| Title: | Pyrrolidine Derivatives and Their Use as Complement Pathway Modulators | ||
| Patent Application Number: | WO 2014/002057 Al | Publication date: | 3 January 2014 |
| Priority Application: | US 61/665,477 | Priority date: | 28 June 2012 |
| US 61/774,241 | 7 March 2013 | ||
| Inventors: | Hommel, U.; Lorthiois, E. L. J.; Maibaum, J. K.; Ostermann, N.; Randl, S. A.; Vulpetti, A.; Rogel, O. | ||
| Assignee Company: | Novartis AG [CH/CH]; Lichtstrasse 35, CH-4056 Basel (CH) | ||
| Disease Area: | Age-related macular degeneration, diabetic retinopathy, and related ophthalmic diseases | Biological Target: | The complement alternative pathway and Factor D |
| Summary: | The invention in this patent application relates to pyrrolidine derivatives represented generally by formula (I), which inhibit the alternative pathway of the complement system and particularly inhibit Factor D activity. These compounds may potentially provide a treatment for patients suffering from conditions and diseases associated with activation of the complement alternative pathway such as age-related macular degeneration (AMD), diabetic retinopathy, and related ophthalmic diseases. | ||
| Macular degeneration is a clinical term that describes progressive loss of central vision associated with abnormalities of Bruch’s membrane, the choroid, the neural retina, and/or the retinal pigment epithelium. Age-related macular degeneration (AMD) is the most prevalent form of macular degeneration that affects older adults and may cause loss of sharp vision. Dry (or atrophic) AMD is an early form of AMD; this form may advance in 10–20% of dry AMD patients to a more severe form known as wet (or neovascular) AMD. Wet AMD may cause destruction of the central retina and potentially can affect blindness. | |||
| The complement system is an important part of the innate immune system. One of the mechanisms that activate this immune system is the alternative complement pathway. It is believed, based on genetic evidence, that the activation of the alternative complement pathway is linked to the pathogenesis of AMD. Inhibition of the alternative complement pathway is thus a viable clinical target for the treatment of AMD. Factor D is a protein that exists in the plasma in very low concentration (about 1.8 μg/mL), and it is known to be involved in the activation of the alternative complement system pathway. Factor D has been shown to be the limiting enzyme for activation of the alternative complement system, and its inhibition makes it a suitable target to inhibit that pathway. | |||
| The inventors emphasized the need of new therapeutic agents for treatment of AMD since the currently used anti-VEGF (Vascular Endothelial Growth Factor) agents such as Lucentis are not an effective medical therapy. Inhibitors of Factor D activity such as the compounds described in this patent application may potentially provide such a needed therapy. | |||
| Important Compound Classes: | 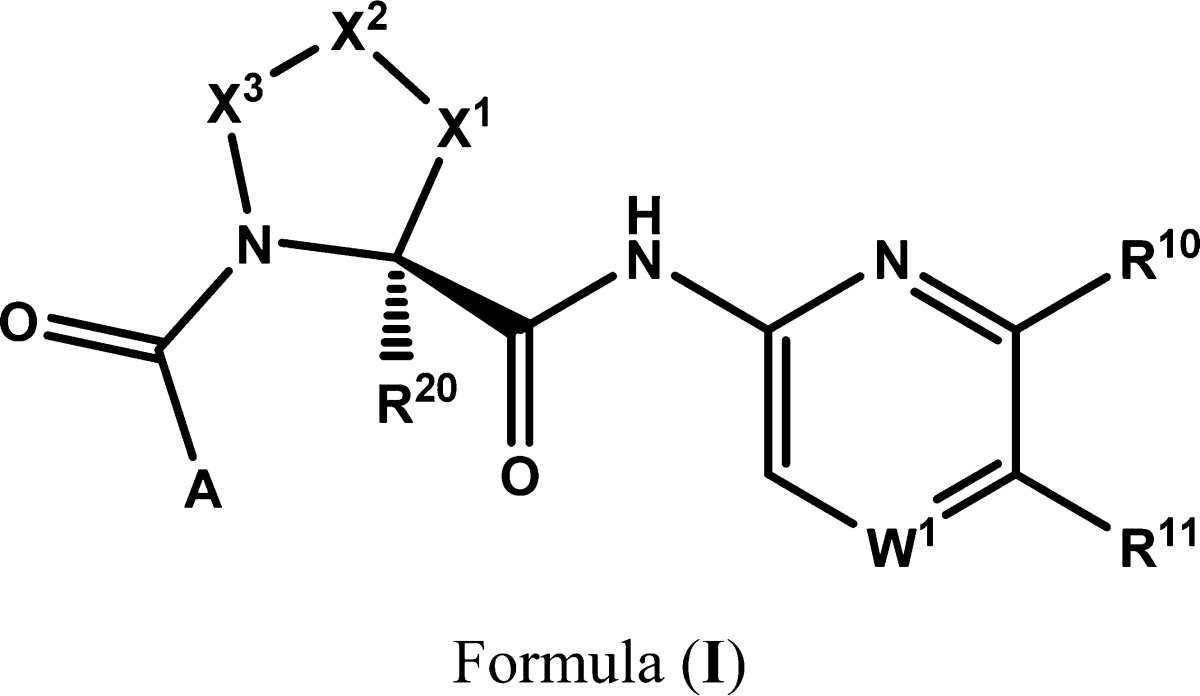 |
||
| Key Structures: | The inventors described the synthesis and structures of 39 examples of formula (I) including compounds 4, 21, and 29 shown below.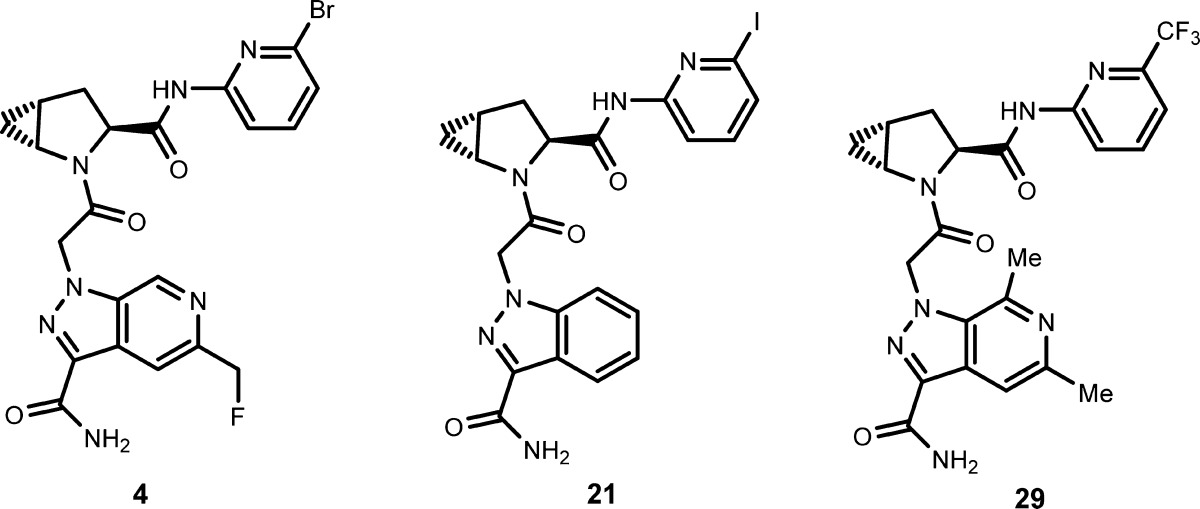
|
||
| Biological Assay: | Human complement Factor D assay (two methods) | ||
| Biological Data: | The Factor D inhibition data were determined as IC50 in μM for all 39 reported examples including compounds 4, 21, and 29 (structures above) listed in the following table: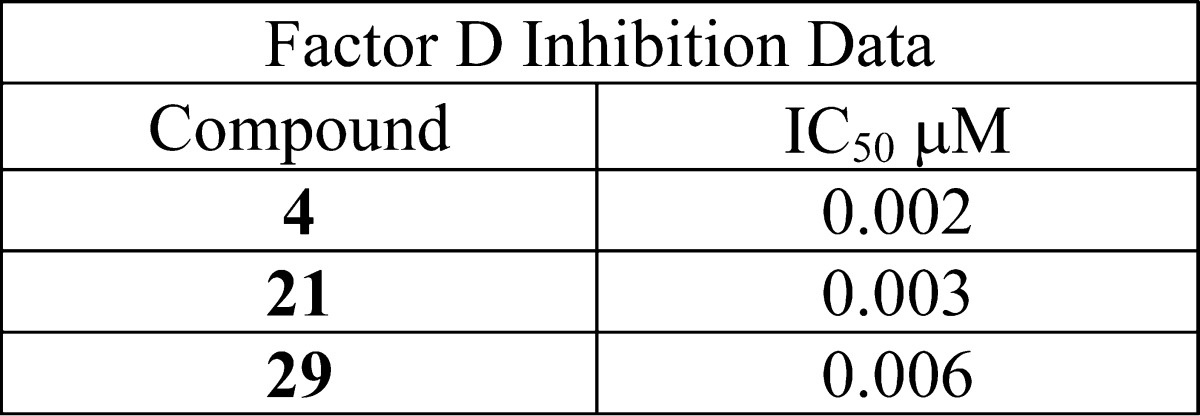
|
||
| Claims: | Claims 1–9: Composition of matter, variations of formula (I) | ||
| Claim 10: 29 specific examples of Formula (I) listed by chemical name | |||
| Claim 11: 9 specific examples of formula (I) listed by chemical name | |||
| Claims 12–13: Pharmaceutical composition | |||
| Claims 14–17: Methods of treatments of diseases and disorders | |||
| Claims 18–21: Use of compounds as medicament | |||
| Recent Review Articles: | 1. Ebrahimi K. B.; Fijalkowski N.; Cano M.; Handa J. T.. J. Pathol. 2013, 229 (5), 729–742. | ||
| 2. Loyet K. M.; DeForge L. E.; Katschke K. J. Jr.; Diehl L.; Graham R. R.; Pao L.; Sturgeon L.; Lewin-Koh S.-C.; Hollyfield J. G.; van Lookeren Campagne M.. Invest. Ophthalmol. Vis. Sci. 2012, 53 (10), 6628–6637. | |||
| 3. Stanton C. M.; Yates J. R. W.; den Hollander A. I.; Seddon J. M.; Swaroop A.; Stambolian D.; Fauser S.; Hoyng C.; Yu Y.; Atsuhiro K.; et al. Invest. Ophthalmol. Vis. Sci. 2011, 52 (12), 8828–8834. | |||
The authors declare no competing financial interest.


