Abstract
Repair of complex ventral hernias frequently results in postoperative complications. This study assessed postoperative outcomes in a consecutive cohort of patients with ventral hernias who underwent herniorrhaphy using components separation techniques and reinforcement with non–cross-linked intact porcine-derived acellular dermal matrix (PADM) performed by a single surgeon between 2008 and 2012. Postoperative outcomes of interest included incidence of seroma, wound infection, deep-vein thrombosis, bleeding, and hernia recurrence determined via clinical examination. Of the 47 patients included in the study, 25% were classified as having Ventral Hernia Working Group grade 1 risk, 62% as grade 2, 2% as grade 3, and 11% as grade 4; 49% had undergone previous ventral hernia repair. During a mean follow-up of 31 months, 3 patients experienced hernia recurrence, and 9 experienced other postoperative complications: 4 (9%) experienced deep-vein thrombosis; 3 (6%), seroma; 2 (4%), wound infection; and 2 (4%), bleeding. The use of PADM reinforcement following components separation resulted in low rates of postoperative complications and hernia recurrence in this cohort of patients undergoing ventral hernia repair.
Key words: Ventral hernia repair, Biologic tissue matrix, Components separation, Synthetic mesh
Abdominal wall repair (AWR) for hernia is a common procedure, with an estimated 1 million or more procedures performed each year in the United States.1 Incisional hernias are a common complication of AWR, with reported incidences ranging from 9% to 20% in prospective studies of patients undergoing abdominal surgery.2–7 Significant advances have been made in surgical repair of abdominal hernias in recent decades, including the use of components separation techniques8,9 and prosthetic mesh and biologic tissue matrix materials to facilitate closure of abdominal wall defects.9 Nevertheless, data from several retrospective studies have shown hernia recurrence remains a significant problem following AWR using components separation techniques, with recurrence rates ranging from 14% to 22%.10–13 Synthetic mesh or biologic tissue matrix materials can be used to provide additional reinforcement in AWR with or without components separation. Reported recurrence rates following repair with prosthetic materials are highly variable14–17 and can be impacted by the complexity of the individual patient case, number of previous hernia repairs, and surgeon's technique.9,18 While there is lack of consensus regarding which mesh or matrix type to use for reinforcement in AWR, according to the Ventral Hernia Working Group (VHWG), synthetic mesh should be avoided in patients classified as having grade 2 risk (e.g., those who are smokers, obese, diabetic, immunosuppressed or have chronic obstructive pulmonary disease) owing to the increased risk of postoperative infection associated with comorbidities.9
Biologic tissue matrices may offer advantages over synthetic mesh for AWR in high-risk patients (e.g., better revascularization, less infection).9,19,20 Non–cross-linked intact porcine-derived acellular dermal matrix (PADM; Strattice Reconstructive Tissue Matrix, LifeCell Corp, Branchburg, New Jersey) is designed to perform as a surgical matrix for soft-tissue repair while serving as a scaffold for the rapid ingrowth of host cells, collagen, and blood vessels.21,22 In our practice, we have observed high complication rates following complex AWR with synthetic mesh in patients who have multiple risk factors with or without potentially contaminated or infected surgical fields. The objective of this study was to assess and describe postoperative outcomes in a consecutive cohort of patients who underwent ventral hernia repair using components separation techniques and reinforcement with PADM.
Patients and Methodology
This retrospective, Institutional Review Board–approved study included consecutive patients who underwent ventral hernia repair with components separation with PADM reinforcement performed by the author at the University of Pittsburgh Medical Center (Pittsburgh, Pennsylvania) and Butler Memorial Hospital (Butler, Pennsylvania). Surgeries were performed between August 2008 and January 2012, with follow-up data available through February 2013. Information on patient demographics, comorbidities, relevant medical and surgical history, presence of previously placed mesh, and length of surgical incision were recorded.
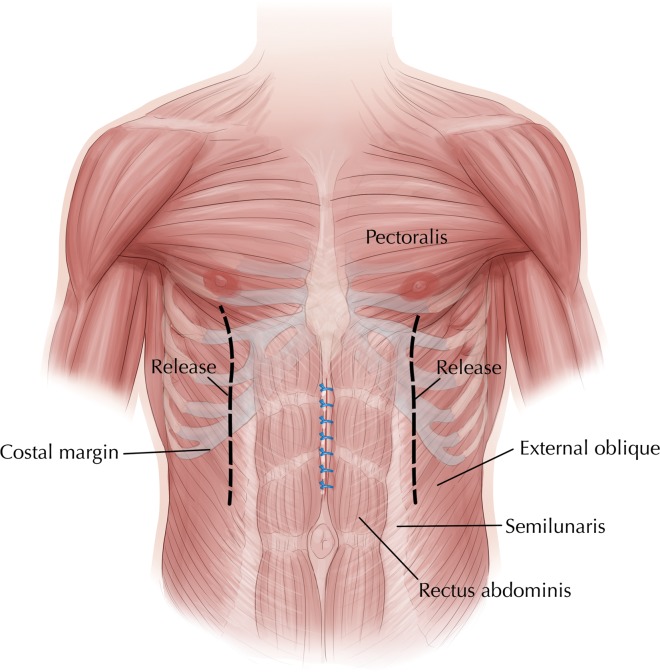
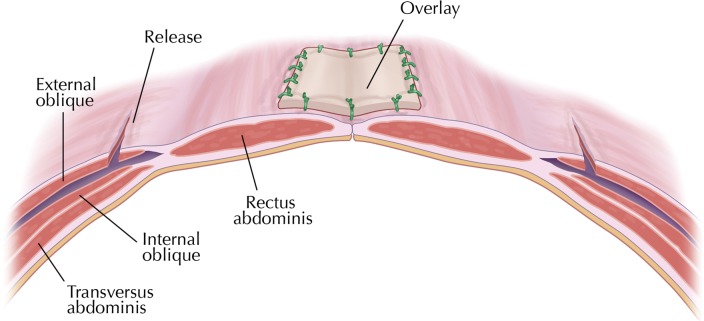
Prior to surgery, smokers were advised regarding smoking cessation, and all patients received prophylactic antibiotics. Ventral hernia repair, including components separation with bilateral dissection of the external oblique to the inferior portion of the pectoralis major muscle (Fig. 1), removal (if possible) of any previously placed mesh, and placement of PADM was performed in all patients according to the author's usual technique. For midline closure, interrupted No. 1 Prolene monofilament nonabsorbable (Ethicon, Inc, Somerville, New Jersey), or No. 1 Surgipro monofilament nonabsorbable (Covidien, Mansfield, Massachusetts) sutures were used. PADM was overlaid in a medial position across the anterior side of the rectus abdominis and sutured into place with interrupted Maxon No. 1 monofilament absorbable sutures (Covidien) to reinforce midline closure (Fig. 2). Two size-19 French Blake drains (Ethicon) were placed in all patients and remained in place until drainage was <30 mL for 2 consecutive days. All patients received intravenous cefazolin for 24 hours after surgery. Postoperative pain was managed with intravenous or orally administered analgesics, as needed.
Fig. 1.
Anterior abdominal wall showing anatomic location of midline incision and components separation.
Fig. 2.
Midline closure showing overlay of non–cross-linked porcine-derived acellular dermal matrix in place.
Postoperative Assessments
Postoperative outcomes assessed included seroma, evidenced by localized accumulation of fluid in the perioperative area, confirmed via computed tomography (CT) evaluation; wound infection, based on presence of erythema, exudate, tenderness, pain, and/or fever, as well as positive blood or tissue culture results; deep-vein thrombosis, identified by clinical signs and symptoms including swelling, pain, and color changes in the affected area, and confirmed based on ultrasound imaging; and bleeding. Hernia recurrence was diagnosed based on clinical or CT evaluation; any patient with a palpable mass, pain, swelling, or bulge extending beyond the reestablished abdominal wall boundary was identified as a possible hernia recurrence and confirmed based on ultrasound examination. Follow-up visits occurred at 1, 3, 6, 9, and 12 months after surgery and yearly thereafter. Complications, including hernia recurrence, were determined through clinical examination.
Results
A total of 47 patients were included in the study. Patient demographics and clinical characteristics are summarized in Table 1. The majority of patients were female (68%) and obese [body mass index (BMI) >30 kg/m2, 60%], and most (64%) were classified as having VHWG grade 2 or grade 3 risk for postoperative complications at the surgical site.9 Other comorbidities that were present in ≥5% of the study population included diabetes (n = 14, 30%), history of cancer (n = 6, 13%), chronic obstructive pulmonary disease (n = 4, 9%), and history of deep-vein thrombosis (n = 3, 6%).
Table 1.
Patient demographics and characteristics

Twenty-three patients (49%) had undergone previous ventral herniorrhaphy. The number of previous herniorrhaphies in the total study sample ranged from 0 to 11 (median, 0); the majority of patients (79%) had ≤1 previous repair. The mean (SD) length of surgical incision was 26 (9) cm. Primary fascial closure was achieved, and PADM was successfully placed in all 47 patients. PADM sheet sizes ranged from 6 × 16 cm to 6 × 20 cm. Herniorrhaphy was performed in the setting of infected previously placed mesh in 5 patients (11%), and the infected mesh was removed during surgery in all of these patients. Concurrent surgical procedures performed included panniculectomy in 3 patients. The mean (SD) length of hospital stay was 8 (6) days (median, 6 days; range, 2–29 days).
Patients were followed for a mean (SD) duration 31 (12) months (median, 34 months; range, 13–54 months). Of the 47 patients in the study, 31 were followed for ≥2 years. Overall, 12 patients experienced a total of 14 postoperative complications (3 hernia recurrences and 11 other complications; Table 2). Of the 3 patients (6%) who experienced hernia recurrence during the follow-up period, 2 had recurrences at 18 months postsurgery and 1 had recurrence at 7 months postsurgery. The patient with recurrence at 7 months was an 85-year-old, nonobese (BMI, 25 kg/m2) man who had 2 previous herniorrhaphies. He had no other relevant comorbidities and was classified as having VHWG grade 1 risk at the time of the most recent surgery. One patient who had a hernia recurrence at 18 months postsurgery was a 50-year-old, morbidly obese (BMI, 46 kg/m2) man with no previous history of hernia repair surgery. He had VHWG grade 2 risk for postsurgical complications and no other relevant comorbidities at the time of surgery. The other patient who experienced hernia recurrence at 18 months after surgery was a 75-year-old obese (BMI, 36 kg/m2) woman who had a history of 11 previous ventral hernia repair procedures. She had a previously placed infected mesh removed during her most recent surgery (VHWG grade 4 risk).
Table 2.
Summary of postoperative complications
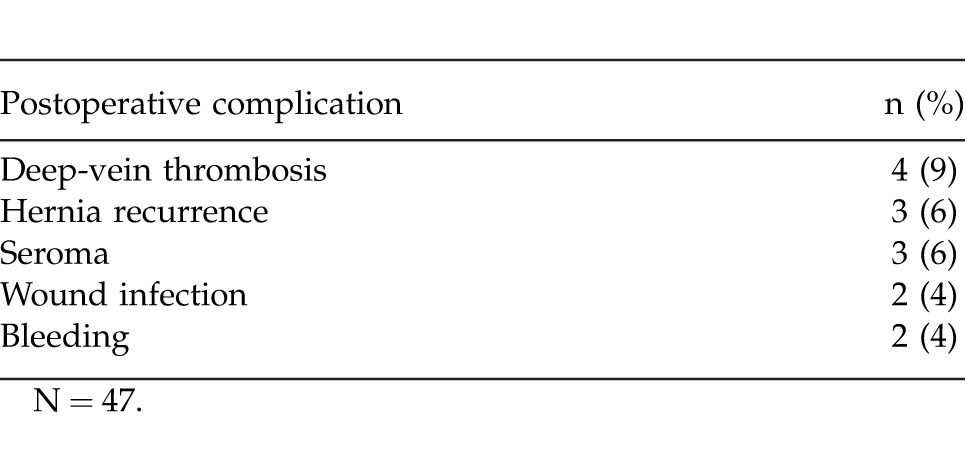
Nine of the 47 (19%) patients experienced 11 other postoperative complications, including deep-vein thrombosis (n = 4), seroma (n = 3), wound infection (n = 2), and bleeding (n = 2).
Discussion
In this cohort of patients, ventral hernia repair with use of components separation and non–cross-linked PADM resulted in low rates of postsurgical complications. Overall, 75% of patients did not experience any postsurgical complications, and only 3 experienced a recurrent hernia during a mean follow-up of approximately 2.5 years. These observations are particularly encouraging given that about half of the patients included in the study had undergone one or more previous ventral hernia repairs. Prior abdominal surgery is a known risk factor for hernia recurrence,23,24 and this risk increases with each subsequent repair.25
The risk of postsurgical complications after AWR with prosthetic mesh placement can be impacted by numerous factors, including patient comorbidities, the complexity of the patient's condition at the time of surgery, the surgeon's techniques for prosthetic mesh placement, the specific physical and/or chemical properties of the individual matrix or mesh,9 and the time elapsing since hernia repair. Reported rates of recurrence across studies of synthetic mesh in AWR range from 4% to 32%,12,14–16,18,20,26–29 with a mean of approximately 19% across studies. Reported recurrence rates following placement of biologic tissue matrix range between 0% and 44%,12,20,30–43 with a mean of approximately 12% across studies, compared with the recurrence rate of 6% observed in the current study.
There have been several previous reports of results from prospective35 and retrospective30,36,42 studies of PADM use in complex AWR. It is not surprising that incidences of postsurgical complications observed in the current study were lower than complication rates in previous studies involving populations that largely comprised patients with potentially contaminated (VHWG grade 3) or infected (VHWG grade 4) surgical sites.30,35,36 One previous study included a population largely comprising patients with VHWG grade 2 risk for postsurgical complications, as in the current study. Patel and colleagues42 retrospectively assessed postsurgical outcomes following AWR with components separation and PADM in a cohort of 41 patients; of whom, 33 (81%) were classified as having VHWG grade 2 risk for postsurgical complications. The authors observed incidences of seroma, wound infection, and hernia recurrence of 7%, 2%, and 0%, respectively, at a mean follow-up of 16 months. In the current study, the incidences of seroma, wound infection, and hernia recurrence were 6%, 4%, and 6%, respectively, in a population that had 62% of patients classified as VHWG grade 2 risk followed for a mean of 31 months. The high incidence of postoperative deep-vein thrombosis (9%) in the current study may be explained by patient histories. Of the 4 patients who experienced deep-vein thrombosis, 1 was a cancer patient who was also a smoker with chronic obstructive pulmonary disease, and he had a previously placed infected mesh removed during the current surgery (i.e., classified as VHWG grade 4 risk). The other 3 patients with deep-vein thrombosis were classified as having grade 2 risk, though all 3 were smokers and 2 were obese.
Interpretation of the results of this study is limited by its retrospective design; the fact that the study included a heterogeneous group of patients undergoing surgery at one health system by a single surgeon; the lack of a control group or comparator; and its limited duration of follow-up. It will be important to continue follow-up on these patients to evaluate long-term outcomes.
Conclusion
The use of non–cross-linked PADM for reinforcement following components separation in complex ventral hernia repair resulted in low rates of postoperative complications and hernia recurrence in this patient cohort. Continued follow-up of these patients will be key for evaluating the long-term success of this approach for ventral hernia repair.
Acknowledgments
Editorial support for this article was provided by Peloton Advantage, LLC, Parsippany, New Jersey, and funded by LifeCell Corp., Branchburg, New Jersey. The opinions expressed in this article are those of the authors. The authors received no honoraria/fee for service or other form of financial support related to the development of this article.
References
- 1.Rutkow IM. Demographic and socioeconomic aspects of hernia repair in the United States in 2003. Surg Clin North Am. 2003;83(5):1045–1051. doi: 10.1016/S0039-6109(03)00132-4. [DOI] [PubMed] [Google Scholar]
- 2.Mudge M, Hughes LE. Incisional hernia: a 10 year prospective study of incidence and attitudes. Br J Surg. 1985;72(1):70–71. doi: 10.1002/bjs.1800720127. [DOI] [PubMed] [Google Scholar]
- 3.Israelsson LA, Jonsson T. Incisional hernia after midline laparotomy: a prospective study. Eur J Surg. 1996;162(2):125–129. [PubMed] [Google Scholar]
- 4.Berretta R, Rolla M, Patrelli TS, Piantelli G, Merisio C, Melpignano M, et al. Randomised prospective study of abdominal wall closure in patients with gynaecological cancer. Aust N Z J Obstet Gynaecol. 2010;50(4):391–396. doi: 10.1111/j.1479-828X.2010.01194.x. [DOI] [PubMed] [Google Scholar]
- 5.Seiler CM, Bruckner T, Diener MK, Papyan A, Golcher H, Seidlmayer C, et al. Interrupted or continuous slowly absorbable sutures for closure of primary elective midline abdominal incisions: a multicenter randomized trial (INSECT: ISRCTN24023541) Ann Surg. 2009;249(4):576–582. doi: 10.1097/SLA.0b013e31819ec6c8. [DOI] [PubMed] [Google Scholar]
- 6.Veljkovic R, Protic M, Gluhovic A, Potic Z, Milosevic Z, Stojadinovic A. Prospective clinical trial of factors predicting the early development of incisional hernia after midline laparotomy. J Am Coll Surg. 2010;210(2):210–219. doi: 10.1016/j.jamcollsurg.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 7.Sugerman HJ, Kellum JM, Jr, Reines HD, DeMaria EJ, Newsome HH, Lowry JW. Greater risk of incisional hernia with morbidly obese than steroid-dependent patients and low recurrence with prefascial polypropylene mesh. Am J Surg. 1996;171(1):80–84. doi: 10.1016/S0002-9610(99)80078-6. [DOI] [PubMed] [Google Scholar]
- 8.Ramirez OM, Ruas E, Dellon AL. “Components separation” method for closure of abdominal-wall defects: an anatomic and clinical study. Plast Reconstr Surg. 1990;86(3):519–526. doi: 10.1097/00006534-199009000-00023. [DOI] [PubMed] [Google Scholar]
- 9.Breuing K, Butler CE, Ferzoco S, Franz M, Hultman CS, Kilbridge JF, et al. Incisional ventral hernias: review of the literature and recommendations regarding the grading and technique of repair. Surgery. 2010;148(3):544–558. doi: 10.1016/j.surg.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Hultman CS, Tong WM, Kittinger BJ, Cairns B, Overby DW, Rich PB. Management of recurrent hernia after components separation: 10-year experience with abdominal wall reconstruction at an academic medical center. Ann Plast Surg. 2011;66(5):504–507. doi: 10.1097/SAP.0b013e31820b3d06. [DOI] [PubMed] [Google Scholar]
- 11.DiCocco JM, Magnotti LJ, Emmett KP, Zarzaur BL, Croce MA, Sharpe JP, et al. Long-term follow-up of abdominal wall reconstruction after planned ventral hernia: a 15-year experience. J Am Coll Surg. 2010;210(5):686–688. doi: 10.1016/j.jamcollsurg.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 12.Ko JH, Wang EC, Salvay DM, Paul BC, Dumanian GA. Abdominal wall reconstruction: lessons learned from 200 “components separation” procedures. Arch Surg. 2009;144(11):1047–1055. doi: 10.1001/archsurg.2009.192. [DOI] [PubMed] [Google Scholar]
- 13.Tong WM, Hope W, Overby DW, Hultman CS. Comparison of outcome after mesh only repair, laparoscopic component separation, and open component separation. Ann Plast Surg. 2011;66(5):551–556. doi: 10.1097/SAP.0b013e31820b3c91. [DOI] [PubMed] [Google Scholar]
- 14.Luijendijk RW, Hop WC, van den Tol MP, de Lange DC, Braaksma MM, IJzermans JN, et al. A comparison of suture repair with mesh repair for incisional hernia. N Engl J Med. 2000;343(6):392–398. doi: 10.1056/NEJM200008103430603. [DOI] [PubMed] [Google Scholar]
- 15.Korenkov M, Sauerland S, Arndt M, Bograd L, Neugebauer EA, Troidl H. Randomized clinical trial of suture repair, polypropylene mesh or autodermal hernioplasty for incisional hernia. Br J Surg. 2002;89(1):50–56. doi: 10.1046/j.0007-1323.2001.01974.x. [DOI] [PubMed] [Google Scholar]
- 16.Burger JW, Luijendijk RW, Hop WC, Halm JA, Verdaasdonk EG, Jeekel J. Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg. 2004;240(4):578–583. doi: 10.1097/01.sla.0000141193.08524.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferzoco SJ. A systematic review of outcomes following repair of contaminated or infected ventral incisional hernias with biologic mesh. Int Surg. 2013;98(4):399–408. doi: 10.9738/INTSURG-D-12-00002.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anthony T, Bergen PC, Kim LT, Henderson M, Fahey T, Rege RV, et al. Factors affecting recurrence following incisional herniorrhaphy. World J Surg. 2000;24(1):95–100. doi: 10.1007/s002689910018. [DOI] [PubMed] [Google Scholar]
- 19.Butler CE. The role of bioprosthetics in abdominal wall reconstruction. Clin Plast Surg. 2006;33(2):199–211. doi: 10.1016/j.cps.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Ghazi B, Deigni O, Yezhelyev M, Losken A. Current options in the management of complex abdominal wall defects. Ann Plast Surg. 2011;66(5):488–492. doi: 10.1097/SAP.0b013e31820d18db. [DOI] [PubMed] [Google Scholar]
- 21.Byrd JF, Jr, Agee N, Getz SB, Lincourt AE, Iannitti DA, Heniford BT. Paper presented at: Annual Meeting of the American Association of Plastic Surgeons; April 9–12. Boca Raton, FL: 2011. Evaluation of porcine-derived grafts (XenMatrix, Strattice, and Permacol) in an in vivo preclinical study [abstract] [Google Scholar]
- 22.Campbell KT, Burns NK, Rios CN, Mathur AB, Butler CE. Human versus non-crosslinked porcine acellular dermal matrix used for ventral hernia repair: comparison of in vivo fibrovascular remodeling and mechanical repair strength. Plast Reconstr Surg. 2011;127(6):2321–2332. doi: 10.1097/PRS.0b013e318213a053. [DOI] [PubMed] [Google Scholar]
- 23.van't Riet M. De Vos Van Steenwijk PJ, Bonjer HJ, Steyerberg EW, Jeekel J. Incisional hernia after repair of wound dehiscence: incidence and risk factors. Am Surg. 2004;70(4):281–286. [PubMed] [Google Scholar]
- 24.Read RC, Yoder G. Recent trends in the management of incisional herniation. Arch Surg. 1989;124(4):485–488. doi: 10.1001/archsurg.1989.01410040095022. [DOI] [PubMed] [Google Scholar]
- 25.Flum DR, Horvath K, Koepsell T. Have outcomes of incisional hernia repair improved with time? A population-based analysis. Ann Surg. 2003;237(1):129–135. doi: 10.1097/00000658-200301000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park A, Birch DW, Lovrics P. Laparoscopic and open incisional hernia repair: a comparison study. Surgery. 1998;124(4):816–821. doi: 10.1067/msy.1998.92102. [DOI] [PubMed] [Google Scholar]
- 27.de Vries Reilingh TS, van Goor H, Charbon JA, Rosman C, Hesselink EJ, van der Wilt GJ, et al. Repair of giant midline abdominal wall hernias: “components separation technique” versus prosthetic repair: interim analysis of a randomized controlled trial. World J Surg. 2007;31(4):756–763. doi: 10.1007/s00268-006-0502-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eid GM, Prince JM, Mattar SG, Hamad G, Ikrammudin S, Schauer PR. Medium-term follow-up confirms the safety and durability of laparoscopic ventral hernia repair with PTFE. Surgery. 2003;134(4):599–603. doi: 10.1016/s0039-6060(03)00283-6. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez R, Rehnke RD, Ramaswamy A, Smith CD, Clarke JM, Ramshaw BJ. Components separation technique and laparoscopic approach: a review of two evolving strategies for ventral hernia repair. Am Surg. 2005;71(7):598–605. [PubMed] [Google Scholar]
- 30.Butler CE, Campbell KT. Minimally invasive component separation with inlay bioprosthetic mesh (MICSIB) for complex abdominal wall reconstruction. Plast Reconstr Surg. 2011;128(3):698–709. doi: 10.1097/PRS.0b013e318221dcce. [DOI] [PubMed] [Google Scholar]
- 31.Buinewicz B, Rosen B. Acellular cadaveric dermis (AlloDerm): a new alternative for abdominal hernia repair. Ann Plast Surg. 2004;52(2):188–194. doi: 10.1097/01.sap.0000100895.41198.27. [DOI] [PubMed] [Google Scholar]
- 32.Espinosa-de-los-Monteros A, de la Torre JI, Marrero I, Andrades P, Davis MR, Vasconez LO. Utilization of human cadaveric acellular dermis for abdominal hernia reconstruction. Ann Plast Surg. 2007;58(3):264–267. doi: 10.1097/01.sap.0000254410.91132.a8. [DOI] [PubMed] [Google Scholar]
- 33.Franklin ME, Jr, Trevino JM, Portillo G, Vela I, Glass JL, Gonzalez JJ. The use of porcine small intestinal submucosa as a prosthetic material for laparoscopic hernia repair in infected and potentially contaminated fields: long-term follow-up. Surg Endosc. 2008;22(9):1941–1946. doi: 10.1007/s00464-008-0005-y. [DOI] [PubMed] [Google Scholar]
- 34.Kolker AR, Brown DJ, Redstone JS, Scarpinato VM, Wallack MK. Multilayer reconstruction of abdominal wall defects with acellular dermal allograft (AlloDerm) and component separation. Ann Plast Surg. 2005;55(1):36–41. doi: 10.1097/01.sap.0000168248.83197.d4. [DOI] [PubMed] [Google Scholar]
- 35.Itani KMF, Rosen M, Vargo D, Awad SS, DeNoto G, Butler CE, et al. Prospective study of single-stage repair of contaminated hernias using a biologic porcine tissue matrix: the RICH study. Surgery. 2012;152(3):498–505. doi: 10.1016/j.surg.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Alicuben ET, DeMeester SR. Onlay ventral hernia repairs using porcine non-crosslinked dermal biologic mesh. Hernia. 2013] doi: 10.1007/s10029-013-1054-2. [published online ahead of print February. Available at http://www.ncbi.nlm.nih.gov/pubmed/23400527. Accessed February 24, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chavarriaga LF, Lin E, Losken A, Cook MW, Jeansonne LO, White BC, et al. Management of complex abdominal wall defects using acellular porcine dermal collagen. Am Surg. 2010;76(1):96–100. [PubMed] [Google Scholar]
- 38.Cobb GA, Shaffer J. Cross-linked acellular porcine dermal collagen implant in laparoscopic ventral hernia repair: case-controlled study of operative variables and early complications. Int Surg. 2005;90((suppl 3)):S24–S29. [PubMed] [Google Scholar]
- 39.Hsu PW, Salgado CJ, Kent K, Finnegan M, Pello M, Simons R, et al. Evaluation of porcine dermal collagen (Permacol) used in abdominal wall reconstruction. J Plast Reconstr Aesthet Surg. 2009;62(11):1484–1489. doi: 10.1016/j.bjps.2008.04.060. [DOI] [PubMed] [Google Scholar]
- 40.Nasajpour H, LeBlanc KA, Steele MH. Complex hernia repair using component separation technique paired with intraperitoneal acellular porcine dermis and synthetic mesh overlay. Ann Plast Surg. 2011;66(3):280–284. doi: 10.1097/SAP.0b013e3181e9449d. [DOI] [PubMed] [Google Scholar]
- 41.Parker DM, Armstrong PJ, Frizzi JD, North JH., Jr Porcine dermal collagen (Permacol) for abdominal wall reconstruction. Curr Surg. 2006;63(4):255–258. doi: 10.1016/j.cursur.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 42.Patel KM, Nahabedian MY, Gatti M, Bhanot P. Indications and outcomes following complex abdominal reconstruction with component separation combined with porcine acellular dermal matrix reinforcement. Ann Plast Surg. 2012;69(4):394–398. doi: 10.1097/SAP.0b013e31822f997b. [DOI] [PubMed] [Google Scholar]
- 43.Pomahac B, Aflaki P. Use of a non-cross-linked porcine dermal scaffold in abdominal wall reconstruction. Am J Surg. 2010;199(1):22–27. doi: 10.1016/j.amjsurg.2008.12.033. [DOI] [PubMed] [Google Scholar]