Abstract
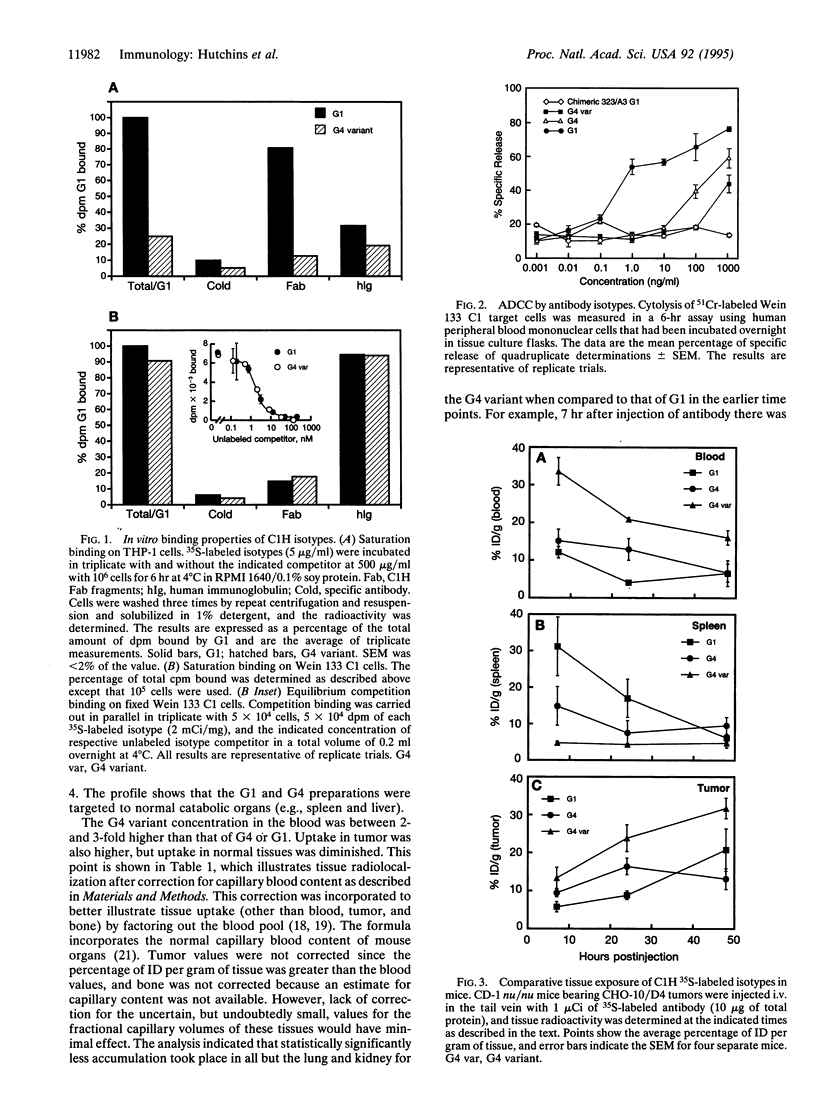
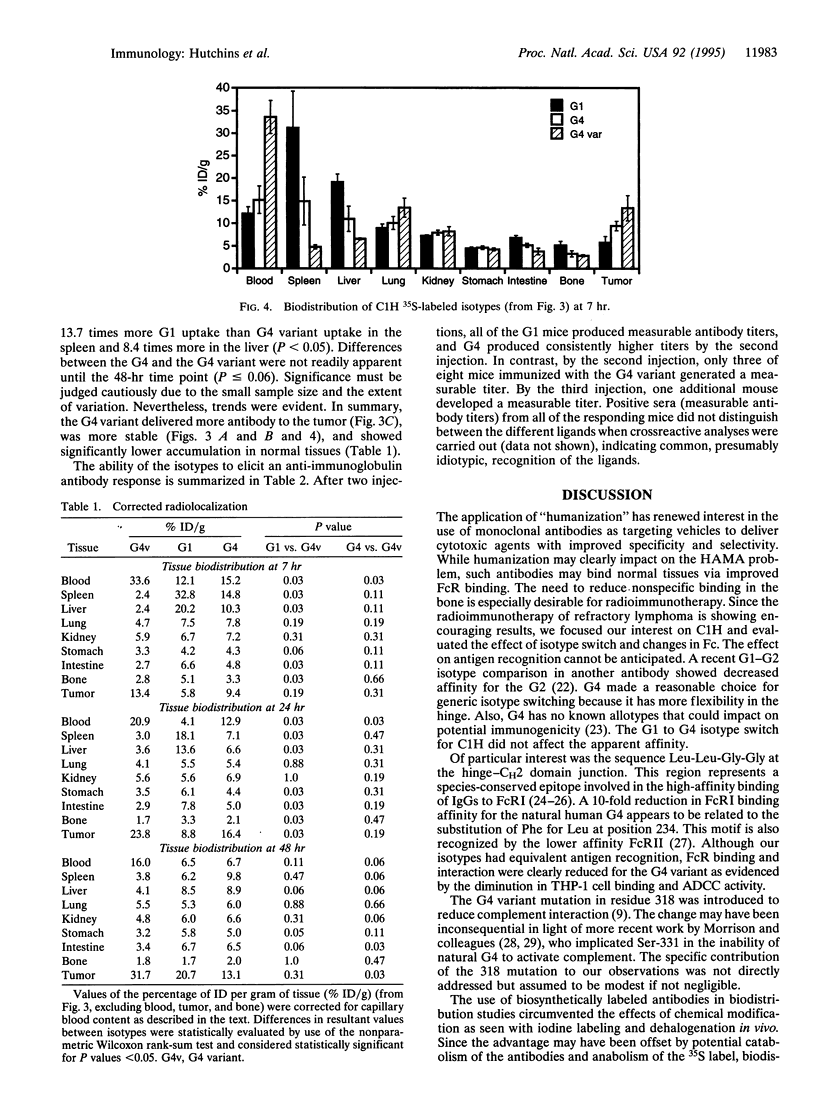
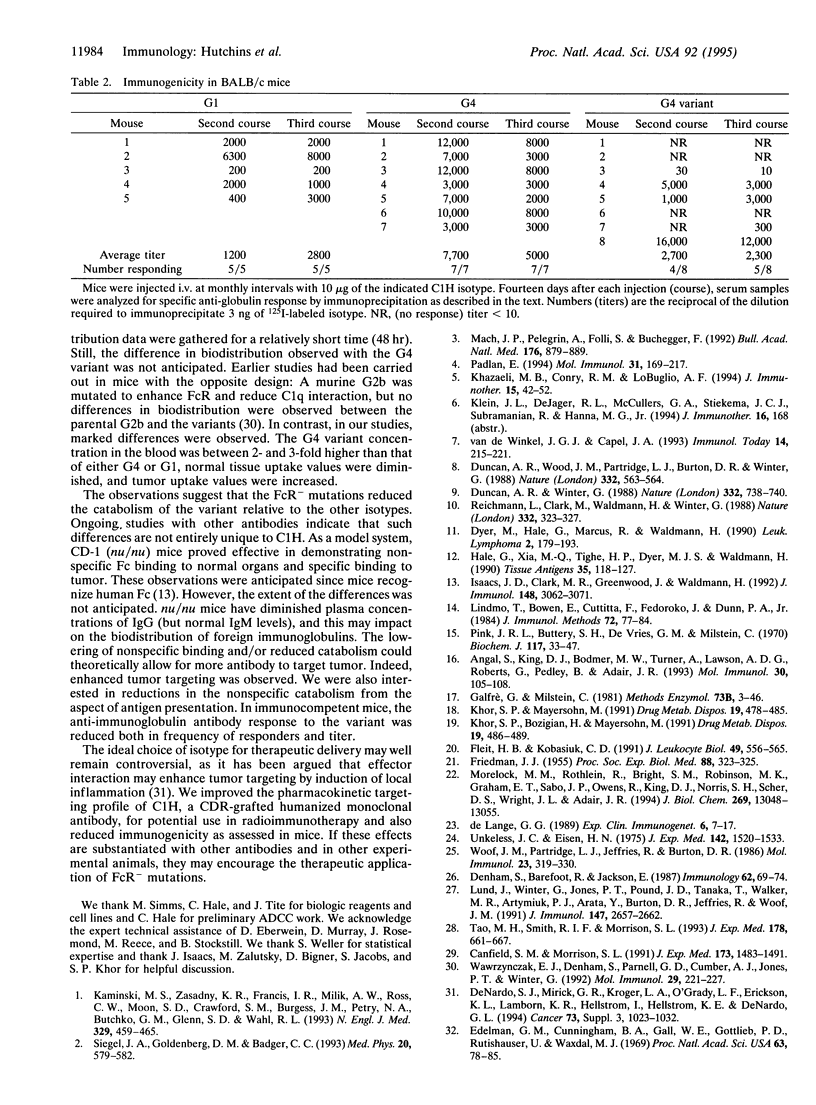
Radiolabeled antibodies have shown promise for the treatment of lymphoma and for solid tumor targeting. Campath-1H is a humanized monoclonal antibody that reacts with the CD52 antigen present on human lymphoid and myeloid cells. Campath-1H is a gamma1 (G1) isotype that induces lymphopenia via an Fc-mediated mechanism(s). Isotype switches were engineered, and the resulting antibodies were expressed in NS0 mouse myeloma cells and biosynthetically radiolabeled with [35S]methionine. The forms included G1, G4, and a G4 variant that contained alanine substitutions at (EU numbering) Leu-235, Gly-237, and Glu-318. All isotypes bound antigen equivalently as assessed by target cell binding in vitro. The G4 variant had a greatly reduced capacity to interact with Fc receptor by virtue of reduced binding to THP-1 human myeloid cells and by a 1000-fold increase in EC50 to intermediate antibody-dependent cellular cytotoxicity. The pharmacokinetics of the isotypes were compared in CD-1 (nu/nu) mice bearing an experimental antigen-expressing tumor. The plasma half-life and tumor uptake were increased for the G4 variant. The G4 variant showed significantly less spleen, liver, and bone uptake but similar uptake in the lung, kidney, and stomach and lower tissue-to-blood ratios. Immunogenicity was assessed after repeated monthly administrations of unlabeled antibody in BALB/c mice. A 50% reduction in the incidence of anti-globulin response was observed for the G4 variant. These properties suggest that antibodies with reduced Fc receptor interaction merit additional study as potential targeting vehicles relative to other isotypes for radioimmunotherapy or situations where diminished normal tissue binding contributes to efficacy.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angal S., King D. J., Bodmer M. W., Turner A., Lawson A. D., Roberts G., Pedley B., Adair J. R. A single amino acid substitution abolishes the heterogeneity of chimeric mouse/human (IgG4) antibody. Mol Immunol. 1993 Jan;30(1):105–108. doi: 10.1016/0161-5890(93)90432-b. [DOI] [PubMed] [Google Scholar]
- Canfield S. M., Morrison S. L. The binding affinity of human IgG for its high affinity Fc receptor is determined by multiple amino acids in the CH2 domain and is modulated by the hinge region. J Exp Med. 1991 Jun 1;173(6):1483–1491. doi: 10.1084/jem.173.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNardo S. J., Mirick G. R., Kroger L. A., O'Grady L. F., Erickson K. L., Yuan A., Lamborn K. R., Hellstrom I., Hellstrom K. E., DeNardo G. L. The biologic window for chimeric L6 radioimmunotherapy. Cancer. 1994 Feb 1;73(3 Suppl):1023–1032. doi: 10.1002/1097-0142(19940201)73:3+<1023::aid-cncr2820731341>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Denham S., Barfoot R., Jackson E. A receptor for monomeric IgG2b on rat macrophages. Immunology. 1987 Sep;62(1):69–74. [PMC free article] [PubMed] [Google Scholar]
- Duncan A. R., Winter G. The binding site for C1q on IgG. Nature. 1988 Apr 21;332(6166):738–740. doi: 10.1038/332738a0. [DOI] [PubMed] [Google Scholar]
- Duncan A. R., Woof J. M., Partridge L. J., Burton D. R., Winter G. Localization of the binding site for the human high-affinity Fc receptor on IgG. Nature. 1988 Apr 7;332(6164):563–564. doi: 10.1038/332563a0. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Cunningham B. A., Gall W. E., Gottlieb P. D., Rutishauser U., Waxdal M. J. The covalent structure of an entire gammaG immunoglobulin molecule. Proc Natl Acad Sci U S A. 1969 May;63(1):78–85. doi: 10.1073/pnas.63.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDMAN J. J. Organ plasma volume of normal unanesthetized mice. Proc Soc Exp Biol Med. 1955 Mar;88(3):323–325. doi: 10.3181/00379727-88-21577. [DOI] [PubMed] [Google Scholar]
- Fleit H. B., Kobasiuk C. D. The human monocyte-like cell line THP-1 expresses Fc gamma RI and Fc gamma RII. J Leukoc Biol. 1991 Jun;49(6):556–565. doi: 10.1002/jlb.49.6.556. [DOI] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Hale G., Xia M. Q., Tighe H. P., Dyer M. J., Waldmann H. The CAMPATH-1 antigen (CDw52). Tissue Antigens. 1990 Mar;35(3):118–127. doi: 10.1111/j.1399-0039.1990.tb01767.x. [DOI] [PubMed] [Google Scholar]
- Isaacs J. D., Clark M. R., Greenwood J., Waldmann H. Therapy with monoclonal antibodies. An in vivo model for the assessment of therapeutic potential. J Immunol. 1992 May 15;148(10):3062–3071. [PubMed] [Google Scholar]
- Kaminski M. S., Zasadny K. R., Francis I. R., Milik A. W., Ross C. W., Moon S. D., Crawford S. M., Burgess J. M., Petry N. A., Butchko G. M. Radioimmunotherapy of B-cell lymphoma with [131I]anti-B1 (anti-CD20) antibody. N Engl J Med. 1993 Aug 12;329(7):459–465. doi: 10.1056/NEJM199308123290703. [DOI] [PubMed] [Google Scholar]
- Khazaeli M. B., Conry R. M., LoBuglio A. F. Human immune response to monoclonal antibodies. J Immunother Emphasis Tumor Immunol. 1994 Jan;15(1):42–52. doi: 10.1097/00002371-199401000-00006. [DOI] [PubMed] [Google Scholar]
- Khor S. P., Bozigian H., Mayersohn M. Potential error in the measurement of tissue to blood distribution coefficients in physiological pharmacokinetic modeling. Residual tissue blood. II. Distribution of phencyclidine in the rat. Drug Metab Dispos. 1991 Mar-Apr;19(2):486–490. [PubMed] [Google Scholar]
- Khor S. P., Mayersohn M. Potential error in the measurement of tissue to blood distribution coefficients in physiological pharmacokinetic modeling. Residual tissue blood. I. Theoretical considerations. Drug Metab Dispos. 1991 Mar-Apr;19(2):478–485. [PubMed] [Google Scholar]
- Lindmo T., Boven E., Cuttitta F., Fedorko J., Bunn P. A., Jr Determination of the immunoreactive fraction of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. J Immunol Methods. 1984 Aug 3;72(1):77–89. doi: 10.1016/0022-1759(84)90435-6. [DOI] [PubMed] [Google Scholar]
- Lund J., Winter G., Jones P. T., Pound J. D., Tanaka T., Walker M. R., Artymiuk P. J., Arata Y., Burton D. R., Jefferis R. Human Fc gamma RI and Fc gamma RII interact with distinct but overlapping sites on human IgG. J Immunol. 1991 Oct 15;147(8):2657–2662. [PubMed] [Google Scholar]
- Mach J. P., Pèlegrin A., Folli S., Buchegger F. Les anticorps monoclonaux radiomarqués en tant que missiles anti-tumeurs, leurs succès diagnostiques, leur potentiel thérapeutique. Bull Acad Natl Med. 1992 Jun;176(6):879–889. [PubMed] [Google Scholar]
- Morelock M. M., Rothlein R., Bright S. M., Robinson M. K., Graham E. T., Sabo J. P., Owens R., King D. J., Norris S. H., Scher D. S. Isotype choice for chimeric antibodies affects binding properties. J Biol Chem. 1994 Apr 29;269(17):13048–13055. [PubMed] [Google Scholar]
- Padlan E. A. Anatomy of the antibody molecule. Mol Immunol. 1994 Feb;31(3):169–217. doi: 10.1016/0161-5890(94)90001-9. [DOI] [PubMed] [Google Scholar]
- Pink J. R., Buttery S. H., De Vries G. M., Milstein C. Human immunoglobulin subclasses. Partial amino acid sequence of the constant region of a gamma 4 chain. Biochem J. 1970 Mar;117(1):33–47. doi: 10.1042/bj1170033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechmann L., Clark M., Waldmann H., Winter G. Reshaping human antibodies for therapy. Nature. 1988 Mar 24;332(6162):323–327. doi: 10.1038/332323a0. [DOI] [PubMed] [Google Scholar]
- Siegel J. A., Goldenberg D. M., Badger C. C. Radioimmunotherapy dose estimation in patients with B-cell lymphoma. Med Phys. 1993 Mar-Apr;20(2 Pt 2):579–582. doi: 10.1118/1.597052. [DOI] [PubMed] [Google Scholar]
- Tao M. H., Smith R. I., Morrison S. L. Structural features of human immunoglobulin G that determine isotype-specific differences in complement activation. J Exp Med. 1993 Aug 1;178(2):661–667. doi: 10.1084/jem.178.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkeless J. C., Eisen H. N. Binding of monomeric immunoglobulins to Fc receptors of mouse macrophages. J Exp Med. 1975 Dec 1;142(6):1520–1533. doi: 10.1084/jem.142.6.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawrzynczak E. J., Denham S., Parnell G. D., Cumber A. J., Jones P. T., Winter G. Recombinant mouse monoclonal antibodies with single amino acid substitutions affecting Clq and high affinity Fc receptor binding have identical serum half-lives in the BALB/c mouse. Mol Immunol. 1992 Feb;29(2):221–227. doi: 10.1016/0161-5890(92)90103-5. [DOI] [PubMed] [Google Scholar]
- Woof J. M., Partridge L. J., Jefferis R., Burton D. R. Localisation of the monocyte-binding region on human immunoglobulin G. Mol Immunol. 1986 Mar;23(3):319–330. doi: 10.1016/0161-5890(86)90059-3. [DOI] [PubMed] [Google Scholar]
- de Lange G. G. Polymorphisms of human immunoglobulins: Gm, Am, Em and Km allotypes. Exp Clin Immunogenet. 1989;6(1):7–17. [PubMed] [Google Scholar]
- van de Winkel J. G., Capel P. J. Human IgG Fc receptor heterogeneity: molecular aspects and clinical implications. Immunol Today. 1993 May;14(5):215–221. doi: 10.1016/0167-5699(93)90166-I. [DOI] [PubMed] [Google Scholar]



