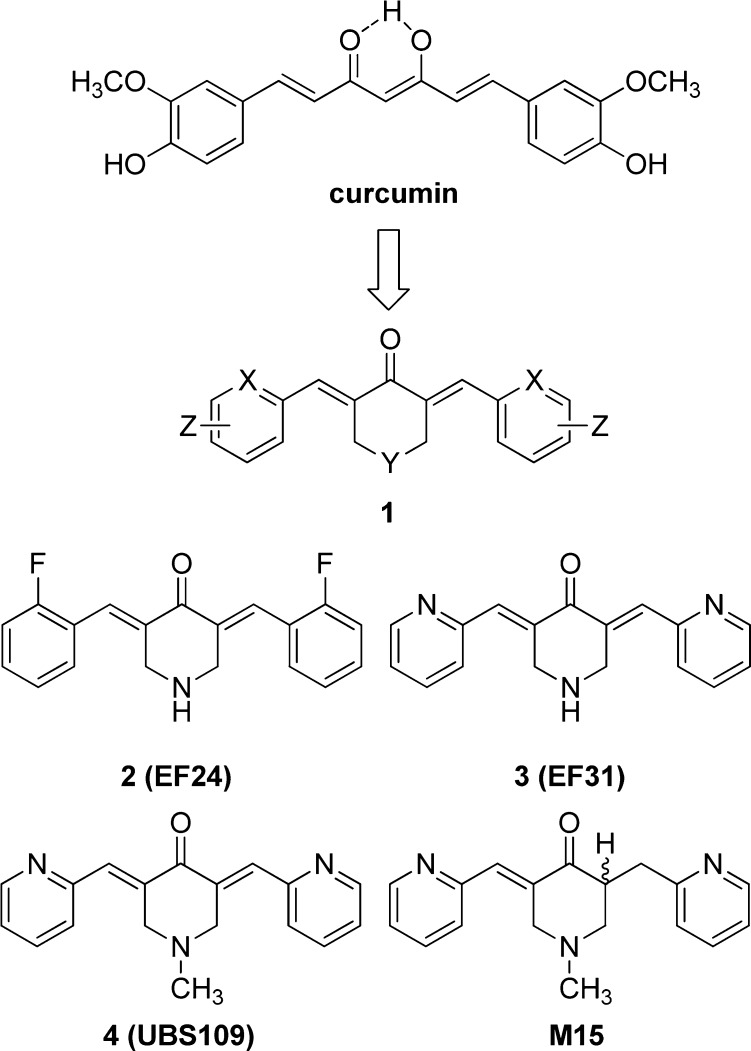
Since 2004, our laboratories have designed, synthesized, and tested numerous variations of the monocarbonyl curcumin analogues 1 (Figure 1) as potential antitumor and anti-inflammatory drugs. As a preliminary, a subset of analogues were subjected to the National Cancer Institute’s 60-cell line assay panel, which monitors tumor cell growth (GI50) by means of in vitro anticancer and antiangiogenesis screens.1
Figure 1.
Curcumin’s deconstruction to monocarbonyl analogues as curcumin mimics (1). Active agents against tumorogenic and angiogenic cell lines (2–4) and the cytotoxic metabolite (M15).
While a number of the compounds showed promising activities, EF24, 2, stood out as a novel lead. The agent has been associated with cell cycle arrest and caspase-mediated apoptosis in human breast cancer,2 prostate cancer,2 colon cancer,3 and gastric adenocarcinoma3 cell lines. EF24 has a substantial effect on markers of oxidative stress2 and suppression of the NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) signaling pathway via direct inhibition of IκB kinase (IKK)-mediated phosphorylation of IκB.4 In addition, it elicits significant regression of breast tumor xenografts (MDA-MB-231) in nude mice.1 In spite of these encouraging results, the aqueous solubility of the compound has prevented its development. As a result, the more soluble compounds 3 and 4 (EF31 and UBS109, respectively) were explored as alternatives. All three analogues are effective as inhibitors of an array of human kinases.5 Analogue 3 proved more efficacious than 2 in an inflammatory model using mouse RAW264.7 macrophages and more potently inhibited the NF-κB pathway by blockade of IKKβ kinase.6 In a separate study, 3 and the considerably more soluble UBS109 have been compared in a human head and neck squamous cell carcinoma (SCC) Tu212 xenograft tumor model established in athymic nude mice. Both EF31 (i.p.) and UBS109 (p.o) inhibit tumor growth significantly over 5–6 week periods in this model.7,8 An accompanying PK study reveals a satisfying in vitro–in vivo correlation in the mid-nM range. In addition, UBS109 is shown to be nontoxic to nonmalignant breast cells (MCF-10A) but cytotoxic for seven cancer cell lines.8 Finally, 3 and UBS109 revealed a suppressive effect on osteoclastogenesis as compared with curcumin, while the latter exhibited a unique stimulatory effect on osteoblastic differentiation and mineralization associated with Smad signaling.9 For these reasons, we selected UBS109 as a potential development candidate. To assist the choice of animal model for preclinical toxicology studies, we determined the metabolites for UBS109 by means of pooled hepatic S9 fractions from five animal species: mouse, rat, dog, cynomolgus monkey, and human.10 Remarkably, the majority of the compound’s metabolism in all species appears to be reductive in nature. Each of the major metabolites was independently synthesized and assayed for cytotoxic activity. Only one agent with a single electrophilic moiety, M15, proved to carry any antitumor activity in cell-based assays.
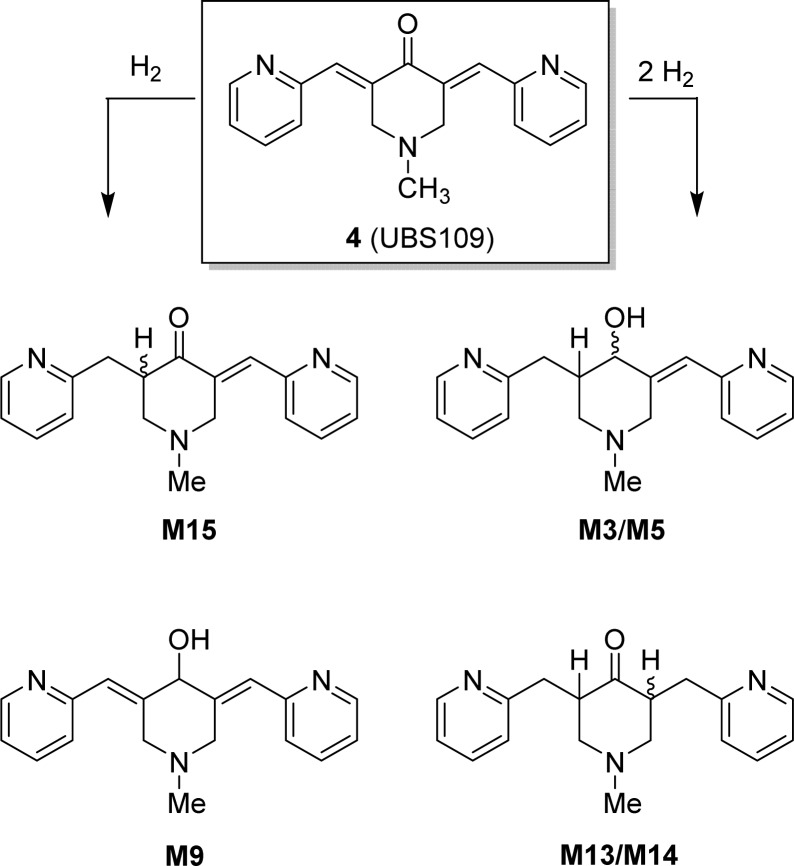
Results for the metabolic stability of UBS109 in S9 fractions and control compounds are presented in the Supporting Information (Table S11). The values obtained are in line with the tabulated acceptance criteria developed by Scynexis, Inc.11 Table S8 (see Supporting Information) provides a summary of putative metabolites detected in each of the five species included in this study. The top five human metabolites in terms of peak areas at the 60 min time point are M15, M14, M5, M9, and M13. These metabolites were selected for fragment interpretation. Structures for M15, M14/M13, M9, and M5/M3 are shown in Scheme 1. These structures have been independently verified using authentic synthetic standards.
Scheme 1. Proposed Structures of Major Metabolites of UBS109.
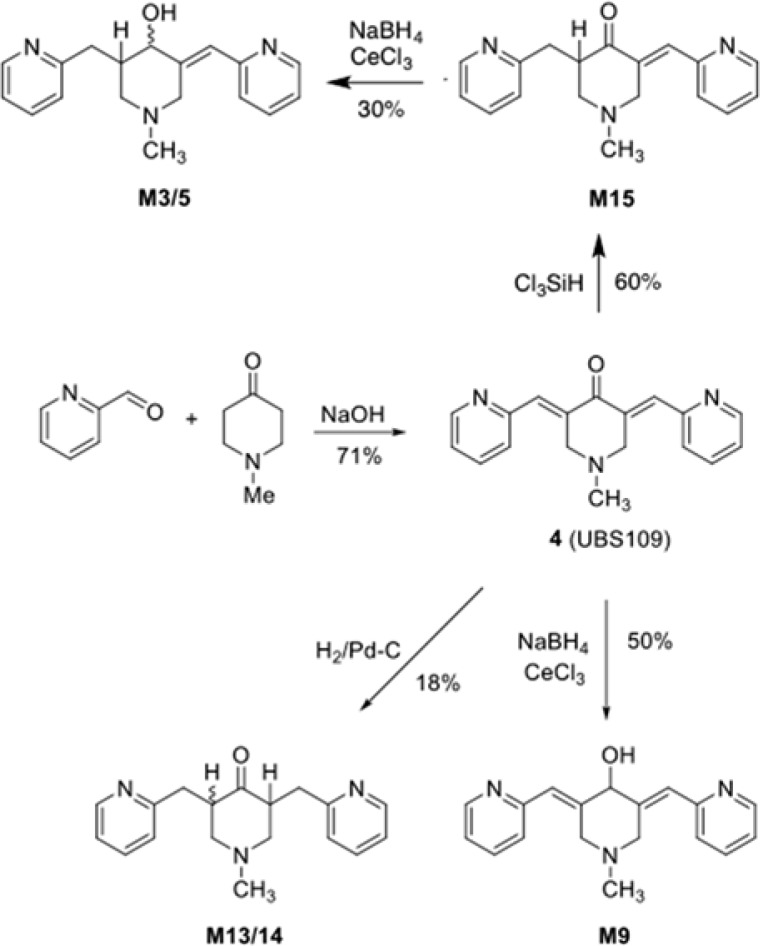
As depicted in Scheme 2, compound UBS109 was synthesized through a base-catalyzed aldol reaction using a modification of our previously reported procedure.1M15 was synthesized by reduction of one of the olefinic double bonds of UBS109 with trichlorosilane based on a previously reported procedure.12,9 We note that inclusion of 20% hexamethylphosphoramide (HMPA) as a Lewis base during this reduction, as carried out in the literature example,12 seemed to have little effect on the outcome of the reaction; moreover, HMPA was very difficult to remove from the product, even after extensive aqueous washes and multiple rounds of column chromatography. A portion of M15 was reduced under Luche conditions to give M3/5, which could be separated to at least an 80:20 diastereomeric ratio by column chromatography, although the structures remain unassigned.13 Similarly, M9 was prepared by Luche reduction of UBS109. M13/14 was prepared as a mixture of diastereomers by catalytic hydrogenation of both olefinic double bonds of the latter dienone.
Scheme 2. Synthesis of UBS109 (4) and Metabolites.
Neutral red cytotoxicity assays using the human tumor cell lines TU212 (head and neck squamous cell carcinoma (SCC)) and MDA-MB-231 (breast adenocarcinoma) were carried out with UBS109, M15, M13/14, M3/5, and M9 at 5 and 10 μM and a DMSO concentration <0.1%. Of these compounds, only UBS109 showed any appreciable activity: the GI50 values14 for M15, M13/14, M5, and M9 were all >10 μM in TU212 cells (Figure 2). However, in MDA-MB-231 cells (Figure 3), the GI50 of M15 was 5–10 and >10 μM for the other metabolites.
Figure 2.
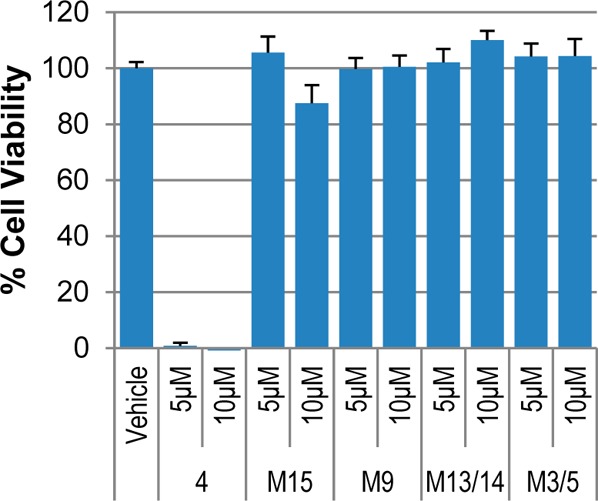
Tu212 neutral red cytotoxicity assay for the metabolites of UBS109 at 5 and 10 μM concentrations. Error bars represent the standard error of the mean. p values were calculated using a two-tailed t test; p < 0.001 for UBS109, 5 and 10 μM; p < 0.05 for 10 μM for M15.
Figure 3.
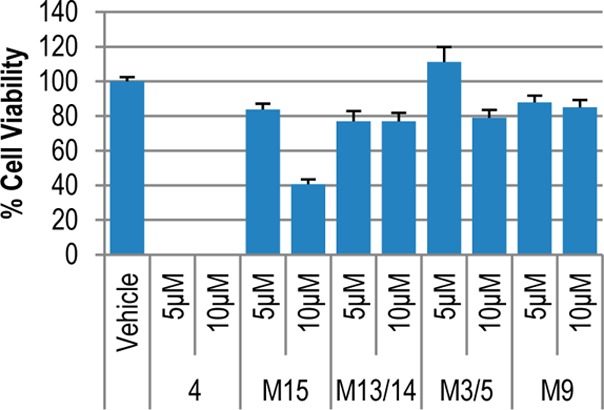
MDA-MB-231 Neutral Red cytotoxicity assay for the metabolites of UBS109 at 5 and 10 μM concentrations. Error bars represent the standard error of the mean. p values were calculated using a two-tailed t test; p < 0.001 for M15 (UBS109) at 10 μM; p < 0.05 for M15 at 5 μM and M9 at 10 μM.
In the in vitro system studied here, UBS109 exhibits a rather short metabolic half-life, from 8 min in pooled cynomolgus monkey liver S9 fractions to 38 min in S9 fractions of the mouse (see Supporting Information); this short half-life and extensive metabolism was also noted in a different study with EF24 (2).15 While the majority of our incubations were conducted in the presence of NADPH, we also determined that there was significant loss of parent in the presence of pooled human S9 fractions and in the absence of NADPH, implying that the metabolism may not occur exclusively via CYP450 enzymes. Relative to +NADPH conditions, peak areas for M5, M9, and M15 were significantly decreased, while M3 and M13/14 were not detected in the absence of NADPH. These results suggest that the first reduction of UBS109 (M9 and M15) occurs in the absence of NADPH, albeit at a slower rate. Secondary reductions were either very low (M5) or undetectable (M3 and M13/14) in the absence of NADPH. The incubations were carried out in the presence of 1% DMSO, but to ensure that there was no substantial inhibitory effect of DMSO on the metabolizing enzymes, we also carried out the human S9 incubation in the presence of 0.2% DMSO. The t1/2 of UBS109 decreased (from 18 to 9 min) under these conditions.
Having prepared authentic samples of some of the metabolites, we were able to assign structures by comparing retention times and fragmentation patterns of standard compounds against metabolites; thus, we have good evidence for the structures proposed (see Supporting Information). Additionally, we carried out a quantitation experiment with metabolites M15 and M5.16 These data suggest that, in the presence of NADPH, 20.2 ± 3.5% and 8.8 ± 0.3% of the mass after one hour was due to M15 and M5, respectively, while 4.1 ± 1.2% of the mass after one hour was due to remaining UBS109. In the absence of NADPH, 27.6 ± 4.7%, 2.6 ± 0.4%, and <0.8% of the mass was calculated to be due to UBS109, M15, and M5, respectively.
Significant reduction of other α,β-unsaturated ketones has been demonstrated in whole blood.17,18 In human, beagle dog, and cynomolgus monkey whole blood, UBS109 shows conversion to the metabolite M15 only (see Figure S13 and S14, Supporting Information). We were unable to quantitate the half-life of UBS109 in whole blood, presumably because the compound reacts in a slowly reversible covalent fashion with blood proteins. Regardless, metabolism seen in whole blood is clearly distinct from that seen in hepatic S9 fractions, except that M15 is the dominant metabolite in both settings.
In addition to the species-dependent half-lives observed, significant differences in the ensemble of metabolites present among species were found. Perhaps surprisingly, the CD1 mouse and Sprague–Dawley rat, with three and five metabolites, respectively, seem to metabolize UBS109 much less avidly than beagle dog, human, and cynomolgus monkey. Each of the latter species generated eight, nine, and 15 metabolites, respectively. Comparison of the human metabolites with those of other species reveals that all nine metabolites produced in the human S9 fractions are also produced in the monkey, but there are six other metabolites present in monkey S9 fractions that are absent in the human. The same comparison between dog and human shows that one of eight dog metabolites is unique to the dog, and two of nine human metabolites are unique to the human. Four metabolites are shared in common between rat and human; one metabolite is rat-specific and five are human-specific. Between the mouse and the human, only one metabolite (M15) is common, leaving two unique mouse metabolites and eight unique human metabolites.
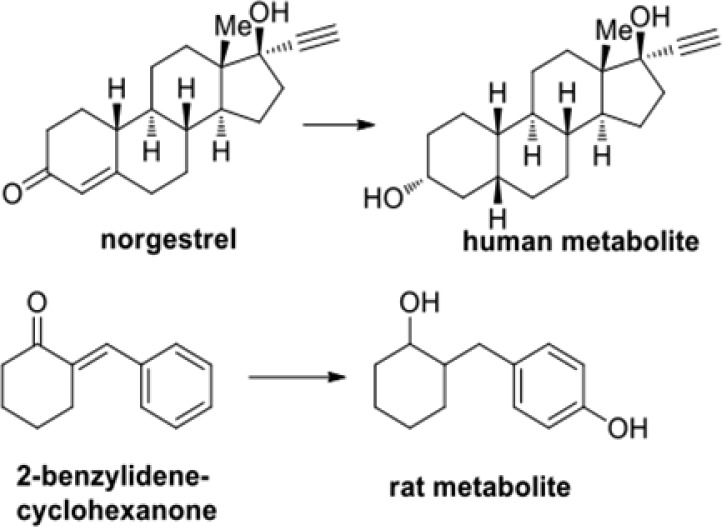
Of the 16 metabolites found in the five species’ S9 fractions studied here, eight are believed to result from reduction alone, one from oxidation alone, six from a mixture of oxidation and reduction, and one from unknown causes. Of the five human metabolites observed to have a detector response >10%19 (M5, M9, M13/M14, and M15), all were the products of hydrogenation. While epoxidation or dihydroxylation might be considered more likely biotransformations for olefinic double bonds, reduction of α,β-unsaturated ketones is a known phenomenon. For instance, the oral contraceptive component norgestrel is known to be reduced to the saturated alcohol in vivo (Scheme 3) analogous to the formation of M14 (Scheme 1).20 In a similar observation, Dimmock and co-workers reported that, in the rat, 2-benzylidenecyclohexanone, a compound that bears structural similarity to UBS109, is metabolized to unknown products and to a metabolite reduced at both its olefinic and carbonyl double bonds. The substance is likewise oxidized at the p-position of the benzene ring (see Scheme 3).1 We hypothesize that the major metabolites of UBS109 might be spared such oxidative aromatic metabolism because of the relatively electron-deficient nature of pyridine, particularly when the aromatic rings in UBS109 are coupled with the electron-withdrawing effect of the conjugated enone.
Scheme 3. Similar Enones from the Literature18 Are Metabolically Reduced to the Corresponding Saturated Alcohols.
Curcumin itself is subject to metabolic reduction similar to the pattern seen here.21−24 In mouse and rat models, curcumin is metabolized to dihydrocurcumin, tetrahydrocurcumin, hexahydrocurcumin, and octahydrocurcumin, as well as the glucuronides and sulfates of these species.
We undertook these studies to choose an appropriate animal model species for toxicology assessment of UBS109 as a prelude to an early stage clinical trial. Unfortunately, there is no nonhuman species that perfectly reproduces the metabolic profile of UBS109 in vitro when compared with human hepatic S9 fractions. Our data suggest that the metabolism of the beagle dog would most closely resemble that of the human, although some of the metabolites produced in the pooled human S9 fractions were missing in the beagle dog S9 fractions. If it were decided that using a species in which the entire array of human metabolites were replicated, one might consider choosing the cynomolgus monkey. However, use of this species would be complicated by the presence of nonhuman metabolites that might prove confounding. In early discussions with the Food and Drug Administration regarding this topic, we were advised that the beagle dog would be a more nearly ideal species for toxicological studies.
The dienone functionality embedded within the curcumin mimics 1–4 is a key contributor to biological activity since its reduction to give the dihydro or tetrahydro analogues diminishes or eliminates antitumor activity1,13 as confirmed by Figures 2 and 3. While it could be that the effects seen here are peculiar to UBS109, the fates of curcumin, a metabolite reported by Dimmock25 and analogue 2(15) would suggest ready metabolic reduction is not a spurious result. There are steps we and others have taken to replace the α,β-unsaturated ketone with structural elements that still confer activity and may dampen metabolic reduction. Others have replaced the enone moiety of curcumin with heterocycles such as pyrazole26 and isoxazole27 and achieved 3–10-fold improvements in cytotoxicity against various cell lines relative to curcumin. A separate strategy by our group has coupled the SH moiety of glutathione to the double bonds of curcumin analogues to render them water-soluble, stable, and, most importantly, as biologically active as the unconjugated parents due to chemical reversibility.28 GSH conjugation effectively saturates the C=C bonds and thereby thwarts metabolic reduction, but nonetheless provides a reservoir of active drug. It needs mentioning that, in spite of the rapid metabolic ablation of unsaturation in 2(15) and UBS109 (Scheme 1), compound class 1 is still capable of efficacious reduction of tumors in mice.1,3,7,8 It is noteworthy that terminal elimination half-lives (t1/2) for 2, 3, and 4 in this species range from 1 to 5 h (UBS109 4–5 h) depending on dose and route of administration.7,8,15 In addition, the protein binding of UBS109 in mouse plasma is 94%,29 a phenomenon subject to reversibility, particularly if plasma protein thiol groups are the binding mediators.28 The prolonged exposure of UBS109 in vivo in parallel with its rapid metabolism and extensive protein binding in vitro suggests that protein sequestration and tissue distribution may serve to protect the agent from overwhelming metabolism in vivo and, thereby, allow it to exert a pharmacological effect by virtue of slow drug release.15 Tissue distribution of UBS109 will be the subject of future investigation.
In conclusion, we have determined the in vitro metabolism of our previously reported anticancer candidate UBS109 (4) in five species (human, cynomolgus monkey, beagle dog, rat, and mouse). The metabolism of UBS109 is rapid, complex, and largely reductive in nature, in that one or more of the double bonds of the molecule is hydrogenated. The in vitro metabolic profile of the beagle dog closely resembles that of the human, although it is possible that the cynomolgus monkey might serve this purpose as well. We have prepared the metabolites that appear to be formed in highest proportion in the human S9 fractions and found that the cytotoxic activity of the hydrogenated metabolites is much reduced when compared to UBS109. Although the majority of the biological activity seems to reside with the latter parent compound, either as the free agent or a GSH conjugate, we will continue to examine whether the major metabolite, M15, shows efficacious in vivo activity. These data reframe the discovery and development of analogues of curcumin and suggest that, moving forward, steps should be taken to address potential metabolic liabilities arising from the α,β-unsaturated ketone.
Acknowledgments
The authors thank Sara Schock, Cindy Rewerts, Steve Wring (Scynexis, Inc.), and Darshil Shah (University of Illinois at Chicago) for helpful discussions and assistance during the revisions of this manuscript, Georgia Chen (Winship Cancer Institute) for generously providing the TU212 cell line, and Dr. Manu Saindane (Emory Institute for Drug Development) for scaling-up M15. We appreciate helpful discussions with Dennis C. Liotta (Department of Chemistry), Richard F. Arrendale (Emory Institute for Drug Development), and Dong Shin (Winship Cancer Institute).
Glossary
ABBREVIATIONS
- DMSO
dimethylsulfoxide
- GSH
glutathione
- HPLC
high-performance liquid chromatography
- IKK
IκB kinase
- LC
(high performance) liquid chromatography
- MS
mass spectrometry
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NR
neutral red
Supporting Information Available
Synthesis and characterization details for metabolites and UBS109, time course metabolite profile tables, in vitro metabolism conditions, LC–MS/MS analysis conditions, cell viability assay procedures, proposed metabolic biotransformations of UBS109, and authentic standard and metabolite spectral comparisons. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Present Address
⊥ Department of Medicinal Chemistry and Pharmacognosy and UIC Cancer Center, University of Illinois-Chicago, Chicago, Illinois 60612, United States.
Author Contributions
The manuscript was written through contributions of all authors.
This work was supported by the National Institutes of Health National Cancer Institute [P50 CA128613 SPORE in Head and Neck Cancer].
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Adams B. K.; Ferstl E. M.; Davis M. C.; Herold M.; Kurtkaya S.; Camalier R. F.; Hollingshead M. G.; Kaur G.; Sausville E. A.; Rickles F. R.; Snyder J. P.; Liotta D. C.; Shoji M. Synthesis and biological evaluation of novel curcumin analogs as anticancer and antiangiogenesis agents. Bioorg. Med. Chem. 2004, 12, 3871–3883. [DOI] [PubMed] [Google Scholar]
- Adams B. K.; Cai J.; Armstrong J.; Herold M.; Lu Y. J.; Sun A.; Snyder J. P.; Liotta D. C.; Jones D. P.; Shoji M. EF24, a novel synthetic curcumin analog, induces apoptosis in cancer cells via a redox-dependent mechanism. Anti-Cancer Drugs 2005, 16, 263–275. [DOI] [PubMed] [Google Scholar]
- Subramaniam D.; May R.; Sureban S. M.; Lee K. B.; George R.; Kuppusamy P.; Ramanujam R. P.; Hideg K.; Dieckgraefe B. K.; Houchen C. W.; Anant S. Diphenyl difluoroketone: a curcumin derivative with potent in vivo anticancer activity. Cancer Res. 2008, 68, 1962–1969. [DOI] [PubMed] [Google Scholar]
- Kasinski A. L.; Du Y.; Thomas S. L.; Zhao J.; Sun S.-Y.; Khuri F. R.; Wang C.-W.; Shoji M.; Sun A.; Snyder J. P.; Liotta D. C.; Fu H. Inhibition of IkappaB kinase-nuclear factor-kappaB signaling pathway by 3,5-bis(2-flurobenzylidene)piperidin-4-one (EF24), a novel monoketone analog of curcumin. Mol. Pharmacol. 2008, 74, 654–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A.; Shi Q.; Moore T. W.; Younghyoun Y.; Prussia A.; Maddox C.; Liotta D. D.; Shim H.; Snyder J. P. Monocarbonyl curcumin analogs: Heterocyclic pleiotropic kinase inhibitors that mediate anti-cancer properties. J. Med. Chem. 2013, 56, 3456–3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera A.; Moore T. W.; Sun A.; Liotta D. C.; Snyder J. P.; Shim H.; Marcus A. I.; Miller A. H.; Pace T. W. W. Inhibition of the NF-κB signaling pathway by the curcumin analog, 3,5-bis(2-pyridinylmethylidene)-4-piperidone (EF31): anti-inflammatory and anti-cancer properties. Int. Immunopharmacol. 2012, 12, 368–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S.; Moore T. W.; Lin X.; Morii N.; Mancini A.; Howard R. B.; Culver D.; Arrendale R. F.; Reddy P.; Evers T. J.; Zhang H.; Sica G.; Chen Z. G.; Sun A.; Fu H.; Khuri F. R.; Shin D. M.; Snyder J. P.; Shoji M. Synthetic curcumin analog EF31 inhibits the growth of head and neck squamous cell carcinoma xenografts. Integr. Biol. 2012, 4, 633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S.; Moore T. W.; Morii N.; Howard R. B.; Culver D.; Arrendale R. F.; Reddy P.; Evers T. J.; Zhang H.; Chen S. G.; Sun A.; Fu H.; Khuri F. R.; Shin D. M.; Snyder J. P.; Shoji M. Synthetic curcumin analog UBS109 inhibits the growth of head and neck squamous cell carcinoma xenografts. Submitted. [DOI] [PubMed]
- Yamaguchi M.; Moore T. W.; Sun A.; Snyder J. P.; Shoji M. Novel curcumin analogue UBS 109 potently stimulates osteoblastogenesis and suppresses osteoclastogenesis: involvement in Smad activation and NF-κB inhibition. Integr. Biol. 2012, 4, 905–913. [DOI] [PubMed] [Google Scholar]
- Wu W.-N.; McKown L. A.. Chapter 11: In vitro drug metabolite profiling using hepatic S9 and human liver microsomes. In Optimization in Drug Discovery: In Vitro Methods (Methods in Pharmacology and Toxicology); Yan Z., Cadwell G. W., Eds.; Humana Press: Totowa, NJ, 2004; pp 163–184. [Google Scholar]
- These acceptance criteria have been developed at Scynexis, Inc. and are gleaned from historical data gathered there.
- Sugiura M.; Sato M.; Kotani S.; Nakajima M. Lewis base-catalyzed conjugate reduction and reductive aldol reaction of α,β-unsaturated ketones using trichlorosilane. Chem. Commun. 2008, 4309–4311. [DOI] [PubMed] [Google Scholar]
- Modzelewska A.; Pettit C.; Achanta G.; Davidson N. E.; Huang P.; Khan S. R. Anticancer activities of novel chalcone and bis-chalcone derivatives. Bioorg. Med. Chem. 2006, 14, 3491–3495. [DOI] [PubMed] [Google Scholar]
- The amount of 20,000 cells/well were seeded, while treatment began after one day in culture. For IC50s, seven drug concentrations ranged from 0.001 to 20 μM.
- Reid J. M.; Buhrow S. A.; Gilbert J. A.; Jia L.; Shoji M.; Snyder J. P.; Ames M. M.. Mouse pharmacokinetics and metabolism of the curcumin analog, 4-piperidione,3,5-bis[(2-fluorophenyl)methylene]-acetate(3E,5E) (EF-24; NSC 716993). Cancer Chemother. Pharmacol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- We did not attempt to quantitate the other metabolites prepared because a mixture of diastereomers was present in our M13/14 and M3 preparations. Additionally, M9 is unstable in solution and converts rather rapidly to M15.
- Lindstrom T. D.; Whitaker G. W. Alpha, beta-ketoalkene reduction. A novel reduction pathway in mammalian tissues. Drug Metab. Dispos. 1984, 12, 72–76. [PubMed] [Google Scholar]
- Lindstrom T. D.; Whitaker G. W. Saturation of an alpha, beta-unsaturated ketone: a novel xenobiotic biotransformation in mammals. Xenobiotica 1984, 14, 503–508. [DOI] [PubMed] [Google Scholar]
- Because the percentages given (see Tables S1–S5 in Supporting Information) are for peak areas based on MS response and because significant differences in ionization may exist even for related compounds, the data given in these tables should not be used to infer relative differences in concentration. To be conservative, we have chosen to focus on all of the metabolites showing a detector response > 10% for that of the parent.
- Gerhards E.; Hecker W.; Hitze H.; Nieuweboer B.; Bellmann O. The metabolism of norethisterone (17-ethinyl-4-estren-17 -ol-3-one) and of dl- and d-norgestrel (18-methyl-17-ethinyl-4-estren-17-ol-3-one) in man. Acta Endrocrinol. 1971, 68, 219–248. [PubMed] [Google Scholar]
- Ireson C.; Orr S.; Jones D. J. L.; Verschoyle R.; Lim C.-L.; Luo J.-L.; Howells L.; Plummer S.; Jukes R.; Williams M.; Steward W. P.; Gescher A. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001, 61, 1058–1064. [PubMed] [Google Scholar]
- Hoehle S. I.; Pfeiffer E.; Sólyom A.; Metzler M. Metabolism of curcuminoids in tissue slices and subcellular fractions from rat liver. J. Agric. Food Chem. 2006, 54, 756–764. [DOI] [PubMed] [Google Scholar]
- Anand P.; Kunnumakkara A. B.; Newman R. A.; Aggarwal B. B. Bioavailability of curcumin: Problems and promises. Mol. Pharmaceutics 2007, 4, 807–818. [DOI] [PubMed] [Google Scholar]
- Marczylo T. H.; Steward W. P.; Gescher A. J. Rapid analysis of curcumin and curcumin metabolites in rat biomatrices using a novel ultraperformance liquid chromatography (UPLC) method. J. Agric. Food Chem. 2009, 57, 797–803. [DOI] [PubMed] [Google Scholar]
- Dimmock R. R.; Hamon N. W.; Hindmarsh K. W.; Sellar A. P.; Turner W. A.; Rank G. H.; Robertson A. J. Evaluation of 2-benzylidenecyclohexanones and 2,6-bis(benzylidene)cyclohexanones for antitumor and cytotoxic activity and as inhibitors of mitochondrial function in yeast: metabolism studies of (E)-2-benyzlidenecyclohexanone. J. Pharm. Sci. 1976, 65, 538–543. [DOI] [PubMed] [Google Scholar]
- Shim J. S.; Kim D. H.; Jung H. J.; Kim J. H.; Lim D.; Lee S. K.; Kim K. W.; Ahn J. W.; Yoo J. S.; Rho J. R.; Shin J.; Kwon H. J. Hydrazinocurcumin, a novel synthetic curcumin derivative is a potent inhibitor of endothelial cell proliferation. Bioorg. Med. Chem. 2002, 10, 2987–2992. [DOI] [PubMed] [Google Scholar]
- Simoni D.; Rizzi M.; Rondanin R.; Baruchello R.; Marchetti P.; Invidiata F. P.; Labbozzetta M.; Poma P.; Carina V.; Notarbartolo M.; Alaimoc A.; Alessandro N. D. Antitumor effects of curcumin and structurally β-diketone modified analogs on multidrug resistant cancer cells. Bioorg. Med. Chem. Lett. 2008, 18, 845–849. [DOI] [PubMed] [Google Scholar]
- Sun A.; Lu Y. J.; Hu H.; Shoji M.; Liotta D. C.; Snyder J. P. Curcumin analog cytotoxicity against breast cancer cells: Exploitation of a redox-dependent mechanism. Bioorg. Med. Chem. Lett. 2009, 19, 6627–6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers T.; Reddy P.; Moore T. W.; Snyder J. P.; Arrendale R. F.. Emory Institute for Drug Discovery. Unpublished.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.