Abstract
Objective We investigated the 5-year postsurgical developmental trajectory of working memory (WM) in children with medulloblastoma using parent and performance-based measures. Method This study included 167 patients treated for medulloblastoma. Serial assessments of WM occurred at predetermined time points for 5 years. Results There was a subtle, statistically significant increase in parental concern about WM, coupled with a statistically significant decrease in age-standardized scores on performance-based measures. However, whole-group mean scores on both parent and performance-based measures remained in the age-expected range. Posterior fossa syndrome was consistently associated with poorer WM. Younger age at treatment and higher treatment intensity were associated with greater negative change in WM performance only. Conclusions Most children treated for medulloblastoma display WM within the age-appropriate range according to parent report and performance. However, the subtle negative changes over time and identified subgroups at increased risk highlight the need for ongoing monitoring of this population.
Keywords: cancer and oncology, cognitive assessment, neurological disorders, neuropsychology
Medulloblastoma, a tumor arising in the brain’s posterior fossa, is the most common type of childhood malignant brain tumor (Crawford, MacDonald, & Packer, 2007). Today, medulloblastoma is often effectively controlled with surgical resection, postsurgical risk-adapted craniospinal irradiation (CSI), and adjuvant chemotherapy, with survival rates now >70% (Gajjar et al., 2006). However, the independent and cumulative effects of disease and treatment factors can disrupt ongoing brain development, and a heightened risk of neuropsychological deficits has been reported in several studies (de Ruiter, van Mourik, Schouten-van Meereren, Grootenhuis, & Oosterlaan, 2012; Ellenberg et al., 2009; Mulhern et al., 2005; Robinson et al., 2010; Robinson, Fraley, Pearson, Kuttesch, & Compas, 2013; Spiegler, Bouffet, Greenberg, Rutka, & Mabbott, 2004). More recent studies have begun to focus on the impact of particular aspects of neurocognitive function beyond general intellectual outcomes, which have dominated the childhood brain tumor literature historically. Some neurocognitive studies (Conklin et al., 2012; Dennis et al., 1992; Kirschen et al., 2008; Palmer et al., 2013a), although not all (Mabbott, Penkman, Witol, Strother, & Bouffet, 2008), have reported that working memory (WM) is detrimentally affected after cranial radiation therapy for brain tumors, including medulloblastomas.
WM represents an important aspect of neurocognition and can be defined as a limited-capacity multicomponent system for the temporary maintenance and manipulation of information (Baddeley, 2012). WM is integral to the registration of new information and therefore to the acquisition of new skills and knowledge. Correspondingly, WM is important for classroom learning and, not unexpectedly, its association with academic achievement has been clearly demonstrated (Alloway, 2009; Gathercole, Pickering, Knight, & Stegmann, 2003; Kibby, Marks, Morgan, & Long, 2004). Most studies assessing WM ability in children treated for medulloblastoma have used performance-based measures, such as span tasks, that require the child to recall and transform an increasing number of sequenced items (Conklin et al., 2012; Dennis et al., 1992; Kirschen et al., 2008; Mabbott et al., 2008; Palmer et al., 2013a). A child’s WM can also be measured by using observer-rating scales assessing everyday behavior, which may increase the ecological validity of a neuropsychological assessment (Anderson, 2002; Chaytor, Schmitter-Edgecombe, & Burr, 2006; Gioia, Isquith, & Kenealy, 2008). Parent ratings of WM theoretically provide an opportunity to explore a child’s capacity for WM in real-life situations not available through performance-based measures administered in the structured clinical environment. A commonly used parent rating scale designed to evaluate everyday behavioral aspects of a range of cognitive functions, including WM, is the Behavior Rating Inventory of Executive Function (BRIEF; Gioia, Isquith, Guy, & Kenworthy, 2000a).Several studies have demonstrated concurrent, predictive, and discriminant validity of the BRIEF in a range of clinical populations (Gioia et al., 2008). However, most studies have found that parent ratings of WM only modestly correlate with performance-based measures, suggesting a lack of direct correspondence (Anderson, Anderson, Northam, Jacobs, & Mikiewicz, 2002; Biggs et al., 2011; Conklin, Salorio, & Slomine, 2008; Gioia & Isquith, 2004; Gioia, Isquith, Retzlaff, & Pratt, 2001; Toplak, Bucciarelli, Jain, & Tannock, 2009; Vriezen & Pigott, 2002).
It is well-evidenced that WM is primarily supported neuroanatomically by a bilateral frontoparietal network (Rottschy et al., 2012). Structural changes in these regions (and their white matter connections) have been related to the development of WM in healthy children (Ostby, Tamnes, Fjell, & Walhovd, 2011; Tamnes et al., 2013). The involvement of subcortical structures, including the basal ganglia, hippocampus, and the cerebellum, in WM tasks has also been demonstrated (Tamnes et al., 2013). Owing to its reliance on white matter connections, WM may be particularly vulnerable to the disease and central nervous system (CNS)-directed treatment effects associated with medulloblastoma (Law et al., 2011). WM deficits in children with medulloblastoma have largely been attributed to the effects of CSI, as its neurotoxic effects on developing cerebral white matter networks are well-established (Filley & Kleinschmidt-DeMasters, 2001). To this end, younger age at diagnosis and higher treatment intensity (e.g., CSI dose) represent the most reliably reported risk factors affecting neurocognitive outcome in medulloblastoma (Robinson et al., 2013). Contemporary treatment protocols are being refined in an attempt to reduce the extent of CSI-related neurocognitive effects, while maintaining the high survival rates that have been achieved (Merchant, Conklin, Wu, Lustig, & Xiong, 2009; Palmer et al., 2013a).
The vast majority of patients with medulloblastoma undergo surgical tumor resection before adjuvant therapy. However, the potential long-term impact of surgery-related complications has been a lesser focus of research to date. After surgical resection and before subsequent treatments, a proportion (estimates of up to 29%) of patients develop a condition known as posterior fossa syndrome (PFS; Gajjar et al., 1996; Robertson et al., 2006). This syndrome (also known as cerebellar mutism syndrome) is characterized by a broad range of neurological symptoms, including speech (e.g., mutism), cognitive (e.g., attention problems), behavioral-affective (e.g., emotional lability), and motoric (e.g., ataxia, decreased movement) disturbances (Pollack, 1997). The nature (transient to unresolving) and severity of PFS are highly variable (Gajjar et al., 1996; Korah et al., 2010; Robertson et al., 2006). Preliminary cross-sectional investigations of the current cohort found that patients with PFS (n = 22) displayed significantly weaker WM skills compared with patients without PFS (n = 22) on performance-based measures at 12 months after diagnosis; although, at this time point, no group differences were identified by parent report of WM (Palmer et al., 2010). Whether PFS poses an increased risk to WM development in medulloblastoma has not yet been investigated longitudinally. Previous studies looking at long-term outcomes in medulloblastoma have excluded patients with PFS (Palmer et al., 2013a) or have not included PFS as a variable (Conklin et al., 2012; Dennis et al., 1992; Kirschen et al., 2008; Mabbott et al., 2008) when describing and quantifying outcomes.
For the current study, we used a prospective, longitudinal design to estimate the trajectory of change in WM based on parent report and performance-based measures during the 5 years after diagnosis of medulloblastoma. We hypothesized that with increasing time since diagnosis, children would perform significantly poorer on WM tasks as rated by parents and on performance measures when compared with age-standardized scores, consistent with the literature suggesting a widening gap between the functional level of children treated for brain tumors and their typically developing same-aged peers over time (de Ruiter et al., 2012). We also explored the influence of variables including PFS, age at diagnosis, and disease-related risk status on parent rating of WM and WM performance and their interaction with time since diagnosis. Given the reviewed literature indicating younger age and higher treatment intensity are associated with greatest risk (Robinson et al., 2013), and preliminary data to suggest that PFS may be another risk factor (Palmer et al., 2010), we hypothesized that these factors would be associated with decline in scores on WM measures over time. We also investigated the relationship between parent report and performance-based measures and anticipated small correlations consistent with the limited relationship between these measures found in other studies.
Method
Participants and Procedures
Participants were enrolled in an international, multisite, institutional review board-approved clinical trial if they were aged 3–21 years with a newly diagnosed embryonal brain tumor. From 2003 to 2011, 318 patients with histologically confirmed medulloblastoma were enrolled with informed consent at nine participating institutions. Patients received postsurgical risk-adapted CSI followed by four cycles of chemotherapy (cyclophosphamide, cisplatin, and vincristine) with stem cell support. In a minority of cases, there was deviation from protocol-based chemotherapy as per clinical judgment. Disease was classified as average risk (≤1.52 residual tumor and no metastatic disease) or high risk (>1.52 residual tumor and/or metastatic disease). Patients with average risk disease received 23.4 Gy CSI, with 3-D conformal boost to the primary site to 55.8 Gy. Patients with high-risk disease received 36–39.6 Gy CSI, with 3-D conformal boost to the primary site to 55.8–59.4 Gy. As part of a separate computerized reading intervention study, a subset of the current cohort was randomized to either an intervention arm or a standard care arm (Palmer et al., 2013b). Analyses of the randomization arm revealed no significant effect on WM among our cohort, and therefore, this variable was excluded from all subsequent analyses.
Neurocognitive assessments followed a predetermined schedule at all participating sites. Initial neurocognitive assessments were scheduled to occur after surgical resection (baseline; mean time after surgery = 35 days, SD = 15 days; and before commencing CSI). At the primary study site, follow-up assessments were scheduled on an annual basis for 5 years after diagnosis. Follow-up assessments at other sites occurred 1, 3, and 5 years after diagnosis. Assessments at each time point were completed within a time window of ±3 months to allow for scheduling. Inclusion of 2- and 4-year postdiagnosis evaluations conducted at the primary study site did not significantly alter results; therefore, data from these time points were included in all analyses to maximize power. Owing to the ongoing nature of this study, 52.12% of the participants had been followed through all 5 years of the protocol at the time of data analysis, with the majority of participants (81.21%) followed through at least 3 years of the protocol.
Participants were included in the current analyses if they had completed two or more assessments of WM over the course of study participation, given longitudinal data modeling. Of note, five participants were <5 years old at baseline and therefore did not complete WM assessment at baseline; however, they have been included in the current study because they completed the WM assessment on at least two occasions once they met age criteria for it to be completed. Further, participants who had experienced PFS (n = 35) were included in the current analyses to increase generalizability to the larger medulloblastoma population. These inclusion criteria differ from previous manuscripts reporting on this cohort in which completion of a baseline assessment was necessary for inclusion and patients with PFS were excluded (Palmer et al., 2013a).
Measures
Parent Report of Working Memory
Parents/caregivers completed the BRIEF. The Working Memory subscale (BRIEF-WM; Gioia et al., 2000a) of the BRIEF was used in the current study. The BRIEF-WM consists of 10 items rated using a 3-point Likert scale (never, sometimes, and often). Items are designed to assess the capacity of the child to hold information in mind for the purposes of completing a task (Gioia et al., 2000a). The BRIEF-WM T-score is age- and gender-standardized, with a mean of 50 (SD = 10), with higher ratings indicative of greater perceived dysfunction and ratings of 60 and above indicative of clinical significance. The BRIEF-WM demonstrates appropriate internal consistency, α = .89–.92, and test–retest reliability, r = .82–.85 (Gioia et al., 2000a; Gioia, Isquith, Guy, & Kenworthy, 2000b). Correlations between BRIEF-WM and relevant published attentional rating scales provide evidence for convergent validity (Gioia et al., 2000a, 2000b, 2001, 2008).
Performance-Based Measure of Working Memory
The Woodcock–Johnson Tests of Cognitive Abilities – Third Edition (WJ-III), Working Memory Composite (WJ-WM) (Woodcock, McGrew, & Mather, 2001) was used to assess WM performance. Age-adjusted standard scores with population mean of 100 (SD = 15) were used for analyses. Lower standard scores indicate poorer performance. Scores from two subtests, Numbers Reversed (a task that requires holding a span of presented numbers in immediate memory while reversing the sequence) and Auditory Working Memory (a task that requires holding a mixed set of numbers and words in immediate memory while reordering into two sequences), are combined to produce a composite measure (WJ-WM). The WJ-III is well-normed from 2 to 19 years, and WJ-WM has demonstrated strong reliability in the age range of 5–19 years, median α = 0.90 (Woodcock et al., 2001).
Statistical Analyses
Descriptive analyses (means and frequencies) of demographic and clinical variables were used to characterize the sample. Linear mixed models (LMMs) using PROC MIXED procedure in SAS Release 9.2 (SAS Institute, Cary, NC) were used to estimate the slope (average rate of change) of the BRIEF-WM and WJ-WM scores with time since diagnosis. LMMs are statistically rigorous techniques that are based on maximum likelihood and can be used to estimate change over time in particular variables. These models can handle variations in the number and timing of measurements per participant. LMMs allow for estimation of the average rate of change (slope) over time (Chambers & Hastie, 1993; Jones, 1993; Little, Milliken, & Stroup, 1996; Rutter & Elashoff, 1994; Searle, 1987; Venables & Ripley, 1997). Exploratory analyses involved extensive visual inspection and spline smoothing of the profile plots. As no deviations from linearity were apparent, linear change was assumed in all models. First, simple models were used to estimate average change over time (slope) in standard scores for each outcome variable. Then, separate unadjusted models were fitted to examine the effects on change over time according to age at diagnosis, disease risk (average versus high), and presence of PFS. LMMs were also used to estimate the change over time in the percentage of children displaying a clinically significant compromised performance on the WJ-WM and BRIEF-WM (i.e., 1 SD from the mean, which equates to <85 for WJ-WM or >60 for BRIEF-WM). Cross-sectional Spearman-ranked correlations were used to examine the relationship between BRIEF-WM and WJ-WM. Strength of the correlation was classified according to Cohen’s (1988) guidelines, where small is r = .10–.29, medium is r = .30–.49, and large is r = .50–1.0. All analyses performed were two-tailed tests at an alpha level of p = .05.
Results
Of the 318 patients who participated in the larger study, 167 were included in the current analyses, resulting in 591 observations of WM (mean number of observations per participant over the 5-year follow-up period = 2.54, SD = 1.45). Reasons for nonparticipation in the WM assessment included being enrolled in a site that did not participate in the neurocognitive component of the study (n = 19), data not yet received from site (n = 2), staffing issues (n = 4), limited English fluency (n = 12), medical status restricting assessment (n = 11), died of disease (n = 2), partial assessment only (n = 2), patient/parents refused testing (n = 6), no informed consent received (n = 8), and consent withdrawn (n = 1). For a further 22 participants, multiple reasons for nonparticipation were provided across the course of their assessments (including medical, staffing, uncooperative, failed to attend, did not consent, partial assessment only, and died of disease). An additional 62 individuals were excluded from current analyses as a result of having only a single evaluation. Comparisons between participants that were included versus not included in the current analyses demonstrated no gender, race, or PFS-status differences between groups; however, statistically significant differences were apparent with respect to age at diagnosis and disease risk (Table I). Participants included in the current analyses were, on average, of older age at diagnosis, with a higher proportion following the average-risk disease arm of the protocol.
Table I.
Comparison Between Patients Who Were Excluded From Versus Included in Present Analyses
| Variable | Excluded (n = 151) | Included (n = 167) | p-value |
|---|---|---|---|
| Age at diagnosis, M (SD) | 7.91 (3.80) | 9.20 (3.90) | .003 |
| Race, % (n) | |||
| White | 73 (110) | 83 (136) | .26 |
| Black | 7 (11) | 7 (12) | |
| Asian | 7 (11) | 4 (7) | |
| Other | 12 (18) | 7 (12) | |
| Risk, % (n) | |||
| Average | 64 (96) | 76 (127) | .02 |
| High | 36 (55) | 24 (40) | |
| Gender, % (n) | |||
| Female | 36 (54) | 37 (62) | .80 |
| Male | 64 (97) | 63 (105) | |
| Posterior fossa syndrome (PFS), % (n) | 26 (40) | 21 (35) | .25 |
Demographic and clinical characteristics of the current study sample are reported in Table I. The included sample was 63% male, with a mean age at diagnosis of 9.2 (SD = 3.9) years. The majority (76%; n = 127) of included patients followed the average-risk protocol, and 21% (n = 35) experienced PFS.
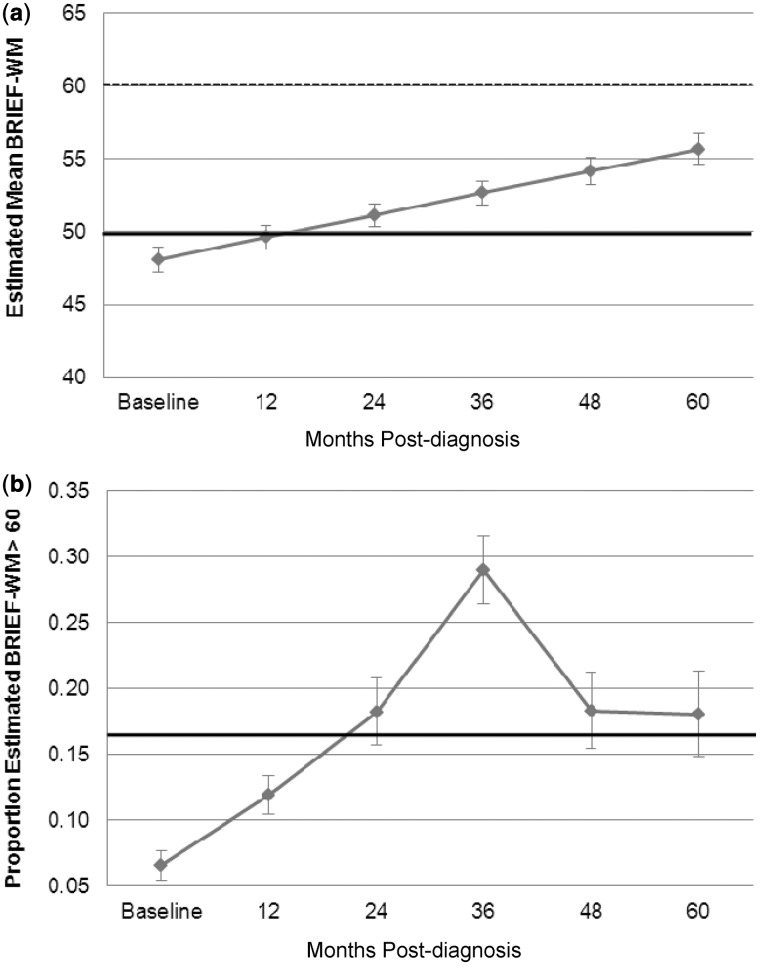
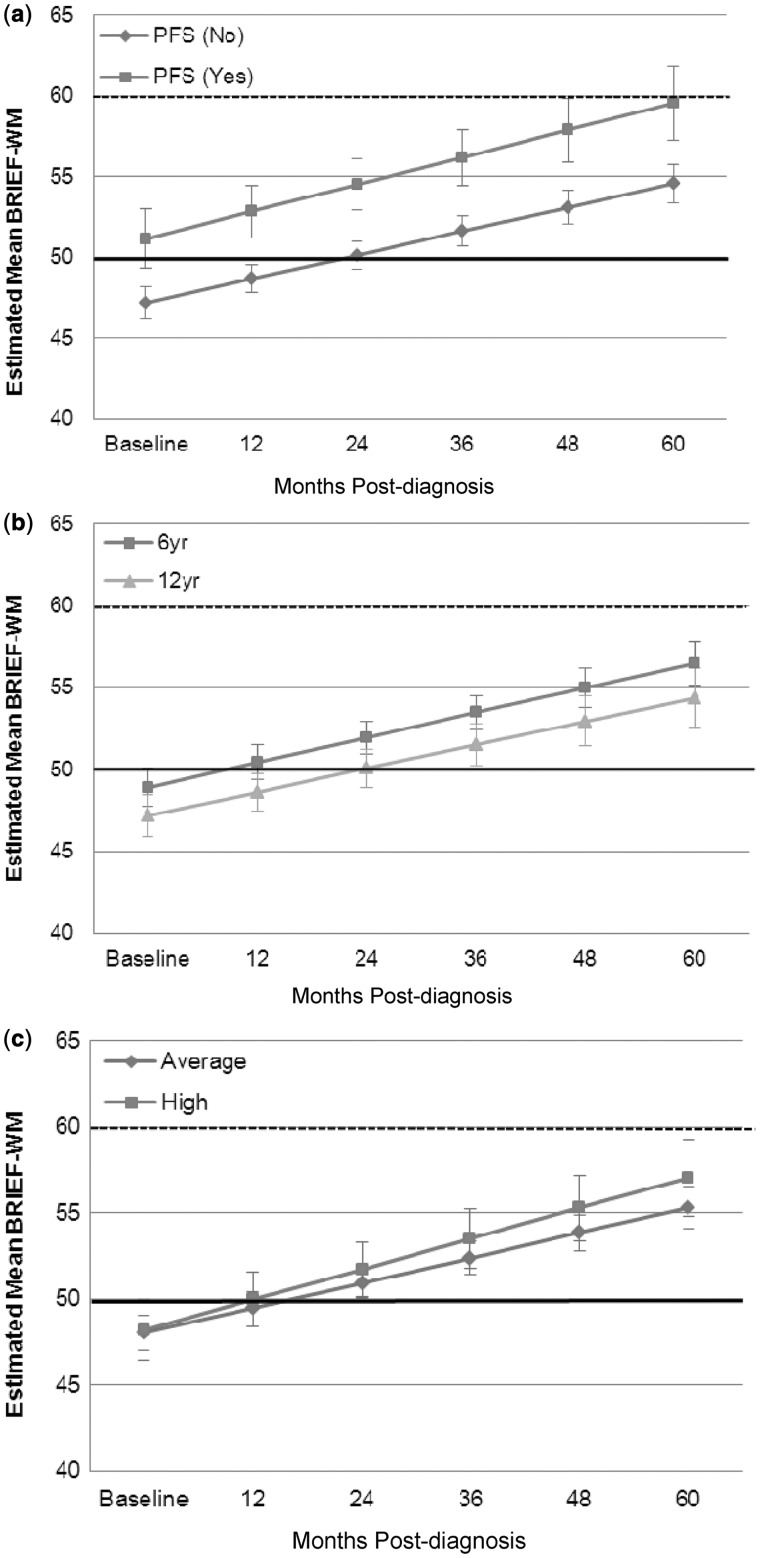
The estimated mean BRIEF-WM score remained within 1 SD of the population mean across the 5-year study period (Figure 1a). Without examination of other covariates, LMM demonstrated that the BRIEF-WM age-standardized scores increased significantly over time since diagnosis (β = 1.51, p < .001), reflecting increasing parental concern about WM. Importantly, LMM demonstrated a statistically significant increase in the percentage of patients falling in the clinically significant range on the BRIEF-WM from baseline through 3 years after diagnosis (β = 0.83, p < .001; Figure 1b), with no significant change from 3 years to 5 years after diagnosis (−0.40, p = .17). Figure 2a shows that PFS was independently related to BRIEF-WM (β = −4.32, p = .02), reflecting greater parental concern about WM problems for children who experienced PFS. The presence of PFS did not contribute to change in BRIEF-WM scores over time (β = −0.20, p = .72). Age at diagnosis and disease risk classification were not significantly associated with BRIEF-WM or change in BRIEF-WM over time since diagnosis (Figure 2b and c). However, the pattern of performance by age was consistent with younger age as a risk factor.
Figure 1.
(a) Estimated means for BRIEF-WM with time since diagnosis: whole group. (b) Estimated proportion of scores 1 SD above population mean on BRIEF-WM with time since diagnosis: whole group. The bold black line indicates the normative mean and the dotted line represents 1 SD above normative mean. Error bars represent standard error of the mean. BRIEF-WM = Behavior Rating Inventory of Executive Function – Working Memory subscale.
Figure 2.
Estimated means for BRIEF-WM with time since diagnosis: (a) PFS and no PFS, (b) age at diagnosis, (c) disease risk (high and average). The bold black line indicates the normative mean and the dotted line represents 1 SD above normative mean. Error bars represent standard error of the mean. PFS = posterior fossa syndrome; BRIEF-WM = Behavior Rating Inventory of Executive Function –Working Memory subscale.
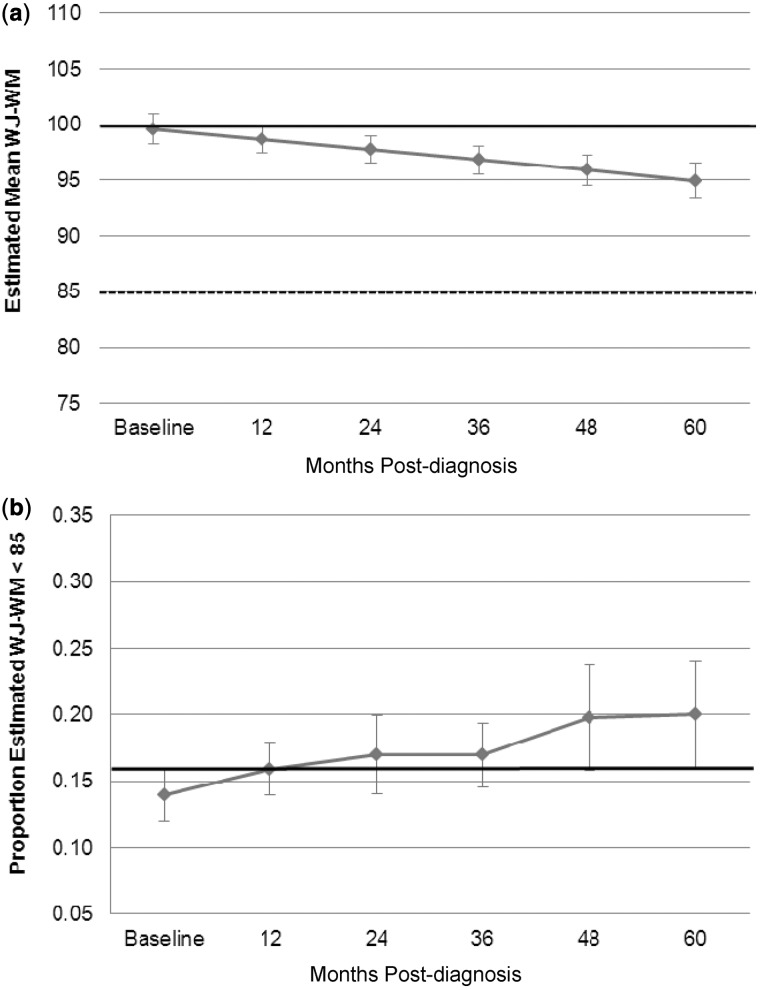
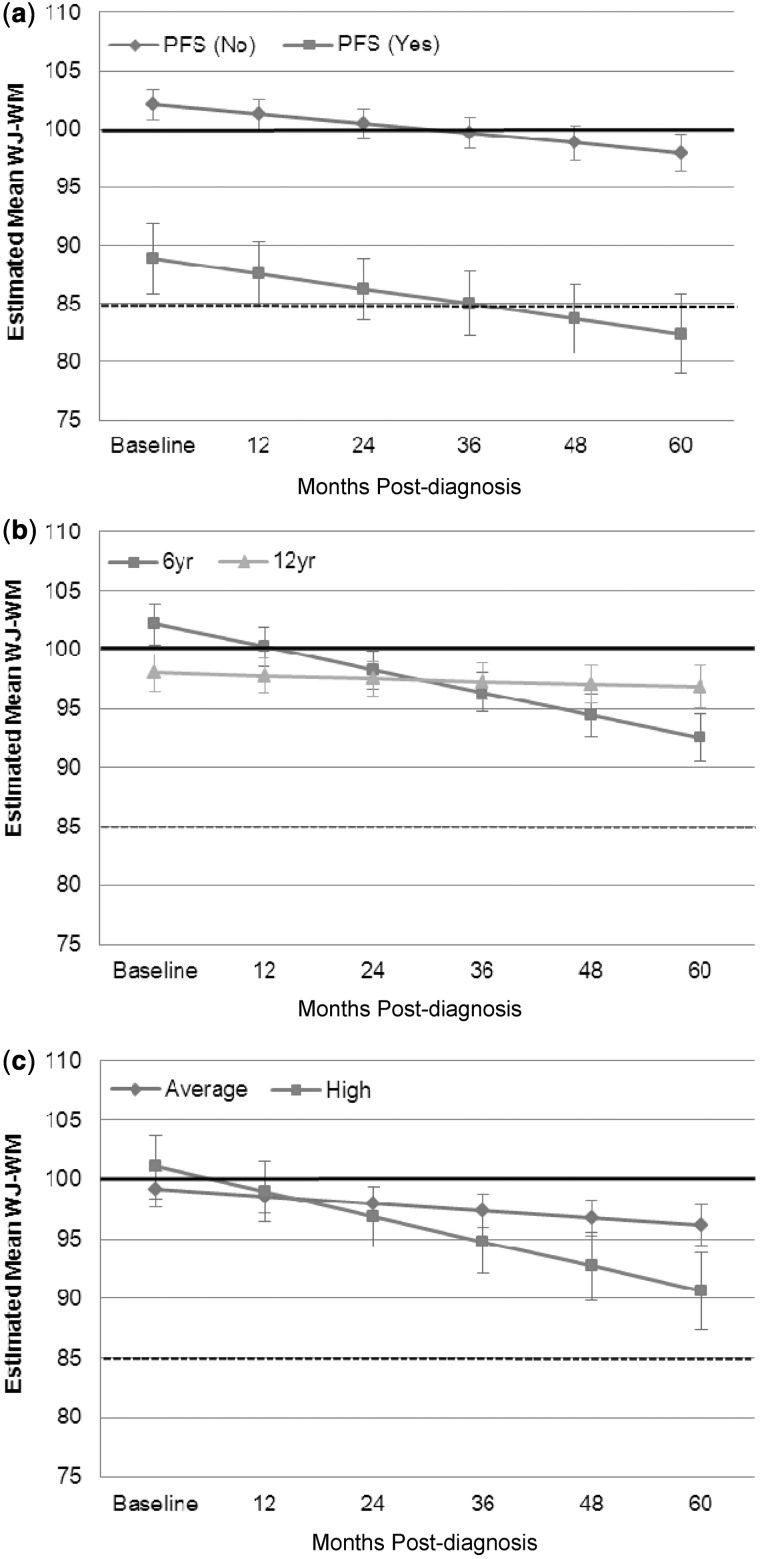
Estimated mean WJ-WM scores were within 1 SD of the population mean across the 5-year study period (Figure 3a). Using LMMs, mean age-standardized scores on WJ-WM decreased significantly over time (β = −0.93, p < .001). However, the percentage of participants performing >1 SD below the population mean on the WJ-WM did not change significantly over time since diagnosis (Figure 3b). Diagnosis of PFS was strongly associated with poorer WJ-WM performance overall (β = 14.15, p < .001) but not associated with change in WJ-WM over time (β = 0.46, p = .55), as reflected in the similar line slopes of Figure 4a. Age at diagnosis (β = −0.10, p = .54) was not independently associated with WJ-WM performance, but an interaction between age at diagnosis and time was observed, with younger age at diagnosis related to a larger decrease in WJ-WM standard scores over time (β = −0.28, p < .001; Figure 4b). There was no significant association between disease risk and WJ-WM when examined in isolation (β = 0.59, p = .84), but the change in WJ-WM over time was significantly greater for patients treated for high-risk disease (β = 1.49, p = .027; Figure 4c).
Figure 3.
(a) Estimated means for WJ-WM with time since diagnosis: whole group. (b) Estimated proportion of scores 1 SD above population mean on WJ-WM with time since diagnosis: whole group. The bold black line indicates the normative mean and the dotted line represents 1 SD below normative mean. Error bars represent standard error of the mean. WJ-WM = The Woodcock–Johnson Tests of Cognitive Abilities – Third Edition, Working Memory Composite.
Figure 4.
Estimated means for WJ-WM with time since diagnosis: (a) PFS and no PFS, (b) age at diagnosis, (c) disease risk (high and average). The bold black line indicates the normative mean and the dotted line represents 1 SD below normative mean. Error bars represent standard error of the mean. PFS = posterior fossa syndrome; WJ-WM = The Woodcock–Johnson Tests of Cognitive Abilities – Third Edition, Working Memory Composite.
Cross-sectional Spearman rank correlation coefficients were of similar magnitude (small to medium) over the six time points (Table II). The correlations were negative (i.e., greater parental concern regarding WM was associated with lower scores on the WJ-WM).
Table II.
Spearman-Rank Correlation Coefficients Between BRIEF-WM and WJ-WM Performance
| Time point | n | r | p-value |
|---|---|---|---|
| Baseline | 92 | −.20 | .06 |
| 1 year | 112 | −.23 | .02 |
| 2 years | 57 | −.29 | .03 |
| 3 years | 80 | −.20 | .07 |
| 4 years | 41 | −.30 | .06 |
| 5 years | 41 | −.34 | .03 |
Note. BRIEF-WM = Behavior Rating Inventory of Executive Function – Working Memory subscale; WJ-WM = The Woodcock–Johnson Tests of Cognitive Abilities – Third Edition (WJ-III), Working Memory Composite.
Discussion
This study aimed to estimate change in parent report and performance measures of WM over 5 years after diagnosis in a large group of children who received postsurgical risk-adapted CNS-directed treatment for medulloblastoma. At a group level, modelling revealed a small but statistically significant increase in WM problems over time as rated by parents. This was reflected in an increase in mean parent BRIEF-WM ratings, as well as an increase in the percentage of children falling in the clinically significant range on the BRIEF-WM. However, estimated mean BRIEF-WM scores over the study period remained in the age-expected range, suggesting that, overall, these changes may not be clinically obvious and the majority of parents continue to rate their child’s WM as within age-expected limits across time. A similar pattern of results was evident on the performance-based measure of WM with a small yet statistically significant decline in age-standardized scores over time. However, although there was a significant increase in children falling in the clinically significant range on the BRIEF-WM over time, no significant increase was observed on the WJ-WM. This lends further support to the notion that, in general, changes in WM over time are likely subtle in this patient group as a whole.
While findings at a group level are modest, current findings suggest that subgroups of children treated for medulloblastoma may be at greater risk of WM changes. PFS appears to be associated with less favorable WM outcomes in this group that remain obvious over time on both parent report and performance measures. The pathophysiological mechanisms underlying PFS are largely unknown. Recent neuroimaging studies provide evidence that bilateral surgical damage to the corticocerebellar tracts may underlie PFS (Miller et al., 2010). Such surgical damage could similarly affect the development of neurocognitive skills reliant on these developing neural networks, such as WM (Law et al., 2011). Although underlying mechanisms remain unclear, findings here suggest that children who experience PFS represent a group who are at increased risk for WM difficulties and therefore require close monitoring in the years after treatment.
The finding that neither age at diagnosis nor disease risk (with associated CSI intensity differences) was significantly associated with parent report of WM is interesting, given that these factors interacted with change over time on performance-based measures. These results may indicate that compared with parent report of WM, objective WM performance was more closely aligned with important developmental and medical variables (i.e., age at diagnosis and disease risk) believed to moderate neurocognitive outcome in medulloblastoma (Robinson et al., 2013). It is possible that parent report may be more susceptible to other intervening or confounding influences that remain unclear. Future research incorporating other variables (e.g., detailed measures of family functioning or socioeconomic position) will be helpful in further elucidating the complexity and interrelationships between factors that may variably influence performance-based assessment and observer-rating of WM function in this vulnerable group.
Interestingly, both measures demonstrated a statistically significant, yet modest, negative change in WM over the 5 years after diagnosis. However, consistent with other studies using parent report alongside performance-based measures, the two tests were not highly correlated (Anderson et al., 2002; Biggs et al., 2011; Conklin et al., 2008; Gioia et al., 2001; Gioia & Isquith, 2004; Toplak et al., 2009; Vriezen & Pigott, 2002).
While both measures appear sensitive to change in function over time in children with medulloblastoma, the limited correlation implies that they may be measuring different facets of function, which may reflect important differences in the behavioral and cognitive dimensions of WM function (Anderson et al., 2002). Alternatively, it has been proposed that parent responses on the BRIEF-WM may reflect more generalized functional abilities as opposed to WM ability specifically (Bodnar, Prahme, Cutting, Denckla, & Mahone, 2007; McAuley, Chen, Goos, Schachar, & Crosbie, 2010).
Strengths, Limitations, and Future Directions
The current study has allowed WM to be examined at multiple, prospectively planned time points in a large clinical sample following the same treatment protocol using both parent report and performance-based measures; however, the limitations of the current study need to be acknowledged. First, this study did not include a control group; therefore, data were compared with normative data from the measures used. Inclusion of a control group would allow for better matching to our patient population and the ability to assess practice effects, particularly given the longitudinal design. Second, while using alternate measurement methods may enhance the validity of current results, the ecological validity of both measurement approaches has been questioned separately. Tasks such as the WJ-WM are conducted in a structured testing environment and performance in this setting may not directly translate into the use of the particular skill in everyday situations (such as the classroom environment). The current study was also limited by reliance on a single informant reporting the behavioral aspects of WM. Given the numerous factors that may influence parental reporting, future research may benefit from using multi-informant approaches (e.g., teacher or self-report) in the assessment of WM in children with medulloblastoma. Third, the visuospatial aspects of WM were not directly assessed in the current study. Future studies incorporating measures of visuospatial WM to complement the more widely used auditory WM tests would provide a more comprehensive understanding of WM function in children treated for medulloblastoma.
Despite a statistically significant change in BRIEF-WM and in WJ-WM scores over time, the change on both measures was relatively small for the group as a whole, with estimated means remaining within 1 SD of the population mean on both performance and parent rating measures over time. While this suggests that the change in WM over time is likely to be subtle, it is important to consider the disproportionate participation of children of younger age and from the high-risk treatment arm in the current sample. As younger age at diagnosis and higher radiation dose have frequently been associated with poorer outcome in the brain tumor literature (Kieffer-Renaux et al., 2000; Merchant et al., 2006; Mulhern et al., 2005; Palmer et al., 2013a; Robinson et al., 2013), and were associated with statistically steeper decline in age-scaled scores over time in the current study, it is possible that the current results underestimate the actual rates and severity of WM deficits in the population of children treated for medulloblastoma. However, the subtle change in WM observed in the current sample is similar to findings of other studies assessing WM in patients receiving CNS-directed radiation therapy for brain tumors, where mean WM scores have also fallen in the age-appropriate range (i.e., within 1 SD of the population mean) in the years after treatment (Conklin et al., 2012; Dennis et al., 1992; Dennis, Hetherington, & Spiegler, 1998; Mabbott et al., 2008; Palmer et al., 2013a).
Conclusion
In summary, most children treated with medulloblastoma continue to display WM abilities that are within the age-appropriate range accordingly to both performance-based measures and parent report in the years after diagnosis. Findings, however, suggest subtle changes in WM over time, as well as particular medical factors that may affect WM function. These subtle changes emphasize the importance of ongoing monitoring to allow for early detection of any changes in WM, particularly in those patients treated at a younger age, those with higher treatment intensity, and/or those who develop PFS. Ideally, neuropsychological assessments should incorporate both performance and rater-based measures to provide a comprehensive assessment of the WM of a particular child. Future research is necessary to determine whether the changes in WM identified in the current study continue or become more significant beyond 5 years after diagnosis.
Funding
National Cancer Institute through a Cancer Center Support (CORE) grant (P30-CA21765), the Noyes Brain Tumor Foundation, Musicians Against Childhood Cancer (MACC), and the American Lebanese Syrian Associated Charities (ALSAC).
Conflicts of interest: None declared.
References
- Alloway T P. Working memory, but not IQ, predicts subsequent learning in children with learning difficulties. European Journal of Psychological Assessment. 2009;25(2):92–98. doi:10.1027/1015-5759.25.2.92. [Google Scholar]
- Anderson P. Assessment and development of executive function (EF) during childhood. Child Neuropsychology. 2002;8:71–82. doi: 10.1076/chin.8.2.71.8724. doi:10.1076/chin.8.2.71.8724. [DOI] [PubMed] [Google Scholar]
- Anderson V A, Anderson P, Northam E, Jacobs R, Mikiewicz O. Relationships between cognitive and behavioral measures of executive function in children with brain disease. Child Neuropsychology. 2002;8:231–240. doi: 10.1076/chin.8.4.231.13509. doi:10.1076/chin.8.4.231.13509. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory: Theories, models, and controversies. Annual Reviews in Psychology. 2012;63:1–29. doi: 10.1146/annurev-psych-120710-100422. doi:10.1146/annurev-psych-120710-100422. [DOI] [PubMed] [Google Scholar]
- Biggs S N, Bourke R, Anderson V A, Jackman A R, Killedar A, Nixon G M, Davey M J, Walker A M, Trinder J, Horne R S. Working memory in children with sleep-disordered breathing: Objective versus subjective measures. Sleep Medicine. 2011;12:887–891. doi: 10.1016/j.sleep.2011.07.003. doi:10.1016/j.sleep.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Bodnar L E, Prahme M C, Cutting L E, Denckla M B, Mahone E M. Construct validity of parent ratings of inhibitory control. Child Neuropsychology. 2007;13:345–362. doi: 10.1080/09297040600899867. doi:10.1080/09297040600899867. [DOI] [PubMed] [Google Scholar]
- Chambers J M, Hastie T J E. Statistical Models in Southern California. Pacific Grove, CA: Wadsworth and Brooks; 1993. [Google Scholar]
- Chaytor N, Schmitter-Edgecombe M, Burr R. Improving the ecological validity of executive functioning assessment. Archives of Clinical Neuropsychology. 2006;21:217–227. doi: 10.1016/j.acn.2005.12.002. doi:10.1016/j.acn.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Conklin H M, Ashford J M, Howarth R A, Merchant T E, Ogg R J, Santana V M, Reddick W E, Xiong X. Working memory performance among childhood brain tumor survivors. Journal of the International Neuropsychological Society. 2012;18:996–1005. doi: 10.1017/S1355617712000793. doi:10.1017/S1355617712000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin H M, Salorio C F, Slomine B S. Working memory performance following paediatric traumatic brain injury. Brain Injury. 2008;22:847–857. doi: 10.1080/02699050802403565. doi:10.1080/02699050802403565. [DOI] [PubMed] [Google Scholar]
- Crawford J R, MacDonald T J, Packer R J. Medulloblastoma in childhood: New biological advances. The Lancet Neurology. 2007;6:1073–1085. doi: 10.1016/S1474-4422(07)70289-2. doi:0.1016/S1474-4422%2807%2970289-2. [DOI] [PubMed] [Google Scholar]
- de Ruiter M A, van Mourik R, Schouten-van Meereren A Y, Grootenhuis M A, Oosterlaan J. Neurocognitive consequences of a paediatric brain tumour and its treatment: A meta-analysis. Developmental Medicine and Child Neurology. 2012;55:408–417. doi: 10.1111/dmcn.12020. doi:10.1111/dmcn.12020. [DOI] [PubMed] [Google Scholar]
- Dennis M, Hetherington C R, Spiegler B J. Memory and attention after childhood brain tumors. Medical and Pediatric Oncology. 1998;30(S1):25–33. doi: 10.1002/(sici)1096-911x(1998)30:1+<25::aid-mpo4>3.0.co;2-a. doi:10.1002/(SICI)1096-911X(1998)30:1+<25::AID-MPO4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Dennis M, Spiegler B, Obonsawin M, Maria B, Cowell C, Hoffman H, Hendrick E B, Humphreys R P, Bailey J D, Ehrlich R. Brain tumors in children and adolescents—III. Effects of radiation and hormone status on intelligence and on working, associative and serial-order memory. Neuropsychologia. 1992;30:257–275. doi: 10.1016/0028-3932(92)90004-6. doi:10.1016/0028-3932(92)90004-6. [DOI] [PubMed] [Google Scholar]
- Ellenberg L, Liu Q, Gioia GA, Yasui Y, Packer RJ, Mertens A, Donaldson S S, Stovall M, Kadan-Lottick N, Armstrong G, Robison L L, Zeltzer L K. Neurocognitive status in long-term survivors of childhood CNS malignancies: A report from the Childhood Cancer Survivor Study. Neuropsychology. 2009;23:705. doi: 10.1037/a0016674. doi:10.1037/a0016674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filley C M, Kleinschmidt-DeMasters B K. Toxic leukoencephalopathy. New England Journal of Medicine. 2001;345:425–432. doi: 10.1056/NEJM200108093450606. doi:10.1056/NEJM200108093450606. [DOI] [PubMed] [Google Scholar]
- Gajjar A, Chintagumpala M, Ashley D, Kellie S, Kun L, Merchant T, Woo S, Wheeler G, Ahern V, Krasin M J, Fouladi M, Broniscer A, Krance R, Hale G A, Stewart C F, Dauser R, Sanford R A, Fuller C, Lau C, Boyett J M, Wallace D, Gilbertson R J. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): Long-term results from a prospective, multicentre trial. Lancet Oncology. 2006;7:813–820. doi: 10.1016/S1470-2045(06)70867-1. doi:10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- Gajjar A, Sanford R A, Bhargava R, Heideman R, Walter A, Li Y, Langston J W, Jenkins J J, Muhlbauer M, Boyett J, Kun L E. Medulloblastoma with brain stem involvement: The impact of gross total resection on outcome. Pediatric Neurosurgery. 1996;25:182–187. doi: 10.1159/000121121. doi:10.1159/000121121. [DOI] [PubMed] [Google Scholar]
- Gathercole S E, Pickering S J, Knight C, Stegmann Z. Working memory skills and educational attainment: Evidence from national curriculum assessments at 7 and 14 years of age. Applied Cognitive Psychology. 2003;18:1–16. doi:10.1002/acp.934. [Google Scholar]
- Gioia G A, Isquith P K. Ecological assessment of executive function in traumatic brain injury. Developmental Neuropsychology. 2004;25(1-2):135–158. doi: 10.1080/87565641.2004.9651925. doi:10.1080/87565641.2004.9651925. [DOI] [PubMed] [Google Scholar]
- Gioia G A, Isquith P K, Guy S C, Kenworthy L. Behavior rating inventory of executive function. Odessa, FL: Psychological Assessment Resources, Inc; 2000a. [Google Scholar]
- Gioia G A, Isquith P K, Guy S C, Kenworthy L. Test review behavior rating inventory of executive function. Child Neuropsychology. 2000b;6:235–238. doi: 10.1076/chin.6.3.235.3152. doi:10.1076/chin.6.3.235.3152. [DOI] [PubMed] [Google Scholar]
- Gioia G A, Isquith P K, Kenealy L E. Assessment of behavioral aspects of executive function. In: Anderson V, Jacobs R, Anderson P, editors. Executive functions and the frontal lobes: A lifespan perspective. New York, NY: Psychology Press; 2008. pp. 179–202. [Google Scholar]
- Gioia G A, Isquith P K, Retzlaff P D, Pratt B M. Modelling executive functions with everyday behaviors: A unitary or fractionated system. Brain and Cognition. 2001;47:203–207. doi:10.1006/brcg.2000.1278. [Google Scholar]
- Jones R H. Longitudinal data with serial correlation: A state-space approach. London, UK: Chapman and Hall; 1993. [Google Scholar]
- Kibby M Y, Marks W, Morgan S, Long C J. Specific impairment in developmental reading disabilities: A working memory approach. Journal of Learning Disabilities. 2004;37:349–363. doi: 10.1177/00222194040370040601. doi:10.1177/00222194040370040601. [DOI] [PubMed] [Google Scholar]
- Kieffer-Renaux V, Bulteau C, Grill J, Kalifa C, Viguier D, Jambaque I. Patterns of neuropsychological deficits in children with medulloblastoma according to craniospatial irradiation doses. Developmental Medicine and Child Neurology. 2000;42:741–745. doi: 10.1017/s0012162200001377. doi:10.1111/j.1469-8749.2000.tb00036.x. [DOI] [PubMed] [Google Scholar]
- Kirschen M P, Davis-Ratner M S, Milner M W, Chen S H, Schraedley-Desmond P, Fisher P G, Desmond J E. Verbal memory impairments in children after cerebellar tumor resection. Behavioural Neurology. 2008;20:39–53. doi: 10.3233/BEN-2008-0216. doi:10.3233/BEN-2008-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korah M P, Esiashvili N, Mazewski C M, Hudgins R J, Tighiouart M, Janss A J, Schwaibold F P, Crocker W J, Jr., Marcus R B., Jr. Incidence, risks, and sequelae of posterior fossa syndrome in pediatric medulloblastoma. International Journal of Radiation Oncology, Biology, Physics. 2010;77:106–112. doi: 10.1016/j.ijrobp.2009.04.058. doi:10.1016/j.ijrobp.2009.04.058. [DOI] [PubMed] [Google Scholar]
- Law N, Bouffet E, Laughlin S, Laperriere N, Brière M E, Strother D, McConnell D, Hukin J, Fryer C, Rockel C, Dickson J, Mabbott D. Cerebello–thalamo–cerebral connections in pediatric brain tumor patients: Impact on working memory. NeuroImage. 2011;56:2238–2248. doi: 10.1016/j.neuroimage.2011.03.065. doi:10.1016/j.neuroimage.2011.03.065. [DOI] [PubMed] [Google Scholar]
- Little R C, Milliken G A, Stroup W W. SAS system for mixed models. Cary, NC: SAS Institute Inc; 1996. [Google Scholar]
- Mabbott D J, Penkman L, Witol A, Strother D, Bouffet E. Core neurocognitive functions in children treated for posterior fossa tumors. Neuropsychology. 2008;22:159. doi: 10.1037/0894-4105.22.2.159. doi:10.1037/0894-4105.22.2.159. [DOI] [PubMed] [Google Scholar]
- McAuley T, Chen S H A, Goos L, Schachar R, Crosbie J. Is the behavior rating inventory of executive function more strongly associated with measures of impairment or executive function? Journal of the International Neuropsychological Society. 2010;16:495–505. doi: 10.1017/S1355617710000093. doi:10.1017/S1355617710000093. [DOI] [PubMed] [Google Scholar]
- Merchant T E, Conklin H M, Wu S, Lustig R H, Xiong X. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: Prospective evaluation of cognitive, endocrine, and hearing deficits. Journal of Clinical Oncology. 2009;27:3691–3697. doi: 10.1200/JCO.2008.21.2738. doi:10.1200/JCO.2008.21.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant T E, Kiehna E N, Li C, Shukla H, Sengupta S, Xiong X, Gajjar A, Mulhern R K. Modeling radiation dosimetry to predict cognitive outcomes in pediatric patients with CNS embryonal tumors including medulloblastoma. International Journal of Radiation Oncology, Biology and Physics. 2006;65:210–221. doi: 10.1016/j.ijrobp.2005.10.038. doi:10.1016/j.ijrobp.2005.10.038. [DOI] [PubMed] [Google Scholar]
- Miller N G, Reddick W E, Kocak M, Glass J O, Lobel U, Morris B, Gajjar A, Patay Z. Cerebellocerebral diaschisis is the likely mechanism of postsurgical posterior fossa syndrome in pediatric patients with midline cerebellar tumors. AJNR American Journal of Neuroradiology. 2010;31:288–294. doi: 10.3174/ajnr.A1821. doi:10.3174/ajnr.A1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulhern R K, Palmer S L, Merchant T E, Wallace D, Kocak M, Brouwers P, Krull K, Chintagumpala M, Stargatt R, Ashley D M, Tyc V L, Kun L, Boyett J, Gajjar A. Neurocognitive consequences of risk-adapted therapy for childhood medulloblastoma. Journal of Clinical Oncology. 2005;23:5511–5519. doi: 10.1200/JCO.2005.00.703. doi:10.1200/JCO.2005.00.703. [DOI] [PubMed] [Google Scholar]
- Ostby Y, Tamnes C K, Fjell A M, Walhovd K B. Morphometry and connectivity of the fronto-parietal verbal working memory network in development. Neuropsychologia. 2011;49:3854–3862. doi: 10.1016/j.neuropsychologia.2011.10.001. doi:10.1016/j.neuropsychologia.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Palmer S L, Armstrong C, Onar A, Wu S, Wallace D, Bonner M, Schreiber J, Swain M, Chapieski L, Mabbott D, Knight S, Boyle R, Gajjar A. Processing speed, attention and working memory after treatment for medulloblastoma: An international, prospective and longitudinal study. Journal of Clinical Oncology. 2013a;31:3494–3500. doi: 10.1200/JCO.2012.47.4775. doi:10.1200/JCO.2012.47.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer S L, Hassall T, Evankovich K, Mabbott D J, Bonner M, Deluca C, Cohn R, Fisher M J, Morris E B, Broniscer A, Gajjar A. Neurocognitive outcome 12 months following cerebellar mutism syndrome in pediatric patients with medulloblastoma. Neuro-Oncology. 2010;12:1311–1317. doi: 10.1093/neuonc/noq094. doi:10.1093/neuonc/noq094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer S L, Leigh L, Ellison S, Onar-Thomas A, Wu S, Qaddoumi I, Armstrong G T, Wright K, Wetmore C, Broniscer A, Gajjar A. Feasibility of a computer based intervention aimed at preventing reading decoding deficits among children undergoing active treatment for medulloblastoma: Results of a randomized trial. Journal of Pediatric Psychology. 2013b doi: 10.1093/jpepsy/jst095. Advance online publication. doi: 10.1093/jpepsy/jst095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack I F. Posterior fossa syndrome. International Review of Neurobiology. 1997;41:411–432. doi: 10.1016/s0074-7742(08)60362-1. [DOI] [PubMed] [Google Scholar]
- Robertson P L, Muraszko K M, Holmes E J, Sposto R, Packer R J, Gajjar A, Dias M S, Allen J C, Children's Oncology Group Incidence and severity of postoperative cerebellar mutism syndrome in children with medulloblastoma: A prospective study by the Children's Oncology Group. Journal of Neurosurgery. 2006;105(6 Suppl):444–451. doi: 10.3171/ped.2006.105.6.444. doi:10.3171/ped.2006.105.6.444. [DOI] [PubMed] [Google Scholar]
- Robinson K E, Fraley C E, Pearson M M, Kuttesch J F, Compas B E. Neurocognitive late effects of pediatric brain tumors of the posterior fossa: A quantitative review. Journal of the International Neuropsychological Society. 2013;1:1–10. doi: 10.1017/S1355617712000987. doi:10.1017/S1355617712000987. [DOI] [PubMed] [Google Scholar]
- Robinson K E, Kuttesch J F, Champion J E, Andreotti C F, Hipp D W, Bettis A, Barnwell A, Compas B E. A quantitative meta-analysis of neurocognitive sequelae in survivors of pediatric brain tumors. Pediatric Blood and Cancer. 2010;55:525–531. doi: 10.1002/pbc.22568. doi:10.1002/pbc.22568. [DOI] [PubMed] [Google Scholar]
- Rottschy C, Langner R, Dogan I, Reetz K, Laird A R, Schulz J B, Fox P T, Eickhoff S B. Modelling neural correlates of working memory: a coordinate-based meta-analysis. NeuroImage. 2012;60:830–846. doi: 10.1016/j.neuroimage.2011.11.050. doi:10.1016/j.neuroimage.2011.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter C M, Elashoff R M. Analysis of longitudinal data: Random coefficient regression modeling. Statistics in Medicine. 1994;13:1211–1231. doi: 10.1002/sim.4780131204. doi:10.1002/sim.4780131204. [DOI] [PubMed] [Google Scholar]
- Searle S R. Linear models for unbalanced data. New York, NY: John Wiley and Sons; 1987. [Google Scholar]
- Spiegler B J, Bouffet E, Greenberg M L, Rutka J T, Mabbott D J. Change in neurocognitive functioning after treatment with cranial radiation in childhood. Journal of Clinical Oncology. 2004;22:706–713. doi: 10.1200/JCO.2004.05.186. doi:10.1200/JCO.2004.05.186. [DOI] [PubMed] [Google Scholar]
- Tamnes C K, Walhovd K B, Grydeland H, Holland D, Ostby Y, Dale A M, Fjell A M. Longitudinal working memory development is related to structural maturation of frontal and parietal cortices. Journal of Cognitive Neuroscience. 2013;25:1611–1623. doi: 10.1162/jocn_a_00434. doi:10.1162/jocn_a_00434. [DOI] [PubMed] [Google Scholar]
- Toplak M E, Bucciarelli S M, Jain U, Tannock R. Executive functions: Performance-based measures and the behavior rating inventory of executive function (BRIEF) in adolescents with attention deficit/hyperactivity disorder (ADHD) Child Neuropsychology. 2009;15:53–72. doi: 10.1080/09297040802070929. doi:10.1080/09297040802070929. [DOI] [PubMed] [Google Scholar]
- Venables W N, Ripley B D. Modern applied statistics. New York, NY: Springer; 1997. [Google Scholar]
- Vriezen E R, Pigott S E. The relationship between parental report on the BRIEF and performance-based measures of executive function in children with moderate to severe traumatic brain injury. Child Neuropsychology. 2002;8:296–303. doi: 10.1076/chin.8.4.296.13505. doi:10.1076/chin.8.4.296.13505. [DOI] [PubMed] [Google Scholar]
- Woodcock R W, McGrew K S, Mather N. Woodcock-Johnson III Tests of Cognitive Abilities. Itasca, IL: Riverside; 2001. [Google Scholar]