Abstract
Because pancreaticocystostomy is a method of exocrine secretion management in pancreas transplantation, a legitimate question is whether a pure pancreatic fistula could be shunted into the bladder. After duodenopancreatectomy for cancer, a pancreaticojejunostomy leakage was treated by pancreas-saving anastomosis disconnection. The resulting pure pancreaticocutaneous fistula was later diverted into the bladder using a Denver valved-pump device. Technical problems necessitated redoing the shunt using a modified technique and device. Although the system did work, catheter displacement outside the bladder finally caused device takedown and external fistula restoration. Our attempt did not succeed mostly because of our inexperience in dealing with an altogether novel issue without appropriate technology. Supposing its feasibility, a pancreatic-bladder shunt might have a role in treating pure pancreatic fistulas or creating an external fistula whenever the pancreatic remnant is unreliable for an anastomosis, or when a leaked anastomosis' disconnection is preferable to completion pancreatectomy.
Key words: Pancreatic fistula, Urinary bladder, Fistula-bladder shunt, Denver device, Pancreatectomy, Anastomotic leak, Pancreatic remnant
Since 1983,1 pancreatic juice drainage into the bladder has been a method of exocrine secretion management in pancreas transplantation. Thus, a legitimate question, never raised before, is whether a pancreatic fistula (PF) could be shunted into the urinary system.
A PF may result from various causes, including accidental or iatrogenic pancreatic traumata and necrotizing pancreatitis; in most cases, however, it is a complication of a pancreatic resection. After duodenopancreatectomy (DP), a pancreatic-digestive anastomosis leak is reported in 2% to more than 20% of cases,2 and, despite recently improved results, it is still the main cause of morbidity, with mortality ranging from 5% to 30%.3 Besides being potentially fatal, a postoperative PF (POPF) may need further procedures or surgery, in particular pancreatic-digestive anastomosis disconnection and completion pancreatectomy.
Here we present the case of a patient with a pure pancreaticocutaneous fistula, which was the late outcome of a disconnected pancreaticojejunostomy (PJS) with pancreatic remnant preservation, in which a shunting of the PF into the bladder was attempted. This report is aimed at raising questions as to the feasibility, suitability, and prospects of such a procedure.
Case Report
A 51-year-old nondiabetic man was admitted to the Vaio Hospital General Surgery Unit, affiliated with the University of Parma Medical School, for silent jaundice due to a 4.5 × 3.5-cm mass of the pancreas head without distant metastases. A DP was performed with end-to-side PJS by the technique described in Hakamada et al4 on the first jejunal loop, end-to-side hepaticojejunostomy 20 cm distally, and Roux-en-Y end-to-side gastrojejunostomy. Histopathology showed a G3, pT4N0MX ampullary adenocarcinoma; margins were free.
The postoperative course was complicated by a POPF, which resulted in a wide subhepatic collection. A computed tomography-guided, then surgical, drainage failed and a new operation was necessary for severe sepsis. The patient refused completion pancreatectomy but accepted pancreas-saving surgery, albeit with the certainty of a residual fistula. PJS takedown and disconnected jejunal segment resection were performed. The pancreatic remnant, after 2-cm shortening, was managed only by closed-suction Jackson-Pratt drainage (SIM Italia, Bologna, Italy). The patient gradually recovered from sepsis. The fistula took on pure pancreatic juice characteristics, with a steady, daily 150- to 200-mL output after an unsuccessful attempt at treatment with octreotide. The patient was discharged from the hospital 3 months after the initial surgery, with the Jackson-Pratt drainage shortened to 5 cm externally to the skin in order to collect the pancreatic juice in a urostomy bag. The secretion did not decrease over time and retained its pure, water-like aspect. No skin irritation occurred, nor did cultural examinations ever show bacterial growth.
After 2 more months, the patient, aware of the poor cancer-related prognosis, still refused the completion pancreatectomy. Thus, the idea arose that the fistula (Fig. 1) could be shunted into the urinary bladder via an implantable valve device. After having been informed that no similar experiences had ever previously been reported and that the procedure would have an altogether experimental character, the patient gave his consent to undergo the shunting attempt that, if successful, would at least improve his quality of life.
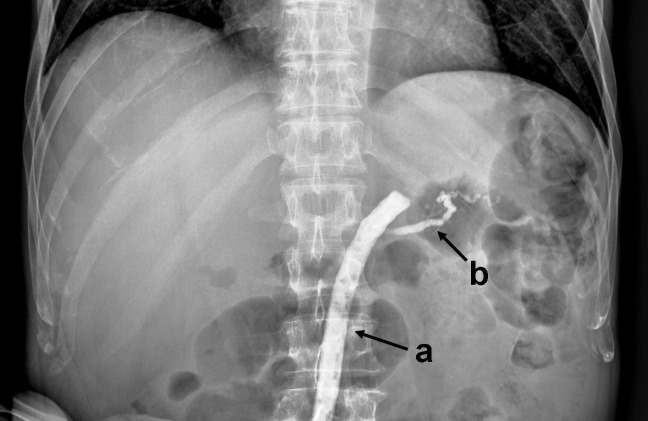
Fig. 1.
Fistulography: (a) Jackson-Pratt drain; (b) main pancreatic duct.
Six months after initial surgery, in the absence of cancer recurrence, the patient underwent fistula-bladder shunt using a Denver device (CareFusion, McGaw Park, Illinois). This is a silicone rubber system for peritoneovenous or pleuroperitoneal shunting (Fig. 2), consisting of a fenestrated collector in continuity with a single or double, flexible, miter-valved pump chamber of 2 cm3 in further continuity with an outlet catheter. The flow through the valve is possible either when the pressure gradient is about 3 cm H2O or when the chamber is manually pumped. The double-valved devices prevent reflux during manual activation and are usually preferred in intravenous shunts. After incision around the fistula orifice, the tube-wrapping fibrous sheath was dissected for 3 cm, and the fenestrated collector of a single-valve Denver system substituted the previous tube. This collector was fixed by a stitch surrounding the fibrous sheath. A second, suprapubic vertical incision was made, and the valved pump chamber was placed into a tunnel between the 2 incisions, under the muscle. The outlet catheter, appropriately shortened, was inserted into the bladder through a transparietal track and fixed inside it.
Fig. 2.
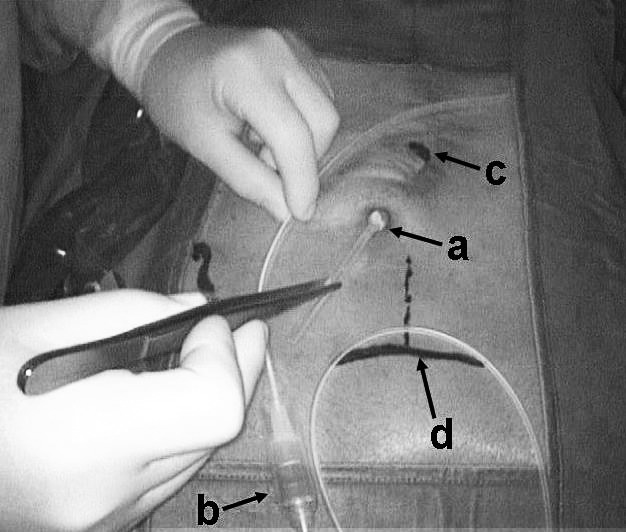
Pancreaticocutaneous fistula shunting procedure: (a) Jackson-Pratt drain, about to be removed; (b) Denver valved-pump device, about to be implanted; (c) umbilicus; and (d) superior aspect of the pubis bone.
For reasons that will be discussed later, this procedure was carried out while paying no attention to placement of the valved chamber in a correct position with respect to a rigid supporting surface needed for manual activation of the pump. As a result, after a few days, a subcutaneous edema and effusion of pancreatic juice from both wounds occurred, which involved further surgery: a leakage between the collector tube and the fibrous sheath was found, likely due to obstruction of the valve chamber by a brown, viscous material; moreover, the outlet tube probably became angled in the seated position. Since it was impossible to activate the pump manually, the shunt was redone. In the fistula track, a new fenestrated collector tube was inserted, independent of the Denver system, that was larger in size and closely adherent to the fibrous wall. It was fixed with 3 laces surrounding the sheath and sealed by cyanoacrylate glue. After shortening the collector, a new pump chamber was fixed to the pubis. Its outlet tube was arranged in an alpha-shaped coil, to avoid possibly becoming angled while the patient was in the seated position, and was then properly shortened and inserted into the bladder through the previous track, but was fixed only externally. Two Jackson-Pratt suction tubes were placed in the preperitoneal space in order to show possible leakages. Finally, the new collector tube and the valve's own collector were connected by interposing an 18 Fr T-tube. Its vertical branch was pulled outside the skin after a brief subcutaneous track in order to allow for temporary external drainage and collection of the pancreatic juice. It would have been removed as soon as the valve pump system was reliable for manual diversion of all the pancreatic juice into the bladder.
For 2 weeks the system fulfilled the theoretical premises, since the pump's activation drained the pancreatic secretion into the bladder and even aspirated the juice collected externally. The Jackson-Pratt drains did not show any leakage. After that time a displacement occurred of the outlet tube outside the bladder. The displacement was suspected on the basis of pancreatic juice appearing from the left Jackson-Pratt drain and was confirmed by a shuntography through the T-tube (Fig. 3). Although this complication would have been easily remediable, the patient asked us to interrupt the attempt and repeat it when a fit device was available. The device was removed, and the external fistula was restored without complications. A bladder catheter allowed for the parietal track healing in 1 week. Antibiograms on effused juice and urine were negative. Unfortunately, an unfavorable oncologic evolution prevented a new shunt attempt, and the patient died 10 months later, 16 months after DP.
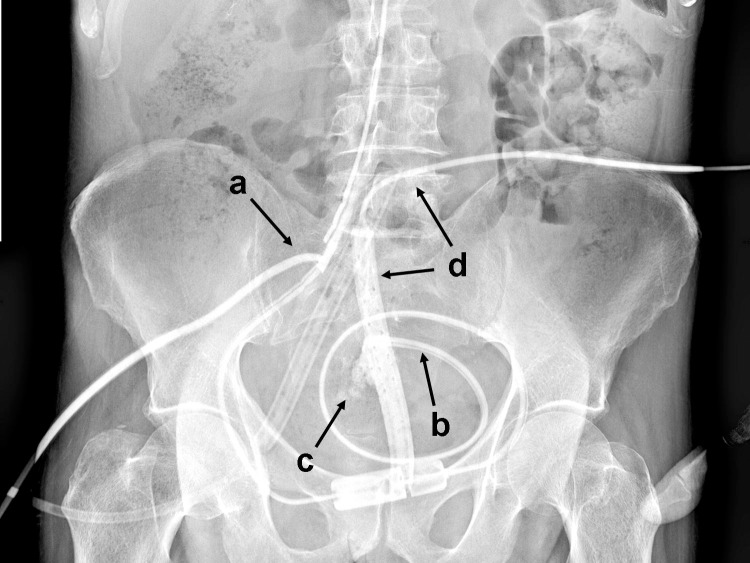
Fig. 3.
Shuntogram through the T-tube (a), showing the upward dislocation of the outlet tube from the bladder (b) and the effusion of the contrast medium in the preperitoneal space (c), which is drained by 1 of the 2 subcutaneous Jackson-Pratt drains (d).
Discussion
Since the presented attempt to shunt the pancreatic secretion into the bladder did not ultimately succeed, not only do the feasibility and effectiveness of such a procedure remain hypothetical, but the reasons for failure should be looked into.
The first operation failed because, without taking into consideration the fact that the Denver device can also work in the absence of a pressure gradient by means of the valve pump manual activation, we assumed that the pressure difference between the two sides was sufficient, as intrapancreatic basal pressure is normally about 11 mm Hg,5 whereas bladder resting pressure is about 10 cm H20, (i.e., 7.35 mm Hg). Even with wide physiologic variations in both values, the continuous pancreatic secretion within a capsuled, impermeable track, the pressure gradient between the two organs, and the gravitational pressure in the upright position were presumed sufficient to allow for a flow toward the bladder.
Such an assumption proved to be erroneous. Thus, in the second operation, not only was manual activation of the pump made possible, but a temporary expedient was also effected by using a T-tube aimed at maintaining a decompression and at evaluating the efficacy of the manual activation of the pump by measuring how much flow over time would be necessary. However, although both measures proved to be important issues, we have not yet fully understood why the shunt did not work after the first attempt. Contrary to what happens in ventriculoperitoneal shunts for hydrocephalus, where a cerebrospinal fluid overdrainage is fearfully possible, the high viscosity of pancreatic juice likely played a determinant role in the shunting malfunction.
The failure of the second operation, namely the outlet tube displacement outside the bladder, was only due to the system's technical inadequacies: the tube was probably badly fixed and, since it was constructed for a different use, was likely pushed out by its elastic tension after the forced alpha-shaped arrangement.
We cannot know whether, in the absence of this last complication, the system would have worked, and for how long, after removal of the T-tube vertical branch. However, in spite of a disappointing conclusion, the partial, temporary success led us to suppose that, in selected cases, a pancreatic-urinary shunt may have a role in pancreatic surgery.
If we make the intriguing hypothesis that a pure, well-stabilized, long-lasting PF may be treated by its diversion into the bladder, 2 theses may follow, both of them, it is hoped, prompting surgeons to adopt, when advisable, a more cautious management of a pancreatic remnant: (1) a pure PF could be deliberately made even during DP, thereby avoiding performing a pancreatic-digestive anastomosis, or a total pancreatectomy, whenever the pancreatic remnant is unreliable for such a procedure, and (2) any mixed pancreatic-biliary PF, as POPFs usually are, could be usefully converted into a pure one, that is, a pancreas-saving leaked anastomosis disconnection and external drainage could be preferred to a risky, redone pancreatic-digestive anastomosis or completion pancreatectomy.
In order to better explain and to support the 2 above theses, we stress the importance of the difference between mixed and pure PFs, as the former have a digestive and destroying potential on contiguous tissues that the latter do not have, because pure pancreatic juice contains enzymes in their inactivated form. This opinion is not universally shared as, for example, the clinical classification of POPFs by the ISGPF2 does not make any distinction between the 2 different characteristics and behaviors. Yet, this difference has practical surgical implications, as reported in various articles indicating that a pure PF may be more easily managed and sometimes deliberately created during DP. Twenty-five years ago, a pancreatic stump external drainage was used in a subset of 19 patients submitted to DP, with only minor morbidity and no mortality.6 In a more recent randomized trial,7 the performance of a controlled, pure, external PF by pancreatic duct and stump closure and peripancreatic drainage resulted in decreased mortality and hospital stays when compared to PJS. Similar results were confirmed in other reports.8,9 Somewhat confirming the “benign” behavior of pure PFs, it should be noted that the external drainage of the pancreatic duct was proposed as an alternative to pancreatic-digestive anastomosis in selected cases: patients with thin pancreatic duct, tender and friable parenchyma, emergency DP for trauma, and elderly or hemodynamically unstable patients who require expeditious completion of the operation.9,10 We could also mention exceptional cases of dramatically bleeding duodenal neoplasms or septic necrotizing cephalic pancreatitis.
PF evolution also deserves the maximum consideration. Although guided, pure PFs are usually temporary and self-limiting, in some cases they can persist and finally become long-lasting complications, thus worsening a patient's quality of life, albeit only in rare exceptions being life-threatening. Mixed POPFs, conversely, are often life-threatening and sometimes require surgical treatment in critical conditions. Completion pancreatectomy, which also implies surgery-induced diabetes, admittedly has a very high rate of complications and mortality, even in very qualified centers.11 In our opinion, a less aggressive approach to a mixed POPF, even more advisable in nondiabetic patients, may be its conversion into a pure fistula, carrying out anastomosis disconnection, pancreatic remnant preservation, and external drainage, as we did in our patient. Surprisingly, this procedure is not considered by the ISGPF,2 which reports only 3 possible reoperation options: leakage site repair by wide pancreatic drainage, conversion to an alternative means of pancreatic-enteric anastomosis, and completion pancreatectomy.
Getting back to our patient, the failure of our incomplete attempt is likely ascribable to poor expertise in dealing with an altogether novel problem and, in addition, to the unsuitable technology. Actually, a suitable technical solution seems to be a necessary condition. At present, a hypothetical prototype should provide: a valve-opening pressure gradient as low as possible; a high-volume valve chamber, in order to increase the efficacy of manual input and decrease its frequency; an implantable, light-suction reservoir of at least half the daily fistula output, in order to limit the need for continuous pump activation by hand, especially overnight; an access port for system patency assessment, possible washing out, and juice drawings; and an outlet catheter natively constructed with a wide coil arrangement and an intravesical locking system. The ideal shunt should be an implantable, electrically activated and telemetrically settable pump allowing for an active, continuous-flow transfer of pancreatic secretion. Such a prototype will have the further, not-inconsiderable advantage of avoiding the need for a reservoir. Suitable devices might possibly derive from the Minnesota peritoneovenous shunt in its US-patented automated version,12 which through current technological advancements could be adapted to the specific characteristics and requirements of PFs.
From a strictly technical viewpoint, an encouraging lesson can be learned from this case, although it is difficult to predict whether a shunt into the bladder can play an actual role in the management of pure PFs, whether occurring postoperatively or intraoperatively programmed. This possibility should be verified, provided a suitable prototype is tested and a more sophisticated technology is developed. To this aim, an experimental preliminary study on animals might also be needed. In any case, the infrequency of indications warrants the collection of occasional experiences from various centers.
Acknowledgments
Partially funded by grants from the Ministero dell'Università e della Ricerca Scientifica e Tecnologica, Repubblica Italiana. No conflict of interest to declare.
References
- 1.Cook K, Sollinger HW, Warner T, Kamps D, Belzer FO. Pancreaticocystostomy: an alternative method for exocrine drainage of segmental pancreatic allografts. Transplantation. 1983;35(6):634–336. [PubMed] [Google Scholar]
- 2.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138(1):8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Paye F. The pancreatic stump after pancreaticoduodenectomy: the “Achille's heel” revisited…. J Visc Surg. 2010;147(1):e13–e20. doi: 10.1016/j.jviscsurg.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Hakamada K, Narumi S, Toyoki Y, Nara M, Ishido K, Miura T, et al. An easier method for performing a pancreatico-jejunostomy for the soft pancreas using a fast-absorbable suture. World J Gastroenterol. 2008;14(7):1091–1096. doi: 10.3748/wjg.14.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fazel A, Geenen JE, MoezArdalan K, Catalano MF. Intrapancreatic ductal pressure in sphincter of Oddi dysfunction. Pancreas. 2005;30(4):359–362. doi: 10.1097/01.mpa.0000160278.11086.89. [DOI] [PubMed] [Google Scholar]
- 6.Funovics JM, Zöch G, Wenzl E, Schulz F. Progress in reconstruction after resection of the head of the pancreas. Surg Gynecol Obstet. 1987;164(6):545–548. [PubMed] [Google Scholar]
- 7.Reissman P, Perry Y, Cuenca A, Bloom A, Eid A, Shiloni E, et al. Pancreaticojejunostomy versus controlled pancreaticocutaneous fistula in pancreaticoduodenectomy for periampullary carcinoma. Am J Surg. 1995;169(6):585–588. doi: 10.1016/s0002-9610(99)80226-8. [DOI] [PubMed] [Google Scholar]
- 8.De Bree E, Melissas J, Schoretsanitis G, Sanidas E, Tsiftsis DD. Pylorus preserving pancreaticoduodenectomy with external pancreatic remnant drainage. Acta Chir Belg. 2004;104(6):668–672. doi: 10.1080/00015458.2004.11679640. [DOI] [PubMed] [Google Scholar]
- 9.Katsaragakis S, Antonakis P, Kanstadoulakis M, Androulakis G. Reconstruction of the pancreatic duct after pancreaticoduodenectomy: a modification of the Whipple procedure. J Surg Oncol. 2001;77(1):26–29. doi: 10.1002/jso.1060. [DOI] [PubMed] [Google Scholar]
- 10.Pisters WT, Evans DB. Commentary [about J Surg Oncol. J Surg Oncol. 2001;2001;7777(1)(1):26–29. 30. [Google Scholar]
- 11.Müller MW, Fiess H, Kleeff J, Dahmen R, Wagner M, Hinz U, et al. Is there still a role for total pancreatectomy? Ann Surg. 2007;246(6):966–975. doi: 10.1097/SLA.0b013e31815c2ca3. [DOI] [PubMed] [Google Scholar]
- 12.Buchwald H, Guzman E, Wigness BD, Dorman FD, Rohde TD. The Minnesota shunt. ASAIO Trans. 1989;35(2):168–170. doi: 10.1097/00002480-198904000-00012. [DOI] [PubMed] [Google Scholar]