Abstract
For treatment of moderate-to-severe active Crohn’s disease, clinicians generally rely on immunosuppressants (including azathioprine and 6-mercaptopurine), corticosteroids, and antibodies against tumor necrosis factor α. However, a significant proportion of patients do not respond to these therapies, lose response over time, or are intolerant to these therapies. In such cases, one of the only remaining pharmacologic treatment options is natalizumab, an α4 integrin-targeted antibody. Unfortunately, 3 cases of progressive multifocal leukoencephalopathy (PML) were reported in natalizumab-treated patients in 2005, shortly after natalizumab’s approval by the US Food and Drug Administration (FDA). Natalizumab was subsequently withdrawn from the market but was then reintroduced in 2006 under close supervision by the FDA. Careful review of postmarketing data revealed 3 major risk factors for the development of natalizumab-associated PML, the most significant of which is prior exposure to the JC virus (JCV). To help identify patients who may be at higher risk for developing natalizumab-associated PML, a JCV antibody assay was developed that can detect anti-JCV antibodies in patients’ blood. Clinicians can now consider a patient’s anti-JCV antibody status together with the other major risk factors for natalizumab-associated PML—duration of natalizumab therapy and prior immunosuppressant use—to more accurately gauge the risks and benefits of natalizumab therapy in a particular patient.
Introduction
Treatment of moderate-to-severe Crohn’s disease has evolved dramatically in recent decades. Previously, patients had to rely upon surgical resection and treatment with immunosuppression and immunomodulatory agents to maintain short, inadequate remissions; now, treatment with anti—tumor necrosis factor α (anti-TNFα) agents allows patients to experience durable remission. Despite the efficacy and benefit associated with anti-TNFα agents, however, a significant proportion of patients either lose response or are intolerant to this therapy. For these patients, novel biologic therapies targeting unique molecules represent a much needed treatment alternative.
Natalizumab for the Treatment of Crohn’s Disease
Natalizumab is a humanized, monoclonal antibody directed against the α4 integrin, a cell adhesion molecule involved in the attachment and passage of cells through cell layers. The first large, published trials of natalizumab in patients with Crohn’s disease were the ENACT-1 and ENACT-2 studies, which were designed to assess the efficacy and safety of natalizumab for induction and maintenance of remission, respectively.1 Both studies were randomized, double-blind, placebo-controlled trials that were conducted at 142 centers between December 2001 and March 2004. Patients with moderate-to-severe active Crohn’s disease were enrolled. Concurrent therapy was permitted (including 5-aminosalicylate, prednisolone or equivalent, budesonide, azathioprine, 6-mercaptopu-rine, methotrexate, and antibiotics), but exposure to an anti-TNFα agent in the 3 months prior to study enrollment was not allowed.
A total of 905 patients were randomized 4:1 to receive induction therapy with either 300 mg natalizumab or placebo at Weeks 0, 4, and 8; these patients were followed through Week 12. Patients in ENACT-1 who showed a response to natalizumab induction therapy at both Week 10 and Week 12 (defined as a reduction in the Crohn’s disease activity index score ≥70 points from Week 0) were then eligible for natalizumab maintenance therapy in ENACT-2. Patients in ENACT-2 were re-randomized 1:1 to maintenance therapy with either 300 mg natalizumab or placebo every 4 weeks through Week 56; these patients were followed through Week 60. Natalizumab-treated patients and placebo-treated patients experienced similar rates of response (56% vs 49%, respectively; P=.05) and remission (37% vs 30%, respectively; P=.12) at Week 10 of induction therapy. However, patients who continued to receive natalizumab as maintenance therapy in ENACT-2 achieved significantly higher rates of response (61% for natalizumab vs 28% for placebo; P<.001) and remission (44% for natalizumab vs 26% for placebo; P=.003) through Week 36.
Another trial of natalizumab, ENCORE, was a global, multicenter, randomized, double-blind, placebo-controlled, parallel-group study conducted at 114 centers between March 2004 and March 2005.2 This study was designed to further test the efficacy of natalizumab induction therapy in 509 Crohn’s disease patients with moderate-to-severe active disease and active inflammation (defined as a C-reactive protein [CRP] level >2.87 mg/L). Patients were randomized 1:1 to receive either 300 mg natalizumab or placebo at Weeks 0, 4, and 8. A significant improvement in the rate of response with natalizumab versus placebo was detectable as early as Week 4 (51% vs 37%; P=.001). Nearly half (48%) of natalizumab-treated patients achieved a response at Week 8 that was sustained through Week 12, compared to only 32% of patients treated with placebo (P<.001). Similarly, remission at Week 8 that was sustained through Week 12 occurred in 26% of natalizumab-treated patients compared to 16% of placebo-treated patients (P=.002). Remission rates were also significantly higher with natalizumab than placebo at Weeks 4, 8, and 12 (P≤.009 for all comparisons).
Natalizumab was initially approved in the United States in December 2004 with a primary indication for relapsing multiple sclerosis. In February 2005, 2 cases of progressive multifocal leukoencephalopathy (PML) were reported among natalizumab-treated patients with multiple sclerosis. Soon thereafter, PML was also reported in a natalizumab-treated patient with Crohn’s disease. As a result of these 3 cases of PML, natalizumab was voluntarily withdrawn from the market in 2005. In 2006, however, natalizumab was reintroduced to the market, under close scrutiny by the US Food and Drug Administration (FDA), in response to patient desire. As part of this reintroduction, a comprehensive risk management plan was developed; termed the TOUCH (Tysabri Outreach: Unified Commitment to Health) Prescribing Program, this plan ensures that only prescribers and patients enrolled in TOUCH can prescribe and receive natalizumab, respectively, and only certain pharmacies and infusion sites can dispense and infuse natalizumab, respectively. In addition, TYGRIS (Tysabri Global Observation Program in Safety) is a voluntary, observational, cohort study that was developed to investigate the long-term safety of natalizumab in the clinical practice setting.
Natalizumab-Associated Progressive Multifocal Leukoencephalopathy
PML is an extremely rare disorder resulting from an opportunistic brain infection with JC virus (JCV). Associated with various immunocompromised states, PML came to clinical prominence during the AIDS era, when the frequency of its occurrence increased 12-fold over the previous decade.3 The epidemiology of PML now appears to be changing again, with PML being increasingly documented among natalizumab-treated patients with autoimmune diseases.
Interestingly, PML appears to be slightly different when it is associated with natalizumab than when it occurs in AIDS patients.3 The most frequent initial clinical presentations of natalizumab-associated PML include cognitive disorders (48%), motor abnormalities (37%), language disturbances (31%), and visual defects (26%). Comparatively, the most frequent initial clinical presentations in patients with AIDS-related PML are weakness (42%), speech abnormalities (40%), cognitive abnormalities (36%), gait abnormalities (29%), sensory loss (19%), and visual impairment (19%). The lesions resulting from natalizumab-associated PML also appear to be more often monofocal instead of multifocal, and they most commonly appear in the frontal lobe. Immune reconstitution inflammatory syndrome (IRIS) has also been reported among patients who develop natalizumab-associated PML; the defining feature of IRIS is a “paradoxical worsening of clinical or radiographic finding with recovery of the immune system.”3,4 These findings may extend to new or increased neurologic deficits, an increase in the number or size of lesions, contrast enhancement of the brain lesions, and brain edema.
The gold standard for diagnosis of PML is brain biopsy demonstrating the triad of histopathologic findings that is a hallmark for this condition: demyelination, bizarre astrocytes, and enlarged oligodendrocyte nuclei. However, a brain biopsy is not required to diagnose a suspected case of PML. Instead, a diagnosis of PML is widely accepted if there is demonstration of JCV DNA in the cerebrospinal fluid, a compatible clinical presentation, magnetic resonance imaging (MRI) findings consistent with PML, and no alternative diagnosis.3
Historically, PML was widely considered to be almost universally fatal. However, like its clinical presentation, the prognosis of natalizumab-associated PML may differ from that of AIDS-related PML. In 1 case series, for example, 71% of 35 patients with natalizumab-associated PML were alive 6 months after diagnosis.5 In comparison, the average mortality rate for AIDS-related cases of PML is 80% within 9 months of disease onset.3 Importantly, a negative patient prognosis appears to be highly dependent on the presence of widespread disease and longer time to diagnosis after the initial appearance of symptoms.
Acknowledgement
Gary R. Lichtenstein, MD, has received consulting fees from Abbott Corporation, Alaven, Centocor Ortho Biotech, Elan Pharmaceuticals, Ferring, Meda Pharmaceuticals, Millennium Pharmaceuticals, Ono Pharmaceuticals, Pfizer Pharmaceuticals, Procter & Gamble, Prometheus Laboratories, Inc., Salix Pharmaceuticals, Santarus, Schering-Plough Corporation, Shire Pharmaceuticals, UCB Pharmaceuticals, Warner Chilcott, and Wyeth. He has also received research funding from Alaven, Bristol-Myers Squibb, Centocor Ortho Biotech, Ferring Proctor & Gamble, Prometheus Laboratories, Inc., Salix Pharmaceuticals, Shire Pharmaceuticals, UCB Pharmaceuticals, and Warner Chilcott.
References
- 1.Sandborn WJ, Colombel JF, Enns R, et al. Natalizumab induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2005;353:1912–1925. doi: 10.1056/NEJMoa043335. [DOI] [PubMed] [Google Scholar]
- 2.Targan SR, Feagan BG, Fedorak RN, et al. International Efficacy of Natalizumab in Crohn’s Disease Response and Remission (ENCORE) Trial Group. Natalizumab for the treatment of active Crohn’s disease: results of the ENCORE trial. Gastroenterology. 2007;132:1672–1683. doi: 10.1053/j.gastro.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 3.Berger JR. The clinical features of PML. Cleve Clin J Med. 2011;78(suppl 2):S8–S12. doi: 10.3949/ccjm.78.s2.03. [DOI] [PubMed] [Google Scholar]
- 4.Kleinschmidt-DeMasters BK, Miravalle A, Schowinsky J, Corboy J, Vollmer T. Update on PML and PML-IRIS occurring in multiple sclerosis patients treated with natalizumab. J Neuropathol Exp Neurol. 2012;71:604–617. doi: 10.1097/NEN.0b013e31825caf2c. [DOI] [PubMed] [Google Scholar]
- 5.Vermersch P, Kappos L, Gold R, et al. Clinical outcomes of natalizumab associated progressive multifocal leukoencephalopathy. Neurology. 2011;76:1697–1704. doi: 10.1212/WNL.0b013e31821a446b. [DOI] [PubMed] [Google Scholar]
Risk of Progressive Multifocal Leukoencephalopathy in Patients Treated with Natalizumab
While the use of natalizumab has been associated with an increased risk for PML, the underlying pathogenesis of PML in natalizumab-treated patients is not yet completely understood.1 For example, it remains unclear whether PML is an off-target adverse event that occurs more frequently with natalizumab or if PML results directly from the drug’s action against very late activation antigen 4. One possible mechanism suggested that the development of PML in natalizumab-treated patients may be due to “contributions by JCV-infected B cells as well as by impaired immune surveillance by JCV-specific cytotoxic lymphocytes.”2 In an earlier paper, Van Assche and colleagues noted that inhibition of α4 integrins may prevent the transport to the central nervous system of JCV-primed CD4+ helper T cells as well as CD8+ cytotoxic T cells, thus leading to fulminant JCV replication.3 These authors further suggested that natalizumab may weaken the blood-brain barrier by targeting the β1 integrin present on the surface of the endothelial cells that comprise this barrier. A weakened blood-brain barrier could then facilitate the infiltration of JCV into the central nervous system.
Risk Factors for Progressive Multifocal Leukoencephalopathy in Natalizumab-Treated Patients
Three factors have been identified that increase the risk of PML in natalizumab-treated patients: longer duration of natalizumab therapy, especially beyond 2 years; prior treatment with an immunosuppressant; and the presence of anti-JCV antibodies (Table 1).4
Table 1.
Risk Factors for the Development of Progressive Multifocal Leukoencephalopathy (PML) in Patients Treated with Natalizumab
| Risk factor | Impact on PML risk |
|---|---|
| Longer duration of natalizumab treatment | Incidence of PML according to duration of natalizumab exposure:
|
| Prior immunosuppressant treatment | Immunosuppressants associated with PML include mitoxantrone, cyclophosphamide, methotrexate, azathioprine, and mycophenolate. |
| Presence of anti-JCV antibodies | Estimated incidence of PML according to anti-JCV antibody status:
|
CI=confidence interval; JCV=JC virus.
Data from Sørensen PS, et al.6
The effect of natalizumab exposure on PML risk was estimated in a study that evaluated the incidence of 193 confirmed cases of PML among 92,200 natalizumab-treated patients.5,6 PML incidence increased with increasing treatment duration, with the highest frequency occurring among patients who had received more than 2 years of natalizumab therapy (corresponding to 25—36 infusions). Specifically, the incidence of PML was 0.04 cases per 1,000 patients (95% confidence interval [CI], 0.01-0.11) for those with 1—11 months of natalizumab treatment, 0.56 cases per 1,000 patients (95% CI, 0.39-0.78) for those with 13—24 months of natalizumab treatment, and 1.99 cases per 1,000 patients (95% CI, 1.59-2.45) for those with 25—36 months of natalizumab treatment.
Prior treatment with an immunosuppressant also appears to confer a heightened risk of PML in natalizumab-treated patients. Of the 125 cases of PML that had been identified among natalizumab-treated patients as of December 2011, 38% occurred in patients who had received immunosuppressant therapy.6,7 The immunosuppressant agent most commonly reported by these patients was mitoxantrone, which had been used by 57% of natalizumab-treated PML patients; patients also reported use of cyclophosphamide (19%), methotrexate (17%), azathioprine (15%), mycophenolate (9%), and other immunosuppressants (13%). The mean duration of prior immunosuppressant use among these patients was 30.6 months (range, 0.03-204 months), and the mean washout period was 24.7 months (range, 2-93 months). Importantly, data from the TYGRIS global risk management plan showed that 45% of patients who developed PML reported prior use of immunosuppressive agents; in comparison, prior immunosuppressant use was reported by only 20% of the overall population of patients in this database.
Because JCV infection is required for the development of PML, the presence of anti-JCV antibodies is a key factor in the evaluation of PML risk in natalizumab-treated patients. In 2010, Gorelik and colleagues used a 2-step approach to assess this risk: They first used an enzyme-linked immunosorbent assay (ELISA) to develop a statistical threshold for the presence of anti-JCV antibodies in the serum and plasma of natalizumab-treated patients with multiple sclerosis, after which they used this assay to retrospectively detect anti-JCV antibodies in archived serum samples from natalizumab-treated patients who had developed PML.8 These archived samples had been collected 16-180 months prior to PML diagnosis.
Approximately half (53.6%) of the overall population of natalizumab-treated patients was found to be positive for anti-JCV antibodies, but ELISA analysis detected anti-JCV antibodies in all cases of PML (100%; P<.0001). In a separate study of nearly 6,000 patients with multiple sclerosis, the overall prevalence of anti-JCV antibodies was found to be 55% (95% CI, 54-56), which confirms the prevalence reported by Gorelik and colleagues.8,9 Importantly, Gorelik and colleagues also showed that the rate of anti-JCV antibody seroconversion is approximately 1—2% per year.8 Using incidence data, the estimated incidence of PML in patients who are negative for anti-JCV antibodies was found to be 0.11 cases per 1,000 patients (95% CI, 0-0.59), which is at least 20-fold lower than the incidence reported for anti-JCV antibody—positive patients (2.16 cases per 1,000 patients; 95% CI, 1.40-3.18; P<.0001).6,10
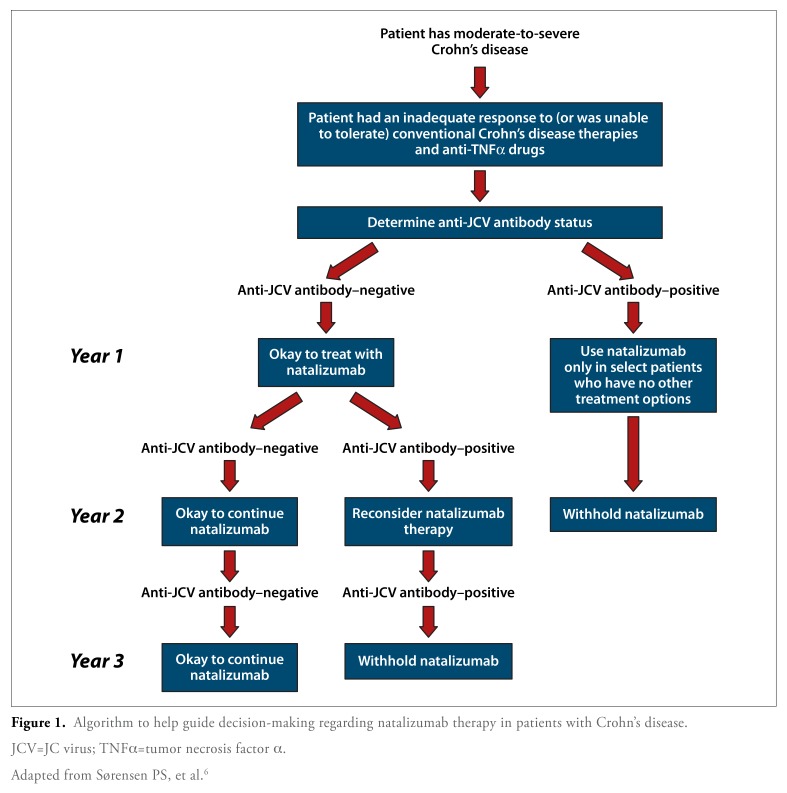
Based on these risk factors, Sørensen and colleagues recently suggested a treatment algorithm for risk stratification of natalizumab-treated patients with multiple sclerosis; this algorithm can be modified for use in patients with Crohn’s disease (Figure 1).6 In this algorithm, patients are first stratified according to their anti-JCV antibody status. Patients who are negative for anti-JCV antibodies may be prescribed natalizumab; due to the 1—2% incidence of seroconversion, these patients should undergo annual anti-JCV antibody testing while on natalizumab therapy. If these patients continue to test negative for anti-JCV antibodies, then natalizumab may be freely continued. However, if a subsequent test result shows evidence of seroconversion to anti-JCV antibody—positive status, then natalizumab treatment should be reconsidered.
Figure 1.
Algorithm to help guide decision-making regarding natalizumab therapy in patients with Crohn’s disease.
JCV=JC virus; TNFα=tumor necrosis factor α.
Adapted from Sørensen PS, et al.6
For patients who initially test positive for the presence of anti-JCV antibodies, short-term natalizumab treatment may be considered regardless of prior immunosuppressant exposure, as the risk of developing PML has been shown to be very low during the first 12 months of treatment. The risk of PML is escalated with the use of prior immunosuppressants in patients who are anti-JCV antibody—positive; prior use of immunosuppressants nearly triples the risk of PML compared to those with no prior immunosuppressant use. If the patient has previously been treated with immunosuppressants, natalizumab therapy should be limited to 1—2 years, as the risk of PML increases significantly beyond that threshold.
Occurrence of Progressive Multifocal Leukoencephalopathy in Clinical Trials
To date, 3 cases of PML have been reported in clinical trials of natalizumab. Two of these cases were reported in the SENTINEL trial (N=1,171), a 2-year, phase III study that evaluated the addition of natalizumab or placebo to interferon β-la therapy in patients with relapsing multiple sclerosis.11 The first case of PML in this trial was a 46-year-old female who received 30 doses of natalizumab (300 mg every 4 weeks) between April 2002 and July 2004 during the randomized portion of this study, followed by an additional 7 doses of natalizumab through January 2005 as part of the open-label extension study.12 This patient first reported new issues with hand-eye coordination and problems with speech in November 2004; her symptoms progressively worsened over the next few months, and she was hospitalized with a significant decline in neurologic status on February 12, 2005. Suspected PML was confirmed when polymerase chain reaction (PCR) testing showed that the patients cerebrospinal fluid was positive for JCV DNA. The patient died on February 24, 2005.
The second case was a 44-year-old male who had received 28 infusions of natalizumab (300 mg every 4 weeks) from October 2002 to December 2004.13 In October 2004, an MRI scan revealed a new nonenhancing lesion in the right frontal lobe. During December 2004, the patient reported difficulties with attention and concentration, and he subsequently developed progressive left hemiparesis, dysarthria, and cognitive impairment. Further MRI scans showed new and extensive abnormalities. PCR testing of the patients cerebrospinal fluid in early February 2005 was positive for JCV DNA, and the patients condition continued to deteriorate despite treatment with cidofovir, corticosteroids, and intravenous immune globulin. Following 1 month of therapy with cytarabine, however, the patient exhibited neurologic improvement. By the end of May 2005, following a second course of cytarabine, the patient was beginning to walk and have meaningful conversations, although he still had disabling ataxia, cognitive impairment, mild neglect, and mild left hemiparesis.
The third case of PML reported in a natalizumab clinical trial occurred in ENACT-1, a 12-week, randomized, double-blind, placebo-controlled trial designed to evaluate the efficacy and safety of natalizumab as induction therapy for moderate-to-severe Crohn’s disease.14 ENACT-1 enrolled a total of 905 patients; those who responded to natalizumab during this study were then eligible for enrollment in ENACT-2, a 48-week maintenance study. The 1 case of PML in this study occurred in a 60-year-old male with Crohn’s disease who had received 3 doses of natalizumab during the ENACT-1 study (monthly infusions of 300 mg), after which he received placebo for 9 months during the ENACT-2 trial.3 After experiencing a disease relapse, the patient resumed open-label natalizumab therapy in February 2003, receiving a total of 5 doses (300 mg every 4 weeks). He had previously received immunosuppressive therapy.
In July 2003, the patient presented to the emergency room with severe confusion and disorientation. A computed tomography (CT) scan of the brain showed a nonenhancing hypodense lesion in the right frontal lobe; MRI scans revealed hyperintense nonenhancing lesions in the right frontal lobe, left frontal lobe, and right temporal lobe. Surgery was performed, and histologic examination of the resected tissue resulted in a diagnosis of grade III astrocytoma. The patient continued to deteriorate postoperatively; despite the initiation of corticosteroid therapy, he died in December 2003. A postmortem analysis of archived serum samples showed that the patient had been positive for JCV DNA in May 2003; by the time he was admitted to the hospital in July 2003, JCV DNA levels had increased by a factor of 12. A high viral load was also observed in a paraffin-embedded tissue sample of the patient’s brain lesion, which had been taken during surgery and archived.
While these 3 patients are the only cases of PML to have occurred in the setting of a clinical trial, additional cases of PML have been reported in the postmarketing setting in both multiple sclerosis patients and Crohn’s disease patients, some of whom were not receiving concomitant immunosuppressive therapy.4 Using this postmarketing surveillance data, the estimated incidence of PML has been calculated for natalizumab-treated patients with various risk factors.4 For anti-JCV antibody—positive patients who receive natalizumab for up to 24 months, the risk of developing PML is relatively low: less than 1 in 1,000 for patients with no prior immunosuppressant use or 2 in 1,000 for patients who do have a history of immunosuppressant use. For anti-JCV antibody—positive patients who are treated with natalizumab for 25-48 months, the risk of developing PML is higher: 4 in 1,000 for patients with no prior immunosuppressant use or 11 in 1,000 for patients with prior immunosuppressant use.
Discussing Natalizumab Use with Patients
When considering whether to initiate natalizumab therapy for a patient with Crohn’s disease, the clinician should first consider the FDA-approved indication for this drug, which states that natalizumab should only be used in patients who had an inadequate response or were unable to tolerate conventional Crohn’s disease therapies (including anti-TNFα agents).4 If the clinician determines that the patient is an appropriate candidate for natalizumab therapy, then the patient’s anti-JCV antibody status should be determined. With this information, the clinician can then have a meaningful discussion with the patient about whether to initiate natalizumab therapy.
For anti-JCV antibody—negative Crohn’s disease patients, the clinician should be cognizant that natalizumab is very effective for inducing and maintaining remission in patients with active moderate-to-severe disease.14 Because of the very low estimated incidence of PML among anti-JCV antibody—negative individuals, many clinicians feel comfortable prescribing natalizumab in this population. When considering natalizumab therapy for Crohn’s disease patients who are anti-JCV antibody—positive, the benefit of the drug remains unchanged, but the risk of PML is greater. In this case, the clinician should have a meaningful discussion with the patient about both the risks and possible benefits of natalizumab therapy, as well as other treatment options that could be considered.
Acknowledgement
Gary R. Lichtenstein, MD, has received consulting fees from Abbott Corporation, Alaven, Centocor Ortho Biotech, Elan Pharmaceuticals, Ferring, Meda Pharmaceuticals, Millennium Pharmaceuticals, Ono Pharmaceuticals, Pfizer Pharmaceuticals, Procter & Gamble, Prometheus Laboratories, Inc., Salix Pharmaceuticals, Santarus, Schering-Plough Corporation, Shire Pharmaceuticals, UCB Pharmaceuticals, Warner Chilcott, and Wyeth. He has also received research funding from Alaven, Bristol-Myers Squibb, Centocor Ortho Biotech, Ferring Proctor & Gamble, Prometheus Laboratories, Inc., Salix Pharmaceuticals, Shire Pharmaceuticals, UCB Pharmaceuticals, and Warner Chilcott.
References
- 1.Yaldizli O, Putzki N. Natalizumab in the treatment of multiple sclerosis. Ther Adv Neurol Disord. 2009;2:115–128. doi: 10.1177/1756285608101861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Houff S, Berger JR. Reply to “‘Thinking without thinking’ about natalizumab and PML.”. J Neurol Set. 2008;264:198–199. doi: 10.1016/j.jns.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 3.Van Assche G, Van Ranst M, Sciot R, et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N Engl J Med. 2005;353:362–368. doi: 10.1056/NEJMoa051586. [DOI] [PubMed] [Google Scholar]
- 4. Tysabri (natalizumab) [prescribing information]. Cambridge, Mass: Biogen Idec Inc.; 2012.
- 5.Sandrock A, Hotermans C, Richman S. Risk stratification for progressive multifocal leukoencephalopathy (PML) in MS patients: role of prior immunosuppressant use, natalizumab treatment duration, and anti-JCF antibody status. Neurology. 2011;76(suppl 4):A248. [Google Scholar]
- 6.Sørensen PS, Bertolotto A, Edan G, et al. Risk stratification for progressive multifocal leukoencephalopathy in patients treated with natalizumab. Mult Scler. 2012;18:143–152. doi: 10.1177/1352458511435105. [DOI] [PubMed] [Google Scholar]
- 7.Kappos L, Foley J, Gold R, et al. Overview of survival outcome and functional status in postmarketing cases of natalizumab-associated progressive multifocal leukoencephalopathy. Mult Scler J. 2011;17:S131. [Google Scholar]
- 8.Gorelik L, Lerner M, Bixler S, et al. Anti-JC virus antibodies: implications for PML risk stratification. Ann Neurol. 2010;68:295–303. doi: 10.1002/ana.22128. [DOI] [PubMed] [Google Scholar]
- 9.Subramanyam M, Plavina T, Lee S, et al. Anti-JCV antibodies are consistently detected prior to and after PML diagnosis in natalizumab-treated MS patients. Neurology. 2011;76:A636–A637. [Google Scholar]
- 10.Bloomgren G, Richman S, Hotermans C, et al. Contribution of natalizumab treatment duration, prior immunosuppressant use, and anti-JC virus antibody status to the risk of progressive multifocal leukoencephalopathy in natalizumab-treated multiple sclerosis patients. Mult Scler J. 2011;17(suppl 10):S451. [Google Scholar]
- 11.Rudick RA, Stuart WH, Calabresi PA, et al. Natalizumab plus interferon beta-la for relapsing multiple sclerosis. N Engl J Med. 2006;354:911–923. doi: 10.1056/NEJMoa044396. [DOI] [PubMed] [Google Scholar]
- 12.Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leuko encephalopathy complicating treatment with natalizumab and interferon beta-la for multiple sclerosis. N Engl J Med. 2005;353:369–374. doi: 10.1056/NEJMoa051782. [DOI] [PubMed] [Google Scholar]
- 13.Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353:375–381. doi: 10.1056/NEJMoa051847. [DOI] [PubMed] [Google Scholar]
- 14.Sandborn WJ, Colombel JF, Enns R, et al. Natalizumab induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2005;353:1912–1925. doi: 10.1056/NEJMoa043335. [DOI] [PubMed] [Google Scholar]
JC Virus Infection and the JC Virus Antibody Assay
Named after John Cunningham, the patient from whom the virus was first isolated in 1971, JCV is a nonenveloped DNA genome virus in the polyomavirus family.1 Found ubiquitously worldwide, this virus is the proven etiologic agent for PML.2 Two genetic forms of JCV have been identified: The archetypal form is found in the kidney, urine, and sewage, while the prototypical form of JCV is associated with PML.3,4 The primary differences between these 2 forms of the virus center on a particular region of the genome responsible for viral transcriptional regulation and DNA replication.2 Because these genetic differences occur only in noncoding regions of the genome, both forms of JCV are recognized by antigenic assays.
A number of studies have evaluated the seroprevalence of JCV, with estimates in the range of 35—39%—and even up to 72%—in healthy adults; however, some of these studies acknowledged that their assays had marked cross-reactivity for other viruses in the polyomavirus family.5-7 Notably, a 2008 study by Verbeeck and colleagues prospectively collected urine, serum, plasma, and buffy coat samples from 331 individuals: 125 patients with Crohn’s disease, 100 patients with other gastrointestinal diseases, and 106 healthy volunteers.8 All of the Crohn’s disease patients had been treated with at least 1 immunomodulator for at least 3 months. This study assayed both the seroprevalence of JCV (using an ELISA) and JCV DNA viral load (using quantitative real-time PCR).
This study reported an overall JCV seroprevalence rate of 65%. While JCV seropositivity was significantly higher among Crohn’s disease patients compared to healthy volunteers (76% vs 51.9%; P<.001), seropositivity in Crohn’s disease patients was not significantly increased compared to patients with other gastrointestinal diseases (64.6%; P=.06). When samples were tested by real-time PCR, the proportion of subjects who tested positive for JCV DNA was significantly higher in the group of patients with other gastrointestinal diseases (44.0%) compared to either Crohn’s disease patients (28.8%; P=.018) or healthy volunteers (25.5%; P=.005). Interestingly, the median viral load in urine was higher among Crohn’s disease patients (7.36 x 106 copies/mL) than in patients with other gastrointestinal diseases (1.86 x 106 copies/mL; P=.011) or healthy volunteers (2.77 x 105 copies/mL; P=.001). A longitudinal assessment of the Crohn’s disease patients showed no major differences in JCV viral load over time, with JCV positivity rates of 28.8% at the first time point, 27.2% at 4-8 Weeks, and 28.8% at 12-16 Weeks.
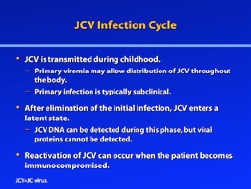
JC Virus Infection Cycle
One model for JCV infection suggests that the virus is commonly transmitted during childhood, at which time it establishes a primary viremia.2 Transmission has been suggested to occur through either the tonsils or the gastrointestinal tract. Distribution of JCV throughout the body may occur during primary viremia, as latent JCV has been identified in a number of tissues, including the kidney, tonsils, and peripheral blood leukocytes.4,9-11 Additionally, several studies have reported detection of JCV in normal brain tissue from patients who do not have PML.12-17 Research suggests that the primary infection is typically subclinical, but it remains unknown whether this infection involves the archetypal and/or prototypical form of the virus. After elimination of the initial infection by the immune system, JCV is thought to enter a latent state in the body. Studies have shown that JCV DNA can be detected during latency using highly sensitive PCR analysis, but viral proteins cannot be detected, suggesting that the virus is truly in a transcriptionally inactive latent phase.
As was recently noted by White and colleagues, “no clear, generally accepted model for JCV reactivation has emerged.”2 Currently, there are 2 primary hypotheses: The first focuses on the immune cells, suggesting that the latent virus is harbored within the B lymphocytes of the bone marrow, which allows the virus to circulate the body and enter the brain. The second hypothesis suggests that the brain is the site for latent JCV and that only during immunosuppression does the virus begin to replicate. Regardless of which theory (if either) is correct, early transcriptional regulatory events are probably critical in the reactivation of JCV within the glial cells; these events thus represent a potential target for future therapies.18
In addition, Berger and colleagues argue that multiple biological barriers are likely in place to prevent JCV reactivation and PML development, given the rarity of PML despite the high frequency of JCV infection.19 Under conditions of immunosuppression, however—as occurs when patients with Crohn’s disease or multiple sclerosis are receiving immunosuppressants—JCV reactivation may occur through an as-yet-unidentified mechanism. In this immunosuppressed state, the virus is allowed to infect the glial cells and replicate, thus resulting in PML.
Anti-JC Virus Antibody Assay
PML first came to clinical prominence during the early years of the AIDS era when effective antiretroviral therapy was not yet available. As this condition became increasingly recognized, PML was also identified as a rare cause of morbidity and mortality in patients who were receiving multidrug immunosuppression in the setting of cancer chemotherapy or organ transplantation. However, because the incidence of PML in these patients appeared low—and because the alternative was often lack of treatment and death—clinicians and patients accepted the small risk of PML as the price for using these life-saving immunosuppression regimens.
As we are now entering an era in which PML occurs more frequently (although still rarely) among patients receiving biologic therapies, the risk of PML has become less tolerable, in part because these patients are less often critically ill and/or may have other treatment options. Combined with evidence showing that JCV infection is required for the development of PML, concern about PML provided the rationale for the development of an assay that could determine whether a patient had prior JCV exposure.
Unlike other chronic viral infections, such as hepatitis, the JCV viral load does not remain sustained over time. Thus, use of molecular testing to measure JCV DNA levels is likely to result in false-negative results. However, intermittent viral shedding is common in patients infected with JCV; this repeated exposure to the virus results in immunogenicity. Measuring seropositivity through the detection of anti-JCV antibodies therefore represents a better way to assess for infection, as antibodies have a longer half-life and are more likely to be detectable in infected patients. One caveat is that seroconversion has been reported to occur at a rate of 1—2% annually.20
The anti-JCV antibody test determines whether a patient has previously been exposed to JCV by detecting whether anti-JCV antibodies are present in the blood. This test is designed as a 2-step assay: The first step is comprised of an ELISA, and the second step consists of a supplemental confirmation test.20,21 For the ELISA, JCV virus-like particles (VLPs) are coated on 96-well microliter plates, after which the patient’s serum or plasma sample is added. An incubation step allows any anti-JCV antibodies present in the sample to recognize and bind to the JCV VLPs coated on the plate. After several washes to remove nonspecific binding, bound anti-JCV antibodies are detected using a secondary antibody that is conjugated to a colorimetric enzymatic reaction; the intensity of this reaction can be measured using a spectrophotometer.
The measured optical density readout of the sample, which is recorded as a normalized value, is directly proportional to the amount of anti-JCV antibodies present. Samples with a normalized value greater than a specified upper threshold are reported as positive, while samples with a normalized value less than a specified lower threshold are reported as negative. Samples with values between the upper and lower thresholds are reported as indeterminate; these samples require further testing using the supplemental confirmation test.
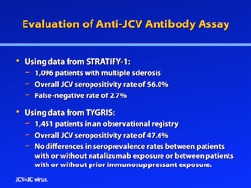
To evaluate this assay, Gorelik and colleagues performed anti-JCV antibody testing in a group of patients with multiple sclerosis.20 Plasma and serum samples were obtained from 831 patients enrolled in the STRATA study, which was designed to evaluate the safety of natalizumab redosing and treatment. Of these patients, 204 were defined as having positive reference sera; they also had detectable levels of JCV DNA in their urine and therefore were definitely infected with JCV. When the ELISA was used to test all patient samples, the median normalized value of the spectrophotometric readout was significantly higher for patients whose urine samples were positive for JCV DNA than for patients whose urine samples were negative (optical density at 450 nm: 0.895 vs 0.131; P<.0001). No patient with a urine sample that was positive for JCV DNA showed an ELISA value below 0.10. In contrast, multiple patients whose urine samples were negative for JCV DNA exhibited ELISA values similar to those observed in patients with JCV DNA-positive urine samples. Among the 204 patients whose urine was positive for JCV DNA, 5 patients were identified who had ELISA values between the upper and lower threshold values and who did not have a positive result on the confirmation assay; thus, the false-negative rate for this test is 2.5%.
More recently, Bozic and colleagues further evaluated this assay using patient samples from both the TYGRIS global risk management plan and STRATIFY-1, an ongoing, longitudinal, observational study of multiple sclerosis patients who are being treated with natalizumab or are considering treatment with natalizumab.22 Among 1,096 patients from STRATIFY-1, the overall JCV seropositivity rate was 56.0%. The false-negative rate was 2.7%, similar to that reported by Gorelik and colleagues.20 Among 1,451 patients from TYGRIS, the overall JCV seropositivity rate was 47.6%. The study by Bozic and colleagues further noted certain trends in seroprevalence, such as a significant increase in seropositivity with increasing age and a lower prevalence of anti-JCV antibodies in females versus males.22 No differences in seroprevalence were observed between patients with or without natalizumab exposure (P=.9709) or between those with or without prior immunosuppressant exposure (P=.6632).
Acknowledgement
William J. Sandborn, MD, has received consulting fees from Abbott Laboratories, Amgen, Elan Pharmaceuticals, Genentech, Janssen, Millennium Pharmaceuticals, Pfizer, and UCB Pharmaceuticals. He has also received fees for non-CME/CE services from Abbott Laboratories, and he has received funds for contracted research from Amgen, Genentech, Janssen, and Pfizer.
References
- 1.Padgett BL, Walker DL, ZuRhein GM, Eckroade RJ, Dessel BH. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971;1:1257–1260. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- 2.White MK, Khalili K. Pathogenesis of progressive multifocal leukoencephalopathy—revisited. J Infect Dis. 2011;203:578–586. doi: 10.1093/infdis/jiq097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frisque RJ, Bream GL, Cannella MT. Human polyomavirus JC virus genome. J Virol. 1984;51:458–469. doi: 10.1128/jvi.51.2.458-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yogo Y, Kitamura T, Sugimoto C, et al. Isolation of a possible archetypal JC virus DNA sequence from nonimmunocompromised individuals. J Virol. 1990;64:3139–3143. doi: 10.1128/jvi.64.6.3139-3143.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kean JM, Rao S, Wang M, Garcea RL. Seroepidemiology of human polyomaviruses. PLoS Pathog. 2009;5:el000363. doi: 10.1371/journal.ppat.1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knowles WA, Pipkin P, Andrews N, et al. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol. 2003;71:115–123. doi: 10.1002/jmv.10450. [DOI] [PubMed] [Google Scholar]
- 7.Stolt A, Sasnauskas K, Koskela P, Lehtinen M, Dillner J. Seroepidemiology of the human polyomaviruses. J Gen Virol. 2003;84(pt 6):1499–1504. doi: 10.1099/vir.0.18842-0. [DOI] [PubMed] [Google Scholar]
- 8.Verbeeck J, Van Assche G, Ryding J, et al. JC viral loads in patients with Crohn’s disease treated with immunosuppression: can we screen for elevated risk of progressive multifocal leukoencephalopathy? Gut. 2008;57:1393–1397. doi: 10.1136/gut.2007.145698. [DOI] [PubMed] [Google Scholar]
- 9.Monaco MC, Jensen PN, Hou J, Durham LC, Major EO. Detection of JC virus DNA in human tonsil tissue: evidence for site of initial viral infection. J Virol. 1998;72:9918–9923. doi: 10.1128/jvi.72.12.9918-9923.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kato A, Kitamura T, Takasaka T, et al. Detection of the archetypal regulatory region of JC virus from the tonsil tissue of patients with tonsillitis and tonsilar hypertrophy. J Neurovirol. 2004;10:244–249. doi: 10.1080/13550280490468663. [DOI] [PubMed] [Google Scholar]
- 11.Dörries K, Vogel E, Günther S, Czub S. Infection of human polyomaviruses JC and BK in peripheral blood leukocytes from immunocompetent individuals. Virology. 1994;198:59–70. doi: 10.1006/viro.1994.1008. [DOI] [PubMed] [Google Scholar]
- 12.White FA, 3rd, Ishaq M, Stoner GL, Frisque RJ. JC virus DNA is present in many human brain samples from patients without progressive multifocal leukoencephalopathy. J Virol. 1992;66:5726–5734. doi: 10.1128/jvi.66.10.5726-5734.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisner C, Dörries K. Evidence of human polyomavirus BK and JC infection in normal brain tissue. Virology. 1992;191:72–80. doi: 10.1016/0042-6822(92)90167-n. [DOI] [PubMed] [Google Scholar]
- 14.Greenlee JE, Clawson SA, Carney HC, O’Neill FJ. Detection of JC virus early region sequences of brains of individuals without progressive multifocal leukoencephalopathy. Ann Neurol. 2005;28(suppl 9):S61. [Google Scholar]
- 15.Mori M, Kurata H, Tajima M, Shimada H. JC virus detection by in situ hybridization in brain tissue from elderly patients. Ann Neurol. 1991;29:428–432. doi: 10.1002/ana.410290414. [DOI] [PubMed] [Google Scholar]
- 16.Mori M, Aoki N, Shimada H, Tajima M, Kato K. Detection of JC virus in the brains of aged patients without progressive multifocal leukoencephalopathy by the polymerase chain reaction and Southern hybridization analysis. Neurosci Lett. 1992;141:151–155. doi: 10.1016/0304-3940(92)90883-9. [DOI] [PubMed] [Google Scholar]
- 17.Perez-Liz G, Del Valle L, Gentilella A, Croul S, Khalili K. Detection of JC vims DNA fragments but not proteins in normal brain tissue. Ann Neurol. 2008;64:379–387. doi: 10.1002/ana.21443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tavazzi E, White MK, Khalili K. Progressive multifocal leukoencephalopathy: clinical and molecular aspects. Rev Med Virol. 2012;22:18–32. doi: 10.1002/rmv.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berger JR, Houff SA, Major EO. Monoclonal antibodies and progressive multifocal leukoencephalopathy. MAbs. 2009;1:583–589. doi: 10.4161/mabs.1.6.9884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorelik L, Lerner M, Bixler S, et al. Anti-JC virus antibodies: implications for PML risk stratification. Ann Neurol. 2010;68:295–303. doi: 10.1002/ana.22128. [DOI] [PubMed] [Google Scholar]
- 21. STRATIFY JCV Antibody ELISA [product insert]. Cypress, Calif: Focus Diagnostics. 2011.
- 22.Bozic C, Richman S, Plavina T, et al. Anti-John Cunnigham virus antibody prevalence in multiple sclerosis patients: baseline results of STRATIFY-1. Ann Neurol. 2011;70:742–750. doi: 10.1002/ana.22606. [DOI] [PubMed] [Google Scholar]
Putting JC Virus Antibody Testing Into Clinical Practice
The approval of natalizumab with an indication for Crohn’s disease coupled with the antiJCV antibody assay’s ability to detect prior JCV exposure prompted a major change in the treatment of Crohn’s disease patients who had failed antiTNFα therapy. Before the antiJCV antibody assay became available, clinicians had difficulty convincing patients with Crohn’s disease to use natalizumab, as patients were understandably concerned about the uncertain risk of developing PML. With the availability of the antiJCV antibody assay, however, clinicians can now identify patients who are negative for antiJCV antibodies and reassure them that they have an exceedingly low estimated risk of developing PML. Given natalizumab’s efficacy for inducing and maintaining disease remission, this additional information often swings the balance in favor of natalizumab therapy. Interestingly, before PML was identified as a rare adverse event associated with natalizumab, this drug was actually considered to be a safer alternative to antiTNFα therapy.
Prior to the development of the antiJCV antibody assay, the primary reason why natalizumab was not widely prescribed to treat Crohn’s disease was the risk of PML associated with this drug. However, subsequent studies have established that the risk of PML is almost zero in patients who are negative for antiJCV antibodies. Thus, Crohn’s disease patients who are negative for antiJCV antibodies are much more likely to elect to begin natalizumab therapy once they understand the implications of their negative test result.
For Crohn’s disease patients who test positive for antiJCV antibodies, 2 trends are observed. If patients had been receiving natalizumab therapy, they generally elect to discontinue treatment once their antiJCV antibody test result comes back positive. Similarly, patients who are considering whether to initiate natalizumab therapy usually elect to pursue alternative treatment options if they learn they are positive for antiJCV antibodies. However, a few antiJCV antibody-positive patients elect to accept the risk of PML; in general, these patients do not have a history of extensive immunosuppression therapy, and they initiate natalizumab with the knowledge that it will be discontinued after 1 year.
Deciding When to Initiate Natalizumab Therapy
The efficacy of natalizumab therapy for the management of Crohn’s disease has now been demonstrated in multiple published clinical reports. In addition to the original clinical trials that established natalizumab as an effective therapy for Crohn’s disease, a recent systematic review and meta-analysis reported that natalizumab was superior to placebo for inducing remission in patients with luminal Crohn’s disease.1-3 In 5 randomized controlled clinical trials that included a total of 1,771 individuals, 65.4% of natalizumab-treated patients and 77.3% of placebo-treated patients failed to achieve remission. Thus, natalizumab treatment was found to reduce the relative risk of not achieving remission (0.88; 95% CI, 0.83-0.94; P=.72).
More recently, Kane and colleagues reported their experience using natalizumab in Crohn’s disease patients who were treated at Mayo Clinic Rochester between April 2008 and September 2010.4 A total of 36 consecutive patients who received natalizumab therapy for active Crohn’s disease were invited to participate in this study; 30 patients agreed to participate and were followed prospectively. These patients had been previously treated with either 1 (23.3%) or 2 (76.7%) anti-TNFα therapies. Nearly half (46%) of patients who received natalizumab achieved a clinical response, as assessed at each monthly infusion using the Harvey-Bradshaw Index. Of the 7 patients who were receiving corticosteroid therapy, 4 were able to taper off corticosteroids. At least 1 adverse event was experienced by each of the 30 patients in this study; however, none of the 13 patients (43%) who discontinued natalizumab therapy did so due to an adverse event. No cases of PML occurred in this study. Overall, the authors of this report concluded that, when natalizumab is used for the treatment of active Crohn’s disease in routine clinical practice, it yields efficacy similar to that reported in clinical trials, and it has a manageable safety profile.
Before initiating natalizumab therapy in a patient with Crohn’s disease, clinicians should first confirm that the patient is losing (or has lost) response to both conventional therapies and anti-TNFα agents. Also, clinicians should consider whether alternatives to natalizumab might be preferable. Many patients who have lost response to biologic therapy have stricturing disease, which makes them excellent candidates for surgical resection. In these cases, surgery is an important alternative to natalizumab therapy, and this option should be considered and discussed with the patient.
For patients with active disease who have lost response to a first or second anti-TNFα agent despite adequate trough levels of the agent, mounting evidence suggests that continuing biologic therapy with yet another anti-TNFα agent is unlikely to elicit a clinical response. Thus, these patients are candidates for natalizumab treatment, and they should undergo anti-JCV antibody testing. In contrast, if a patient with active Crohn’s disease has lost response to an anti-TNFα agent because of the development of antibodies to that agent, the optimal strategy is to switch to an alternative anti-TNFα drug. Currently, 3 anti-TNFα agents have been approved by the FDA for treatment of Crohn’s disease—infliximab, adalimumab, and certolizumab pegol—so clinicians have several choices in this drug category. In addition, a number of new anti-TNFα agents are currently under investigation in multiple clinical trials, suggesting that new options may become available in the future.
Management of Crohn’s Disease Patients According to Anti-JC Virus Antibody Status
Once the patient and the clinician have agreed that natalizumab represents the best option for continued treatment of the patient’s disease, then the previously described ELISA should be used to determine the patient’s anti-JCV antibody status. For anti-JCV antibody-negative patients, natalizumab therapy can be recommended with little-to-no fear for the development of PML, and long-term administration of natalizumab is an option. Being able to safely continue therapy for many years is an especially important consideration for patients with Crohn’s disease, as this lifelong disease requires ongoing therapy in order for patients to maintain remission.
Under its current indication, natalizumab should not be used in combination with immunosuppressants (including 6-mercaptopurine, azathioprine, cyclosporine, or methotrexate) or anti-TNFα agents.5 However, aminosalicylate can be continued during natalizumab therapy. If the patient is receiving chronic treatment with oral corticosteroids at the time he or she initiates natalizumab, then steroid tapering should begin as soon as the patient establishes a therapeutic benefit with natalizumab. Natalizumab should be discontinued if the patient is unable to complete tapering of oral corticosteroids within 6 months of initiating natalizumab. However, in clinical practice, this guidance may not be an ultimatum; many patients are able to markedly reduce their steroid requirements while on natalizumab (similar to therapy with anti-TNFα biologies), and for these patients, the risk/benefit of continuing natalizumab should be taken into consideration. On the other hand, discontinuation of natalizumab should be considered if patients require use of “significant” steroid doses or courses of steroids to control their Crohn’s disease in excess of 3 months per calendar year.
In anti-JCV antibody—negative Crohn’s disease patients who begin natalizumab therapy, anti-JCV antibody status should be reassessed every 6 months.5 This strategy will allow the clinician to detect a new JCV infection promptly and also helps to mitigate the small risk that a false-negative result may have occurred during previous testing. Because the risk of developing PML becomes relatively greater after the first year of natalizumab exposure, reassessing the patient’s anti-JCV antibody status every 6 months will allow sufficient time to make decisions about withdrawing natalizumab and to identify an alternative treatment strategy, if necessary.
For anti-JCV antibody-positive patients, the risk of PML often leads patients to forgo natalizumab therapy; unfortunately, lack of effective alternatives often means that these patients will continue to experience active Crohn’s disease or steroid dependence. Given that natalizumab is only indicated for patients who have either had an inadequate response to or were unable to tolerate conventional therapies and anti-TNFα agents, patients who are considering natalizumab therapy have often exhausted other medical therapies.5 In this situation, the patient’s history should be carefully reviewed to make certain that he or she has been optimally treated with conventional therapies, including immunomodulators; for example, methotrexate may be considered as an alternative strategy for a patient who did not respond to azathioprine. Additionally, clinicians should learn why anti-TNFα therapy was discontinued and determine whether the patient might benefit from another course of anti-TNFα therapy, either with the same medication or a different agent. Finally, patients who are not suitable candidates for natalizumab therapy could consider enrollment in an appropriate clinical trial, which might allow them access to a new treatment option; indeed, patients who are not candidates for natalizumab represent the largest population of patients currently entering Crohn’s disease clinical trials.
In select cases, an anti-JCV antibody—positive patient with Crohn’s disease may elect to initiate natalizumab therapy. Typically, these patients are faced with very difficult circumstances, such as a complete lack of any other treatment options and/or extensive disease that prevents them from being surgical candidates. In these cases, natalizumab may be chosen as a temporary treatment option, with the understanding that its use will be limited to 1 year. After this period, a novel therapeutic alternative may have emerged— either a newly approved drug or an agent under investigation—or the limited course of natalizumab may have improved the patient’s condition enough to make surgery a viable option (if the patient was previously not a candidate for surgery).
Acknowledgement
Stephen B. Hanauer, MD, has received consulting fees and clinical research funding from Elan Pharmaceuticals, Millennium Pharmaceuticals, and Takeda.
References
- 1.Sandborn WJ, Colombel JF, Enns R, et al. Natalizumab induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2005;353:1912–1925. doi: 10.1056/NEJMoa043335. [DOI] [PubMed] [Google Scholar]
- 2.Targan SR, Feagan BG, Fedorak RN, et al. Natalizumab for the treatment of active Crohn’s disease: results of the ENCORE trial. Gastroenterology. 2007;132:1672–1683. doi: 10.1053/j.gastro.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 3.Ford AC, Sandborn WJ, Khan KJ, Hanauer SB, Talley NJ, Moayyedi P. Efficacy of biological therapies in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:644–659. doi: 10.1038/ajg.2011.73. [DOI] [PubMed] [Google Scholar]
- 4.Kane SV, Horst S, Sandborn WJ, et al. Natalizumab for moderate to severe Crohn’s disease in clinical practice: the Mayo Clinic Rochester experience. Inflamm Bowel Dis. doi: 10.1002/ibd.22943. 2012 Mar 14. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 5.Cambridge, Mass:: Biogen Idec Inc.; 2012. Tysabri (natalizumab) [prescribing information]. [Google Scholar]
Question-and-Answer Forum
G&H If a patient with active Crohn’s disease has failed 2 anti-TNFα agents, would you consider treatment with a third anti-TNFα agent or would you consider starting natalizumab therapy?
Stephen B. Hanauer, MD This decision is primarily dependent on why the patient failed prior anti-TNFα therapy. In cases of failure due to the development of immunogenicity, switching to an alternative anti-TNFα agent often results in a favorable response. In contrast, if patients failed anti-TNFα therapy even in the setting of adequate serum trough levels of the drug, then they are unlikely to benefit from switching agents. These latter patients may be candidates for natalizumab treatment.
Gary R. Lichtenstein, MD The GAIN study evaluated induction therapy with adalimumab in patients with Crohn’s disease who had previously been treated with infliximab.1 In these patients, adalimumab induction therapy resulted in a remission rate of 21% at Week 4, compared to a remission rate of only 7% among patients who received placebo (P<.001). These results provide a rationale for attempting treatment with more than 1 anti-TNFα agent in the clinical setting.
William J. Sandborn, MD Crohn’s disease patients who are primary nonresponders to anti-TNFα therapy may be candidates for natalizumab. A primary nonresponder would be defined as a patient who received the initial induction regimen but failed to achieve a clinical response. Although this group has not been extensively evaluated, there is little evidence to suggest that these patients will derive benefit from an alternative anti-TNFα therapy.
G&H What criteria should be used to determine whether a Crohn’s disease patient has responded to natalizumab?
GRL The basic criteria used to determine response to conventional Crohn’s disease therapies can also be useful in the setting of natalizumab treatment. For example, the ability of the patient to taper off and/or remain off of corticosteroid therapy is an important indicator of response. Other indicators that suggest an improvement in inflammation are reduction in CRP level and lack of hyperenhancement on CT enterography.
SBH The first goal of therapy is symptomatic relief and corticosteroid withdrawal, with a primary objective being corticosteroid-free clinical remission. An added benefit of therapy, over and above symptom control, is mucosal healing as assessed by endoscopy.
WJS Currently, natalizumab can only be administered to patients in the context of the TOUCH program. As part of TOUCH, patients are required to be reassessed at regular intervals (every 3 months at the start of natalizumab therapy and every 6 months thereafter) to determine if continued use of natalizumab is warranted. In my experience, natalizumab is a somewhat slower-acting drug compared to other Crohn’s disease therapies. The time to mucosal healing was not rigorously assessed in clinical trials of natalizumab; however, these studies showed that patients generally achieved their maximum benefit after approximately 3 months or slightly thereafter. I generally will continue natalizumab as long as there is evidence that the patient is experiencing some subjective improvement in symptoms—such as a reduction in stool frequency or abdominal pain—at the 3-month follow-up visit.
G&H Under what circumstances would you start or maintain natalizumab therapy in a Crohn’s disease patient who is anti-JCV antibody-positive?
WJS In general, I would not start or continue natalizumab in a patient with Crohn’s disease who is positive for anti-JCV antibodies because of their risk for developing PML.
GRL Natalizumab may be useful for treating patients with extensive disease who are not currently candidates for surgery. A relatively short course of natalizumab (up to 1 year) may be sufficient to induce enough of a response so that surgery becomes an option for these patients.
SBH An important point to remember is that, in addition to longer duration of natalizumab treatment, prior exposure to immunosuppressive agents is another factor that increases the risk of PML in anti-JCV antibody-positive patients. Because nearly 100% of Crohn’s disease patients who are considering natalizumab therapy will have previously tried immunosuppressive therapy, this group is already at a heightened risk for the development of PML.
G&H How might the development of vedolizumab alter considerations of risk and benefit with natalizumab?
WJS Because natalizumab is not selective for the α4β7 integrin, it acts in both the gut and the brain; specifically, it is thought to prevent lymphocyte trafficking to the central nervous system by targeting the α4β1 integrin, which may be part of the mechanism by which natalizumab is involved in JCV reactivation and subsequent development of PML. In contrast, vedolizumab’s effects appear to be more gut-specific, as it is selective for the α4β7 integrin; this selectivity suggests that vedolizumab may pose little-to-no increased risk of JCV reactivation and PML.2 Vedolizumab is currently under investigation as a possible treatment for Crohn’s disease, but it has not yet been approved. Other agents that target leukocyte migration and adhesion are also in various stages of clinical development as treatments for Crohn’s disease, including AJM300, etrolizumab, PF-00547659, and CCX282-B.2
GRL To date, PML has not been reported in vedolizumabtreated patients. If vedolizumab eventually receives FDA approval for treatment of Crohn’s disease, postmarketing surveillance will be needed to determine if vedolizumab is actually safer than natalizumab in terms of the risk of PML. If it is approved and not found to be associated with PML, then I believe that vedolizumab will be preferentially selected for patients who are anti-JCV antibody-positive. For anti-JCV antibody—negative patients, the selection of vedolizumab over natalizumab (or vice versa) will depend on the availability of further data comparing the 2 agents.
SBH Even if vedolizumab gains approval for the treatment of Crohn’s disease, natalizumab will still play an important role in treating this condition. The development of immunogenicity will always be an issue with biologic therapies, which creates a need for multiple agents targeting the same class of molecules.
In contrast, switching to a different anti-TNFα agent might be beneficial for patients who initially achieved a response with an anti-TNFα agent but then subsequently experienced an attenuation or loss of response (or became intolerant to therapy). However, the likelihood of benefit is lower in these patients than in patients who are naive to anti-TNFα therapy. Thus, clinicians should discuss both the likelihood of benefit and the risks of therapy with these patients, as switching anti-TNFα agents exposes the patient to all the risks associated with the anti-TNFα agent but with a potentially considerable attenuation in benefit.
Acknowledgement
Stephen B. Hanauer, MD, has received consulting fees and clinical research funding from Elan Pharmaceuticals, Millennium Pharmaceuticals, and Takeda. Gary R. Lichtenstein, MD, has received consulting fees from Abbott Corporation, Alaven, Centocor Ortho Biotech, Elan Pharmaceuticals, Ferring, Meda Pharmaceuticals, Millennium Pharmaceuticals, Ono Pharmaceuticals, Pfizer Pharmaceuticals, Procter & Gamble, Prometheus Laboratories, Inc., Salix Pharmaceuticals, Santarus, Schering-Plough Corporation, Shire Pharmaceuticals, UCB Pharmaceuticals, Warner Chilcott, and Wyeth. He has also received research funding from Alaven, Bristol-Myers Squibb, Centocor Ortho Biotech, Ferring Proctor & Gamble, Prometheus Laboratories, Inc., Salix Pharmaceuticals, Shire Pharmaceuticals, UCB Pharmaceuticals, and Warner Chilcott. William J. Sandborn, MD, has received consulting fees from Abbott Laboratories, Amgen, Elan Pharmaceuticals, Genentech, Janssen, Millennium Pharmaceuticals, Pfizer, and UCB Pharmaceuticals. He has also received fees for non-CME/CE services from Abbott Laboratories, and he has received funds for contracted research from Amgen, Genentech, Janssen, and Pfizer.
References
- 1.Sandborn WJ, Rutgeerts P, Enns R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med. 2007;146:829–838. doi: 10.7326/0003-4819-146-12-200706190-00159. [DOI] [PubMed] [Google Scholar]
- 2.Thomas S, Baumgart DC. Targeting leukocyte migration and adhesion in Crohn’s disease and ulcerative colitis. Inflammopharmacology. 2012;20:1–18. doi: 10.1007/s10787-011-0104-6. [DOI] [PubMed] [Google Scholar]
Biographies



Slide Library










For a free electronic download of these slides, please direct your browser to the following web address: http://www.clinicaladvances.com/index.php/our_publications/gastro-hep-issue/gh_november_2012/
Notes
Footnotes
Supported through an educational grant from Elan Pharmaceuticals, Inc.
Sponsored by the Postgraduate Institute for Medicine
Disclosure of Conflicts of Interest:Postgraduate Institute for Medicine (PIM) assesses conflict of interest with its instructors, planners, managers, and other individuals who are in a position to control the content of Continuing Medical Education (CME) activities. All relevant conflicts of interest that are identified are thoroughly vetted by PIM for fair balance, scientific objectivity of studies utilized in this activity, and patient care recommendations. PIM is committed to providing its learners with high-quality CME activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of a commercial interest.
The faculty reported the following financial relationships or relationships to products or devices they or their spouse/life partner have with commercial interests related to the content of this CME activity:
Stephen B. Hanauer, MD, has received consulting fees and clinical research funding from Elan Pharmaceuticals, Millennium Pharmaceuticals, and Takeda.
Gary R. Lichtenstein, MD, has received consulting fees from Abbott Corporation, Alaven, Centocor Ortho Biotech, Elan Pharmaceuticals, Ferring, Meda Pharmaceuticals, Millennium Pharmaceuticals, Ono Pharmaceuticals, Pfizer Pharmaceuticals, Procter & Gamble, Prometheus Laboratories, Inc., Salix Pharmaceuticals, Santarus, Schering-Plough Corporation, Shire Pharmaceuticals, UCB Pharmaceuticals, Warner Chilcott, and Wyeth. He has also received research funding from Alaven, Bristol-Myers Squibb, Centocor Ortho Biotech, Ferring, Proctor & Gamble, Prometheus Laboratories, Inc., Salix Pharmaceuticals, Shire Pharmaceuticals, UCB Pharmaceuticals, and Warner Chilcott.
William J. Sandborn, MD, has received consulting fees from Abbott Laboratories, Amgen, Elan Pharmaceuticals, Genentech, Janssen, Millennium Pharmaceuticals, Pfizer, and UCB Pharmaceuticals. He has also received fees for non-CME/CE services from Abbott Laboratories, and he has received funds for contracted research from Amgen, Genentech, Janssen, and Pfizer.
The planners and managers reported the following financial relationships or relationships to products or devices they or their spouse/life partner have with commercial interests related to the content of this CME activity:
The following PIM planners and managers, Laura Excell, ND, NP, MS, MA, LPC, NCC; Trace Hutchison, PharmD; Samantha Mattiucci, PharmD, CCMEP; Jan Schultz, RN, MSN, CCMEP; and Patricia Staples, MSN, NP-C, CCRN hereby state that they or their spouse/life partner do not have any financial relationships or relationships to products or devices with any commercial interest related to the content of this activity of any amount during the past 12 months.
Kay Downer: No real or apparent conflicts of interest.
Disclosure Of Unlabeled Use:This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the US Food and Drug Administration. Postgraduate Institute for Medicine (PIM), Gastroenterology & Hepatology, and Elan Pharmaceuticals, Inc., do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of PIM, Gastro-Hep Communications, Inc., or Elan Pharmaceuticals, Inc. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer:Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Funding for this Clinical Roundtable Monograph has been provided through an educational grant from Elan Pharmaceuticals, Inc. Support of this monograph does not imply the supporter's agreement with the views expressed herein. Every effort has been made to ensure that drug usage and other information are presented accurately; however, the ultimate responsibility rests with the prescribing physician. Gastro-Hep Communications, Inc., the supporter, and the participants shall not be held responsible for errors or for any consequences arising from the use of information contained herein. Readers are strongly urged to consult any relevant primary literature. No claims or endorsements are made for any drug or compound at present under clinical investigation.
Contributor Information
Gary R. Lichtenstein, Director, Center for Inflammatory Bowel Diseases Professor of Medicine University of Pennsylvania Philadelphia, Pennsylvania.
Stephen B. Hanauer, Professor of Medicine and Clinical Pharmacology Director, Section of Gastroenterology and Nutrition University of Chicago Chicago, Illinois.
William J. Sandborn, Chief, Division of Gastroenterology Director, UCSD IBD Center UC San Diego Health System La Jolla, California.