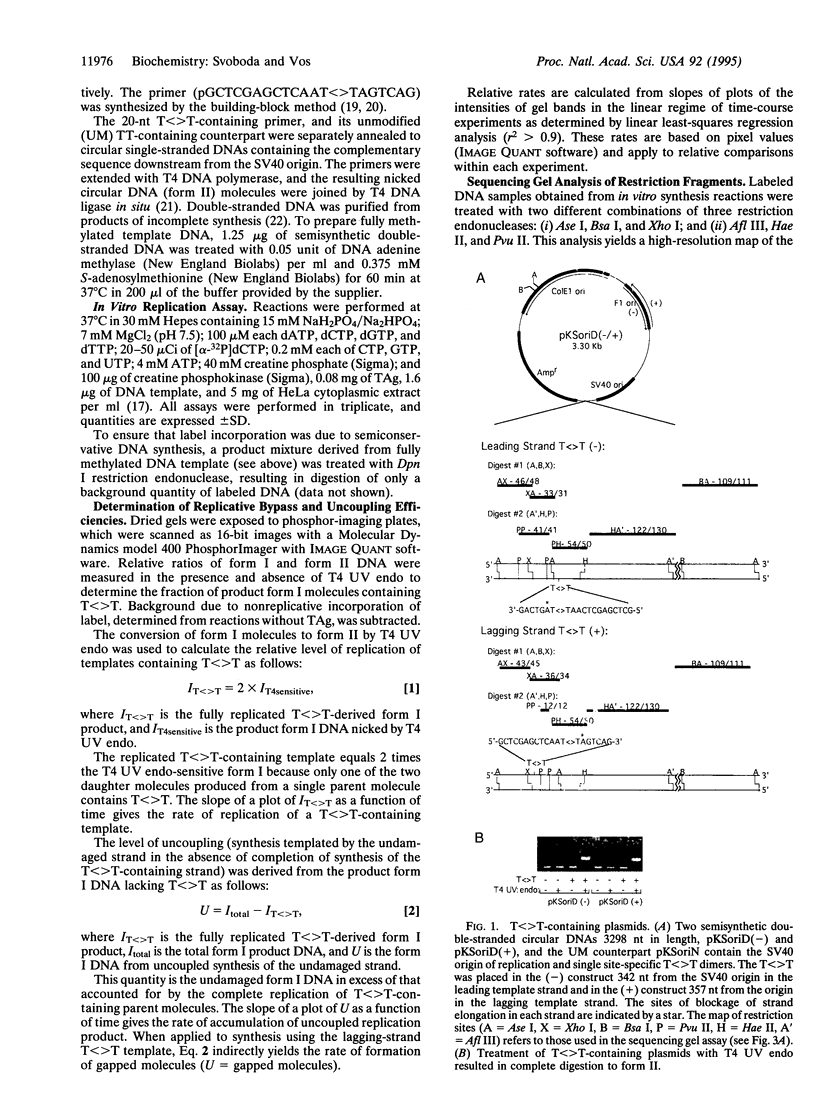
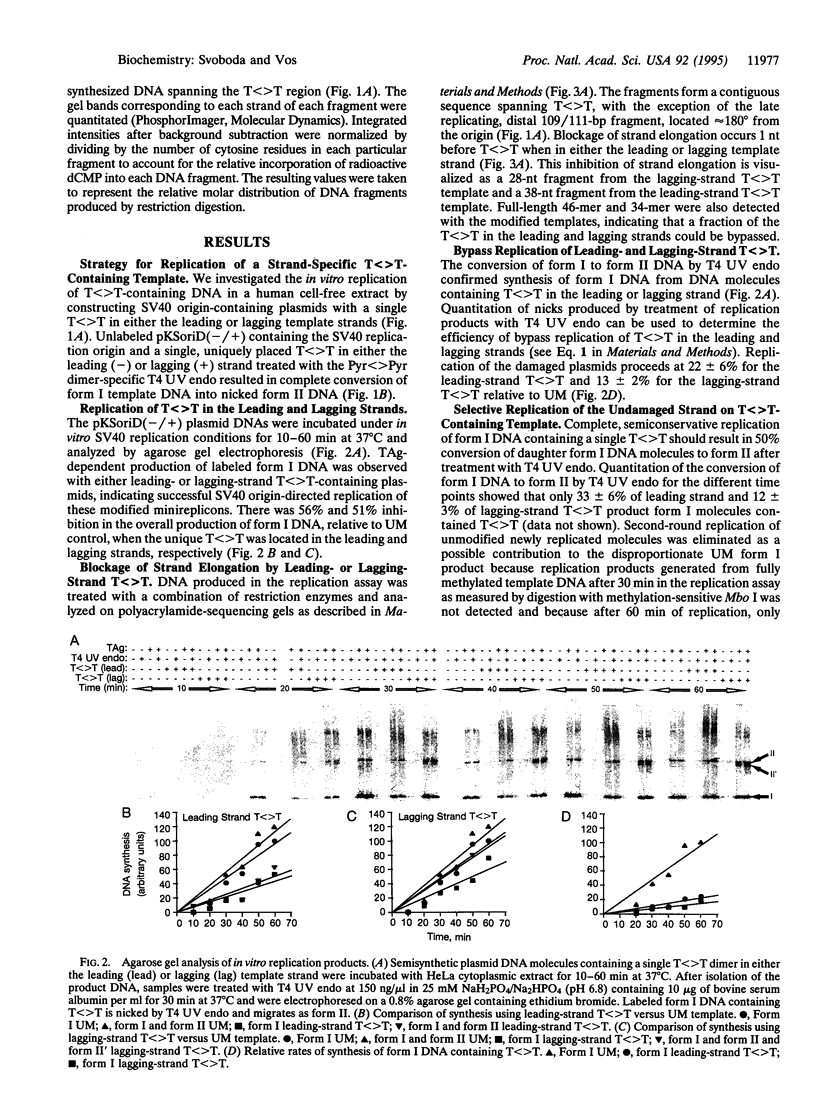
Abstract
We have constructed simian virus 40 minireplicons containing uniquely placed cis,syn-thymine dimers (T <> T) for the analysis of leading- and lagging-strand bypass replication. Assaying for replication in a human cell-free extract through the analysis of full-size labeled product molecules and restriction fragments spanning the T <> T site resulted in the following findings: (i) The primary site of synthesis blockage with T <> T in either the leading or lagging strand was one nucleotide before the lesion. (ii) Replicative bypass of T <> T was detected in both leading and lagging strands. The efficiency of synthesis past T <> T was 22% for leading-strand T <> T and 13% for lagging-strand T <> T. (iii) The lagging-strand T <> T resulted in blocked retrograde synthesis with the replication fork proceeding past the lesion, resulting in daughter molecules containing small gaps (form II' DNA). (iv) With T <> T in the leading-strand template, both the leading and lagging strands were blocked, representing a stalled replication fork. Uncoupling of the concerted synthesis of the two strands of the replication fork was observed, resulting in preferential elongation of the undamaged lagging strand. These data support a model of selective reinitiation downstream from the lesion on lagging strands due to Okazaki synthesis, with no reinitiation close to the damage site on leading strands [Meneghini, R. & Hanawalt, P.C. (1976) Biochim. Biophys. Acta 425, 428-437].
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benbow R. M. Chromosome structures. Sci Prog. 1992;76(301-302):425–450. [PubMed] [Google Scholar]
- Berger C. A., Edenberg H. J. Pyrimidine dimers block simian virus 40 replication forks. Mol Cell Biol. 1986 Oct;6(10):3443–3450. doi: 10.1128/mcb.6.10.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjursell G., Gussander E., Lindahl T. Long regions of single-stranded DNA in human cells. Nature. 1979 Aug 2;280(5721):420–423. doi: 10.1038/280420a0. [DOI] [PubMed] [Google Scholar]
- Carty M. P., Hauser J., Levine A. S., Dixon K. Replication and mutagenesis of UV-damaged DNA templates in human and monkey cell extracts. Mol Cell Biol. 1993 Jan;13(1):533–542. doi: 10.1128/mcb.13.1.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg H. J. Inhibition of DNA replication by ultraviolet light. Biophys J. 1976 Aug;16(8):849–860. doi: 10.1016/S0006-3495(76)85735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. C., Svoboda D. L., Reardon J. T., Sancar A. Human nucleotide excision nuclease removes thymine dimers from DNA by incising the 22nd phosphodiester bond 5' and the 6th phosphodiester bond 3' to the photodimer. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3664–3668. doi: 10.1073/pnas.89.8.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodadek T., Gamper H. Efficient synthesis of a supercoiled M13 DNA molecule containing a site specifically placed psoralen adduct and its use as a substrate for DNA replication. Biochemistry. 1988 May 3;27(9):3210–3215. doi: 10.1021/bi00409a013. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R. Postreplication repair of DNA in ultraviolet-irradiated mammalian cells. J Mol Biol. 1972 May 28;66(3):319–337. doi: 10.1016/0022-2836(72)90418-4. [DOI] [PubMed] [Google Scholar]
- Li J. J., Kelly T. J. Simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6973–6977. doi: 10.1073/pnas.81.22.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneghini R., Hanawalt P. T4-endonuclease V-sensitive sites in DNA from ultraviolet-irradiated human cells. Biochim Biophys Acta. 1976 Apr 2;425(4):428–437. doi: 10.1016/0005-2787(76)90007-1. [DOI] [PubMed] [Google Scholar]
- Mezzina M., Menck C. F., Courtin P., Sarasin A. Replication of simian virus 40 DNA after UV irradiation: evidence of growing fork blockage and single-stranded gaps in daughter strands. J Virol. 1988 Nov;62(11):4249–4258. doi: 10.1128/jvi.62.11.4249-4258.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. D., Bose K. K., Rabkin S. D., Strauss B. S. Sites of termination of in vitro DNA synthesis on ultraviolet- and N-acetylaminofluorene-treated phi X174 templates by prokaryotic and eukaryotic DNA polymerases. Proc Natl Acad Sci U S A. 1981 Jan;78(1):110–114. doi: 10.1073/pnas.78.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. M., Tjian R. Construction and analysis of simian virus 40 origins defective in tumor antigen binding and DNA replication. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6491–6495. doi: 10.1073/pnas.77.11.6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Day C. L., Burgers P. M., Taylor J. S. PCNA-induced DNA synthesis past cis-syn and trans-syn-I thymine dimers by calf thymus DNA polymerase delta in vitro. Nucleic Acids Res. 1992 Oct 25;20(20):5403–5406. doi: 10.1093/nar/20.20.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabkin S. D., Moore P. D., Strauss B. S. In vitro bypass of UV-induced lesions by Escherichia coli DNA polymerase I: specificity of nucleotide incorporation. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1541–1545. doi: 10.1073/pnas.80.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B. W., Gluzman Y. Replication and supercoiling of simian virus 40 DNA in cell extracts from human cells. Mol Cell Biol. 1985 Aug;5(8):2051–2060. doi: 10.1128/mcb.5.8.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B. Smart machines at the DNA replication fork. Cell. 1994 Sep 9;78(5):725–728. doi: 10.1016/s0092-8674(94)90362-x. [DOI] [PubMed] [Google Scholar]
- Taylor J. S., Brockie I. R. Synthesis of a trans-syn thymine dimer building block. Solid phase synthesis of CGTAT[t,s]TATGC. Nucleic Acids Res. 1988 Jun 10;16(11):5123–5136. doi: 10.1093/nar/16.11.5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. S., O'Day C. L. cis-syn thymine dimers are not absolute blocks to replication by DNA polymerase I of Escherichia coli in vitro. Biochemistry. 1990 Feb 13;29(6):1624–1632. doi: 10.1021/bi00458a038. [DOI] [PubMed] [Google Scholar]
- Thomas D. C., Nguyen D. C., Piegorsch W. W., Kunkel T. A. Relative probability of mutagenic translesion synthesis on the leading and lagging strands during replication of UV-irradiated DNA in a human cell extract. Biochemistry. 1993 Nov 2;32(43):11476–11482. doi: 10.1021/bi00094a002. [DOI] [PubMed] [Google Scholar]
- Waga S., Stillman B. Anatomy of a DNA replication fork revealed by reconstitution of SV40 DNA replication in vitro. Nature. 1994 May 19;369(6477):207–212. doi: 10.1038/369207a0. [DOI] [PubMed] [Google Scholar]
- White J. H., Dixon K. Gap filling and not replication fork progression is the rate-limiting step in the replication of UV-damaged simian virus 40 DNA. Mol Cell Biol. 1984 Jul;4(7):1286–1292. doi: 10.1128/mcb.4.7.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]