Abstract
The journey from the discoveries of lymphotoxin (LT) and tumor necrosis factor (TNF) to the present day age of cytokine inhibitors as therapeutics has been an exciting one with many participants and highs and lows; the saga is compared to that in “The Wizard of Oz”. This communication summarizes the contributions of key players in the discovery of the cytokines and their receptors, the changes in nomenclature, and the discovery of the LT family’s crucial role in secondary and tertiary lymphoid organs. The remarkable advances in therapeutics are detailed as are remaining problems. Finally, special tribute is paid to two pioneers in the field who have recently passed away: Byron H. Waksman and Lloyd Old.
Keywords: The Wizard of Oz, Lymphotoxin, Tumor necrosis factor, Secondary lymphoid organs, Tertiary lymphoid organs, Therapeutic inhibitors of LT and TNF, Byron H. Waksman, Lloyd Old
1. Introduction
1.1. Purpose
Our knowledge of the lymphotoxin (LT)/tumor necrosis factor (TNF) family has been gained over the course of many years. I was asked to provide some insight into the early days of the field as one who has been involved for a long time. “The Wizard of Oz” by Frank Baum [1] is a popular book and movie about Dorothy from Kansas and her friends who encounter many obstacles and much excitement as they travel in search of their hearts’ desires, to be fulfilled by the great and powerful Oz in the Emerald City. Here I provide a somewhat biased account of the adventures of a group of travelers who journey along the “yellow brick road” and unlock the mysteries of LT and TNF from the discoveries of the molecules and receptors, to understanding their beneficial and harmful functions, to developing therapeutics that have transformed treatment of some autoimmune diseases. Special attention will be given to two pioneers: Byron H. Waksman and Lloyd Old, who were key movers in the LT/TNF field.
1.2. Description of the LT/TNF family
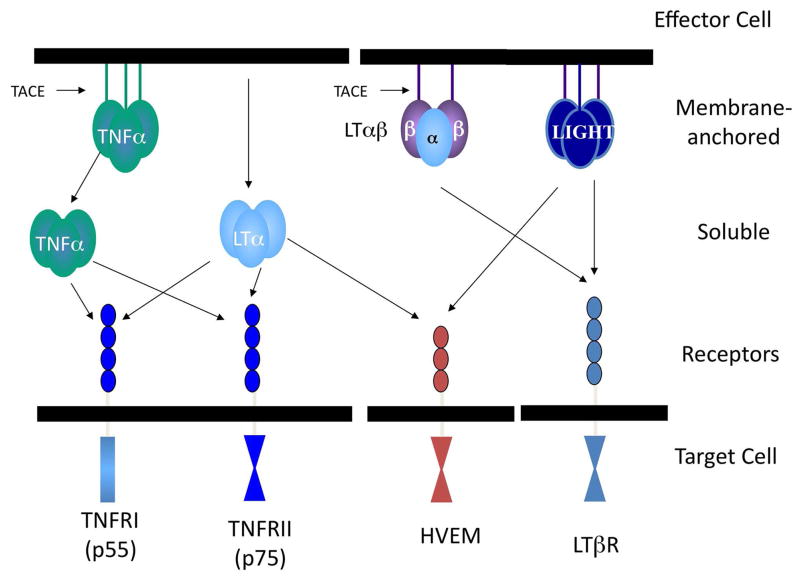
The immediate LT/TNF family consists of three tightly liked genes within the major histocompatibility complex [2]: TNFα, LTα, and LTβ. TNF is produced as a membrane bound molecule that is clipped by the TNF converting enzyme (TACE) to be released as a homotrimer to bind to one of two receptors, TNFR1 or TNFR2. LTα is released as a homotrimer and also binds to the two TNF receptors, hence explaining its similar activities to TNF. LTα3 also binds to an additional receptor, the herpes virus entry mediator (HVEM) as does LIGHT, which is not a member of the immediate LT/TNF immediate gene family. LTα is crucial for the transport of LTβ to the cell surface [3], resulting in the expression of the cell surface the LTα1β2 complex that binds to the LTβR. A recent report indicates that the LTα1β2 complex can be released via a metalloproteinase [4]. The interactions of ligands and receptors are depicted in Figure 1. Distinctions between the ligands include their regulation and cells or origin. A wide range of cells produces TNFα; this includes macrophages after stimulation by Toll-like receptors and CD4 and CD8 T cells after interaction with antigen. A more limited range of cells, including CD4 and CD8 T cells, B cells [5], and notably, lymphoid tissue inducer (LTi) cells [6], produces LTα and LTα1β2.
Figure 1.
A schematic depiction of the members of the ligands and receptors of the members of the lymphotoxin (LT)/tumor necrosis factor (TNF) family.
2. Discovery
2.1. Lymphotoxin
The 1960s saw the description of a secreted cytotoxic material produced by lymphocytes after stimulation by mitogen [7] or interaction with a specific antigen [8, 9]. Granger and his colleagues named this factor lymphotoxin [10]. (In fact, it is likely that these culture supernatants also contained TNFα). Aggarwal’s purification of human LT from a lymphoblastoid cell line [11] provided information for its cloning in 1984 by Patrick Gray [12]; murine LT was cloned in 1987 [13, 14]. Werner Lesslauer’s group’s resolution of the crystal structure of secreted LTα3 with TNFRI [15] led the way to an understanding of the interaction of the many ligands of the extended TNF family with their receptors. Along the way, LT’s name was somewhat arbitrarily changed to TNFβ [16]. The published rationale for this confusing change in nomenclature was that the same in vitro assay (killing L929 cells) was used to evaluate these molecules [16]. Later it was apparent that they were duplicated genes. The change in nomenclature was protested [17], but to no avail until the discovery of LTβ and the LTαβ complex by Browning and Ware [18] and the exciting realization that its biologic activity in lymphoid organ development) differed from that of TNFα [19] (see below). This resulted in renaming TNFβ back to LT (but now LTα!) and the demise of the name TNFβ. This back and forth has continued to engender confusion and frustration for students of this field for many years!
2.2. Tumor necrosis factor
TNF was discovered by Lloyd Old’s group as a factor in serum in response to endotoxin that caused necrosis when directly injected into tumors [20]. As noted above, this somewhat cumbersome assay was replaced by in vitro cytotoxicity against L929 cells. LPS-stimulated macrophages were a major source of TNF (later called TNFα). The Genentech group cloned the gene for murine [21] and human TNF [22]. Beutler and Cerami isolated a factor from a macrophage cell line that affected adipocytes in culture, which they called cachectin [23]. A sequence comparison determined that this was TNF. The observation that TNF was produced under septic conditions and that it might contribute to wasting led to rethinking about its role and trepidation concerning its use as an anti-tumor agent.
2.3. TNF and LTβ Receptors
A material that inhibited TNF was isolated from human urine by David Wallach in 1989 [24]. This was determined to be a TNF receptor. The groups of Loetscher and Lesslauer [25, 26] cloned the p55 (TNFRI) and p75 (TNRFII) receptors and it was revealed that both receptors bind TNFα and TNFβ (LTα). The gene for LTβR was cloned by the Immunex group of Smith and Goodwin [27] and found to bind both the LTα1β2 complex and LIGHT. The cloning of the receptors and ligands resulted in an explosion of knowledge concerning the signaling pathways of the immediate LT/TNF family and also those members of the extended TNF family.
2.4. Which cytokine is more important? Fashions come and go
Several years of research following the original descriptions of LT and TNF revealed important information about their cellular source of origin, mechanism of cytotoxicity through DNA fragmentation [28, 29], and signaling through the classical and alternative NFκB pathways. However, the original dream that TNFα and TNFβ (LT) would be useful as anti-tumor agents was not realized, as it was apparent that TNFα was a mediator in sepsis. The LT field lagged behind that TNF field for several reasons. Although recombinant human LT was available, murine LT proved difficult to prepare and thus signaling studies were not undertaken.. Furthermore, the most widely used monoclonal antibody to mouse TNF appeared to also neutralize LT [30] and for many years there was no antibody specific for murine LTα. TNF’s implication in sepsis suggested that its inhibition might have clinical benefit; LT is not produced by macrophages after LPS and its inhibition was thus not an appropriate target for sepsis. Although both LT and TNF are clearly pro-inflammatory [31, 32] with effects on chemokine induction and changes in endothelial cells [33, 34], many researchers concluded that LT was a weaker, less important member of the family, and it languished in semi-obscurity with its new name, TNFβ. The generation of the LT and TNF transgenic and knock out mice and the discovery of LTβ resulted in LT enjoying resurgence in popularity as a subject of study and potential clinical relevance.
3. Roles in Secondary and Tertiary Lymphoid Organs
3.1. LT is crucial for secondary lymphoid organ development
In order to determine whether there were biologically significant differences between LT and TNF, and whether either molecule could induce Type 1 diabetes, mice transgenic for LTα or TNF under the control of the rat insulin promoter (RIP) were produced [31]. Both mice exhibit florid infiltrates in the islets of Langerhans that were later realized, at least in the RIPLTα mouse, to resemble lymphoid organs [35] (see below). Although the morphological appearance of the infiltrates differs slightly in the RIPLT and RIPTNF mice [31], this alone did not reveal for a major difference in the biologic activity of the molecules. Neither mouse line developed diabetes unless a co-stimulator molecule unless the β cells also made a co-stimulator molecule [36]. Even though there was little difference when the transgenic mice were compared, the analysis of the knock out mice revealed dramatic differences in biologic activity. LTα knock out mice have major defects in SLOs with no lymph nodes, no Peyer’s patches, highly disorganized spleens [37, 38], and defective nasal associated lymphoid tissue [39]. Mice deficient in LTβ have a similar, but slightly less drastic phenotype in that they retain mesenteric, cervical and sacral lymph nodes [19, 40] indicating that the LTαβ complex plays a major role in secondary lymphoid organs, with some role for LTα3 alone. Additional data indicating that LTα has unique activities as LTα3 in addition to its contribution to the LTα1β2 complex derives from the observation that LTα3 from innate lymphoid cells regulates IgA in the gut by regulating homing of T cells, and that this occurs through TNFRI and TNFRII [41]. There is also an alteration in the gut microbiome. These events occur independently of LTβ, which even though it also regulates IgA production, it does so in a T cell-independent manner. Mice deficient in TNF exhibit a much less severe SLO phenotype when compared to mice deficient in LTα or LTβ [42]. There are reductions in marginal zone macrophages, but the lymphoid organs are all present.
LT regulates lymphoid organs in ontogeny by its production by lymphoid tissue inducer cells (LTi cells, also called ILC3 cells) acting on stromal lymphoid tissue organizer cells (LTo) [6, 43] by means of their induction of lymphoid chemokines [44] and endothelial adhesion molecules [45–48] during development. In the adult, LT maintains lymphoid organs through its production by T cells, B cells, and DCs.
3.2. LT induces tertiary lymphoid organs
TLOs, or more accurately, tertiary or ectopic lymphoid tissues, are accumulations of cells that arise in non-lymphoid organs during chronic immune stimulation in autoimmunity, graft rejection, atherosclerosis, microbial infection, and some tumors [47, 49, 50]. These tissues have many characteristics of SLOs including T and B cell compartmentalization, lymphoid chemokines, antigen presenting cells, conduits, high endothelial venules and lymphatic vessels [51] and appear to act as sites of local antigen presentation. Mice transgenic for LTα under the control of the rat insulin promoter (RIPLTα mice) exhibit such infiltrates [31, 32, 35], as do mice transgenic for both RIPLTα and RIPLTβ, but not RIPLTβ alone [48]. The most obvious difference between the infiltrates in the pancreata of these two types of mice has to do with the nature of the HEVs. Those in RIPLTα infiltrates express MAdCAM-1, but their peripheral node addressin (PNAd) is only expressed abluminally, whereas those in the double transgenics express PNAd luminally and abluminally [48]. These differences are due to differences in expression of GlyCAM-1 and HecGlcNac6st2 (also termed HEC6ST, gene name chst4) [46, 48]. The LTαβ complex is crucial for these genes whose expression is necessary for luminal and abluminal PNAd [52] characteristic of a mature HEV [6] that can attract L-selectin+ naïve and memory cells to populate LNs and TLOs. This in turn allows presentation of antigen at the local site-beneficial in infection, but detrimental in autoimmunity where it can give rise to determinant spreading and disease exacerbation.
3.3. Exploiting information from SLOs and TLOs to develop mice for in vivo imaging
We were struck by the presence of HEVs and LVs in TLOs that appeared to be very similar to those in SLOs and resolved to determine if their functions and regulation were actually the same. In these ongoing experiments we are studying their regulation and function and have developed mice that have green fluorescent HEVs and red fluorescent LVs. This was accomplished by means of the pCLASPER recombineering technique [53] to isolate regulatory elements of Hec6St in the case of HEVs [54, 55] or Prox-1 in the case of lymphatic vessels [56] to drive reporter genes. In the case of the Hec6st reporter mice, the expression of both the endogenous gene and the transgene are inhibited by treatment LTβR-Ig, an inhibitor of LTαβ signaling [54]. The transgene is regulated identically to the endogenous gene in development and is expressed in HEVs in TLOs [54]. These data indicate that regulation of the HEVs by LTαβ is similar in TLOs and SLOs. Lymph nodes of mice with green fluorescent HEVs have been imaged in vivo [53, 57], demonstrating that it is possible to image events in real time in TLOs and determine if and how HEVs in that context act as portals for naïve cells to exacerbate autoimmunity or defend against tumors.
ProxTom mice with their red (tdTomato) fluorescent lymphatic vessels have also been successfully imaged in vivo [56]. Previous studies of sections of lymph nodes revealed remarkable plasticity of lymphatic vessels [58, 59] with robust lymphangiogenesis that occurs at early times after immunization and gradually resolves [59]. Interestingly, these early lymphatic vessels are defective in their ability to transport DCs [59] due to defects in lymphatic contraction [60]. We have demonstrated such lymphangiogenesis after immunization by in vivo imaging of lymph nodes of ProxTom mice [57].
4. Therapeutics
4.1. TNFα inhibitors
Once it became apparent that TNF would not be an effective anti-tumor agent because of its unfortunate activity that mimicked septic shock, attempts were made to develop reagents that could inhibit sepsis. Robert Schreiber and colleagues developed an anti-mouse TNF antibody that also appeared to have anti LT activity that was effective against sepsis in mice, but only if administered before LPS. Vilçek and colleagues developed a monoclonal mouse human chimeric monoclonal antibody, cA2 [61], which neutralized cachexia in mice transgenic for human TNF [62]. An alternative approach is to use a truncated portion of the p55 TNFRI in an Fc fusion protein. Originally called Lenercept, this is also protective against sepsis in mice. Later, Etanercept (EMBREL®) was developed using a similar strategy; in this case, the material is a truncated version of the p75 (TNFRII)-Fc fusion protein. Completely humanized versions of the receptor fusion proteins have also been developed (summarized in [63]).
Early attempts to inhibit TNF in situations other than sepsis included murine models of cerebral malaria and multiple sclerosis (MS). Georges Grau, Pierre Vassalli, and colleagues demonstrated that rabbit anti-TNF antibody protected mice against cerebral malaria even if administered 4 days after exposure to Plasmodium berghei. Unfortunately, this is not effective in humans suffering from malaria [64]. My group in collaboration with that of Bob Clark used the Schreiber monoclonal anti-TNF antibody in to inhibit transfer of experimental autoimmune encephalomyelitis (EAE) [65] and later with G. Jeanette Thorbecke to inhibit relapsing EAE [66]. These results suggested that inhibition of TNF might be efficacious in human MS. Unfortunately, Lenercept protein was ineffective in a clinical trial of relapsing-remitting MS and in fact led to exacerbation of the disease in some individuals. The field carried on with the hope that inhibition of TNF might be effective in other autoimmune diseases. Mark Feldmann, Fionula Brennan, and Tini Maini were struck by the high levels of TNF in the joints of RA patients [67] and Feldmann and Maini conducted the first successful anti-TNF randomized trial against RA using cA2 (Infliximab) [68]. The anti-TNF therapies have revolutionized the treatment for RA, psoriasis, and inflammatory bowel disease.
Lenercept and etanercept inhibit both TNFα and LTα, thus expanding their range beyond the anti-TNFα antibodies. It has recently been reported that etanercept is effective at reducing both TNFα and LTα in the synovium of RA patients, particularly those who are high clinical responders [69]. Infliximab, the anti-TNF antibody, is less effective at reducing LTα levels. These observations are consistent with a direct effect of the TNF receptor blockers against both TNF and LT rather than a secondary reduction due to reduction in LT-producing cells infiltrating the joint. Whatever the mechanism, the data suggest another look at combined therapies is warranted.
4.2. LT inhibitors
4.2.1. LTβR-Ig
An LTβR-Ig fusion protein developed by Browning and colleagues [70] inhibits signaling of both LTα1β2 and LIGHT. It prevents development of most lymph nodes when administered to pregnant mice [71] with particularly striking results on blocking HEV maintenance through effects on GlyCAM-1 and Hec6ST [45, 59]. This reagent, has been effectively used in several mouse models of autoimmunity, including collagen arthritis [70] and salivary and lacrimal gland inflammation in the NOD mouse model of Sjögren’s syndrome [72, 73]. Because so many chronic autoimmune diseases exhibit TLO characteristics, and because LTα1β2 is so crucial for HEV development and maintenance, it was thought that an inhibitor of this pathway might be efficacious in treatment of autoimmune diseases. However, the original promise of Baminercept, the material administered to humans [74], was not realized as it failed to meet its endpoint in a phase II trial in RA. Nevertheless, based on the success in treatment of salivary and lacrimal gland inflammation in mice, a Phase II trial is currently underway aimed at human Sjögren’s syndrome (http://clinicaltrials.gov/ct2/show/study/NCT01552681).
4.2.2. Anti-LTα antibody
Jane Grogan’s group has developed a humanized anti-LTα monoclonal antibody, designated MLTA3698A or Pateclizumab that reacts with both LTα3 and LTα1β2 [75]. The existence of a dual recognition molecule suggests that an approach may be useful that goes beyond inhibiting just one aspect of the LT family. Encouraging results reported in a phase I clinical trial in RA patients [76] provide even greater optimism for a multipronged approach.
4.3. Summary and future directions
Much work remains with regard to inhibition of the LT/TNF pathways in therapeutics. Why are some RA patients resistant to anti-TNF therapy? Perhaps the armamentarium could be increased to include reagents that target all three members of the LT/TNF family. How do we minimize the side effects that include reactivation of latent tuberculosis? How do we target TNF and LT at the local site while sparing the beneficial effects of these factors? Caution is warranted to prevent drastic effects on SLOs, given the crucial role of LT in their induction and maintenance.
In some cases chronic inflammation is beneficial. Breast cancer is a striking example where there exists a positive correlation of beneficial outcomes (long term survival, fewer metastases and deaths) with TLOs in the tumor, particularly if the density of HEVs is high [49]. Presumably, the TLO acts as a site for priming of naïve cells and thus induces resistance to the tumor. Thus, the future may include therapeutics that actually encourage the development of HEVs at the site of a tumor to allow generation of a local defense.
5. A Tribute to Two Pioneers
5.1. Introduction
In the majority of this communication, I have paid tribute to many of our fellow travelers. Here, for special notice, are two of the early champions of the field who are known for so much more than a single discovery and who have died since the last TNF Congress.
5.2. Byron H. Waksman (1918–2012)
Byron Waksman’s early studies were on the role of the thymus in delayed type hypersensitivity in rats [77–80] and he can be considered a discoverer of the functions of that hitherto mysterious organ. He revealed the role of the thymus in tolerance by injecting soluble protein antigens into the thymus and demonstrating selective lack of reactivity to those antigens [81]. These experiments were precursors to our understanding of the exquisite control of self-antigen expression by Aire in the thymus [43]. He was a student of many models of autoimmunity including EAE and RA. His interest in understanding mechanisms of inflammation was crucial in the discovery of LT (called cytotoxic factor) with me [9] and IL-1 (called lymphocyte activating factor) with I gal Gery [82]. For many years Dr. Waksman was Chair of the Microbiology Department at Yale University School of Medicine. He joined the National Multiple Sclerosis Society as Director of Research and Medicine and served as President of the Waksman Foundation for Microbiology established by his father, Selman Waksman, the Nobel Prize winner for the discovery of streptomycin. In his later years, well into his 90s, Byron Waksman continued his involvement at New York University and Harvard University, attending lab meetings and giving seminars. Byron Waksman was above all a scientific communicator. He founded a program for scientific journalism at the Marine Biological Laboratory at Woods Hole and the European Initiative for the Communication of Science at the Max Planck Institute in Munich, Germany. In summary, Byron Waksman made crucial scientific contributions and was always aware of the broader clinical and societal implications of his work.
5.3. Lloyd Old (1933–2011)
Lloyd Old, considered by some to be the “father of cancer immunology” grew up in San Francisco where he aspired to be a classical violinist. He pursued that dream in Paris but returned to the United States where he pursued his interests in biology and medicine at the University of California at Berkley and the University of California at San Francisco where he graduated in 3 years at the top of his class. He did postdoctoral work with Baruj Benacerraf at Memorial Sloan Kettering where he remained for the rest of his career. His life’s work was devoted to answering three questions: 1) is there an immune reaction to cancer? 2) if so, what are the targets? 3) how can you stimulate that immunity? Dr. Old’s more than 800 publications included the discovery of TNF; the identification of the TL antigens, later called Ly1,2, and 3, eventually called CD4 and CD8; and the identification if the cancer testis antigens- NY-ESO-1.
Lloyd Old was tremendously influenced by the work of William Coley, a surgeon who injected bacterial lysates into cancer patients and in some cases showed remarkable reduction in tumor burdens. We now know that this material called “Coley’s Toxins” likely included substances such as LPS and other activators of Toll-like receptors and induced cytokines such as IL-1 and TNF. Lloyd Old took his fascination with Coley’s toxins along with Helen Coley Nauts, Dr. Coley’s daughter, to the establishment of the Cancer Research Institute (CRI) an organization that has provided crucial support in the form of postdoctoral fellowships and research grants for individuals in the TNF field. Dr. Old was instrumental in the Cancer Vaccine Collaborative, a joint program between the CRI and the Ludwig Institute for Cancer Research. This group is a network of world wide clinical trials and immune monitoring. In all these endeavors Lloyd Old laid the foundation and in fact provided answers to his three questions.
6. The Yellow Brick Road from Coley’s Toxins to therapeutidcs
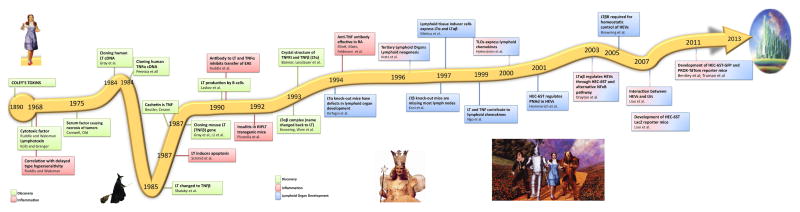
In this communication, I have presented a brief history of the LT/TNF field with high and low points along the way. These are summarized in Figure 2. I leave it to the reader to decide who embodies the characteristics of the Good Witch Glinda, who could be the Wicked Witch of the North, and who are the most likely embodiments of Dorothy, the Tin Woodman, the Cowardly Lion, and the Scarecrow. In all seriousness, the field has brought out the best in the travelers who have persisted in the face of discouragement and changes in research trends and have shown a remarkably cooperative spirit as they move the field to its present prominence and level of accomplishment. We may not have yet reached the Emerald City, but we are well on our way.
Figure 2.
The time line of the key discoveries in the field of LT and TNF depicted schematically with homage to “The Wizard of Oz” [1]. The insert depict the 4 main characters in the book, Dorothy, the Tin Woodman, the Cowardly Lion, and the Scarecrow.
Acknowledgments
These studies were supported by: NIH R21HL098711, NIH U19-AI082713, and JDRF 4-2007-1059
I acknowledge the excellent graphic support of Miriam Hill.
Footnotes
Conflict of interest: The author declares there are no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baum LF. The wonderful wizard of Oz. Chicago: George M. Hill Co; 1900. [Google Scholar]
- 2.Spies T, et al. Genes for the tumor necrosis factors alpha and beta are linked to the human major histocompatibility complex. Proc Natl Acad Sci U S A. 1986;83(22):8699–702. doi: 10.1073/pnas.83.22.8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Androlewicz MJ, Browning JL, Ware CF. Lymphotoxin is expressed as a heteromeric complex with a distinct 33-kDa glycoprotein on the surface of an activated human T cell hybridoma. J Biol Chem. 1992;267(4):2542–7. [PubMed] [Google Scholar]
- 4.Young J, et al. Lymphotoxin-alphabeta heterotrimers are cleaved by metalloproteinases and contribute to synovitis in rheumatoid arthritis. Cytokine. 2010;51(1):78–86. doi: 10.1016/j.cyto.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Laskov R, et al. Production of tumor necrosis factor (TNF-alpha) and lymphotoxin (TNF-beta) by murine pre-B and B cell lymphomas. J Immunol. 1990;144(9):3424–30. [PubMed] [Google Scholar]
- 6.Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3− LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7(4):493–504. doi: 10.1016/s1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]
- 7.Granger GA, Williams TW. Lymphocyte cytotoxicity in vitro: activation and release of a cytotoxic factor. Nature. 1968;218(5148):1253–4. doi: 10.1038/2181253a0. [DOI] [PubMed] [Google Scholar]
- 8.Ruddle NH, Waksman BH. Cytotoxic effect of lymphocyte-antigen interaction in delayed hypersensitivity. Science. 1967;157(3792):1060–2. doi: 10.1126/science.157.3792.1060. [DOI] [PubMed] [Google Scholar]
- 9.Ruddle NH, Waksman BH. Cytotoxicity mediated by soluble antigen and lymphocytes in delayed hypersensitivity. 3. Analysis of mechanism. J Exp Med. 1968;128(6):1267–79. doi: 10.1084/jem.128.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams TW, Granger GA. Lymphocyte in vitro cytotoxicity: lymphotoxins of several mammalian species. Nature. 1968;219(5158):1076–7. doi: 10.1038/2191076a0. [DOI] [PubMed] [Google Scholar]
- 11.Aggarwal BB, Moffat B, Harkins RN. Human lymphotoxin. Production by a lymphoblastoid cell line, purification, and initial characterization. J Biol Chem. 1984;259(1):686–91. [PubMed] [Google Scholar]
- 12.Gray PW, et al. Cloning and expression of cDNA for human lymphotoxin, a lymphokine with tumour necrosis activity. Nature. 1984;312(5996):721–4. doi: 10.1038/312721a0. [DOI] [PubMed] [Google Scholar]
- 13.Li CB, et al. Cloning and expression of murine lymphotoxin cDNA. J Immunol. 1987;138(12):4496–501. [PubMed] [Google Scholar]
- 14.Gray PW, et al. The murine tumor necrosis factor-beta (lymphotoxin) gene sequence. Nucleic Acids Res. 1987;15(9):3937. doi: 10.1093/nar/15.9.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banner DW, et al. Crystal structure of the soluble human 55 kd TNF receptor-human TNF beta complex: implications for TNF receptor activation. Cell. 1993;73(3):431–45. doi: 10.1016/0092-8674(93)90132-a. [DOI] [PubMed] [Google Scholar]
- 16.Shalaby MR, et al. Activation of human polymorphonuclear neutrophil functions by interferon-gamma and tumor necrosis factors. J Immunol. 1985;135(3):2069–73. [PubMed] [Google Scholar]
- 17.Ruddle NH. A new name for lymphotoxin. J Immunol. 1986;136(6):2335–6. [PubMed] [Google Scholar]
- 18.Browning JL, et al. Lymphotoxin beta, a novel member of the TNF family that forms a heteromeric complex with lymphotoxin on the cell surface. Cell. 1993;72(6):847–56. doi: 10.1016/0092-8674(93)90574-a. [DOI] [PubMed] [Google Scholar]
- 19.Koni PA, et al. Distinct roles in lymphoid organogenesis for lymphotoxins alpha and beta revealed in lymphotoxin beta-deficient mice. Immunity. 1997;6(4):491–500. doi: 10.1016/s1074-7613(00)80292-7. [DOI] [PubMed] [Google Scholar]
- 20.Carswell EA, et al. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975;72(9):3666–70. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pennica D, et al. Cloning and expression in Escherichia coli of the cDNA for murine tumor necrosis factor. Proc Natl Acad Sci U S A. 1985;82(18):6060–4. doi: 10.1073/pnas.82.18.6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray PW, et al. Cloning of human tumor necrosis factor (TNF) receptor cDNA and expression of recombinant soluble TNF-binding protein. Proc Natl Acad Sci U S A. 1990;87(19):7380–4. doi: 10.1073/pnas.87.19.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beutler B, et al. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature. 1985;316(6028):552–4. doi: 10.1038/316552a0. [DOI] [PubMed] [Google Scholar]
- 24.Engelmann H, et al. A tumor necrosis factor-binding protein purified to homogeneity from human urine protects cells from tumor necrosis factor toxicity. J Biol Chem. 1989;264(20):11974–80. [PubMed] [Google Scholar]
- 25.Loetscher H, et al. Molecular cloning and expression of the human 55 kd tumor necrosis factor receptor. Cell. 1990;61(2):351–9. doi: 10.1016/0092-8674(90)90815-v. [DOI] [PubMed] [Google Scholar]
- 26.Dembic Z, et al. Two human TNF receptors have similar extracellular, but distinct intracellular, domain sequences. Cytokine. 1990;2(4):231–7. doi: 10.1016/1043-4666(90)90022-l. [DOI] [PubMed] [Google Scholar]
- 27.Crowe PD, et al. A lymphotoxin-beta-specific receptor. Science. 1994;264(5159):707–10. doi: 10.1126/science.8171323. [DOI] [PubMed] [Google Scholar]
- 28.Schmid DS, et al. Target cell DNA fragmentation is mediated by lymphotoxin and tumor necrosis factor. Lymphokine Res. 1987;6(3):195–202. [PubMed] [Google Scholar]
- 29.Schmid DS, Tite JP, Ruddle NH. DNA fragmentation: manifestation of target cell destruction mediated by cytotoxic T-cell lines, lymphotoxin-secreting helper T-cell clones, and cell-free lymphotoxin-containing supernatant. Proc Natl Acad Sci U S A. 1986;83(6):1881–5. doi: 10.1073/pnas.83.6.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheehan KC, Ruddle NH, Schreiber RD. Generation and characterization of hamster monoclonal antibodies that neutralize murine tumor necrosis factors. J Immunol. 1989;142(11):3884–93. [PubMed] [Google Scholar]
- 31.Picarella DE, et al. Transgenic tumor necrosis factor (TNF)-alpha production in pancreatic islets leads to insulitis, not diabetes. Distinct patterns of inflammation in TNF-alpha and TNF-beta transgenic mice. J Immunol. 1993;150(9):4136–50. [PubMed] [Google Scholar]
- 32.Picarella DE, et al. Insulitis in transgenic mice expressing tumor necrosis factor beta (lymphotoxin) in the pancreas. Proc Natl Acad Sci U S A. 1992;89(21):10036–40. doi: 10.1073/pnas.89.21.10036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cuff CA, et al. Lymphotoxin alpha3 induces chemokines and adhesion molecules: insight into the role of LT alpha in inflammation and lymphoid organ development. J Immunol. 1998;161(12):6853–60. [PubMed] [Google Scholar]
- 34.Madge LA, Pober JS. TNF signaling in vascular endothelial cells. Exp Mol Pathol. 2001;70(3):317–25. doi: 10.1006/exmp.2001.2368. [DOI] [PubMed] [Google Scholar]
- 35.Kratz A, et al. Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. J Exp Med. 1996;183(4):1461–72. doi: 10.1084/jem.183.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guerder S, et al. Costimulator B7–1 confers antigen-presenting-cell function to parenchymal tissue and in conjunction with tumor necrosis factor alpha leads to autoimmunity in transgenic mice. Proc Natl Acad Sci U S A. 1994;91(11):5138–42. doi: 10.1073/pnas.91.11.5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Togni P, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264(5159):703–7. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- 38.Liepinsh DJ, et al. Novel lymphotoxin alpha (LTalpha) knockout mice with unperturbed tumor necrosis factor expression: reassessing LTalpha biological functions. Mol Cell Biol. 2006;26(11):4214–25. doi: 10.1128/MCB.01751-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ying X, et al. Lymphotoxin plays a crucial role in the development and function of nasal-associated lymphoid tissue through regulation of chemokines and peripheral node addressin. Am J Pathol. 2005;166(1):135–46. doi: 10.1016/S0002-9440(10)62239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soderberg KA, et al. MAdCAM-1 expressing sacral lymph node in the lymphotoxin beta-deficient mouse provides a site for immune generation following vaginal herpes simplex virus-2 infection. J Immunol. 2004;173(3):1908–13. doi: 10.4049/jimmunol.173.3.1908. [DOI] [PubMed] [Google Scholar]
- 41.Kruglov AA, et al. Nonredundant function of soluble LTalpha3 produced by innate lymphoid cells in intestinal homeostasis. Science. 2013;342(6163):1243–6. doi: 10.1126/science.1243364. [DOI] [PubMed] [Google Scholar]
- 42.Pasparakis M, et al. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med. 1996;184(4):1397–411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akirav EM, Ruddle NH, Herold KC. The role of AIRE in human autoimmune disease. Nat Rev Endocrinol. 2011;7(1):25–33. doi: 10.1038/nrendo.2010.200. [DOI] [PubMed] [Google Scholar]
- 44.Ngo VN, et al. Lymphotoxin alpha/beta and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J Exp Med. 1999;189(2):403–12. doi: 10.1084/jem.189.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Browning JL, et al. Lymphotoxin-beta receptor signaling is required for the homeostatic control of HEV differentiation and function. Immunity. 2005;23(5):539–50. doi: 10.1016/j.immuni.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 46.Drayton DL, et al. I kappa B kinase complex alpha kinase activity controls chemokine and high endothelial venule gene expression in lymph nodes and nasal-associated lymphoid tissue. J Immunol. 2004;173(10):6161–8. doi: 10.4049/jimmunol.173.10.6161. [DOI] [PubMed] [Google Scholar]
- 47.Drayton DL, et al. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol. 2006;7(4):344–53. doi: 10.1038/ni1330. [DOI] [PubMed] [Google Scholar]
- 48.Drayton DL, et al. Ectopic LT alpha beta directs lymphoid organ neogenesis with concomitant expression of peripheral node addressin and a HEV-restricted sulfotransferase. J Exp Med. 2003;197(9):1153–63. doi: 10.1084/jem.20021761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martinet L, Garrido I, Girard JP. Tumor high endothelial venules (HEVs) predict lymphocyte infiltration and favorable prognosis in breast cancer. Oncoimmunology. 2012;1(5):789–790. doi: 10.4161/onci.19787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stranford S, Ruddle NH. Follicular dendritic cells, conduits, lymphatic vessels, and high endothelial venules in tertiary lymphoid organs: Parallels with lymph node stroma. Front Immunol. 2012;3:350. doi: 10.3389/fimmu.2012.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruddle NH. Lymphatic vessels and tertiary lymphoid organs. Journal of Clinical Investigation. 2014 doi: 10.1172/JCI71611. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hemmerich S, et al. Sulfation of L-selectin ligands by an HEV-restricted sulfotransferase regulates lymphocyte homing to lymph nodes. Immunity. 2001;15(2):237–47. doi: 10.1016/s1074-7613(01)00188-1. [DOI] [PubMed] [Google Scholar]
- 53.Bentley KL, et al. A yeast-based recombinogenic targeting toolset for transgenic analysis of human disease genes. Ann NY Acad Sci. 2010;1207:E58–E68. doi: 10.1111/j.1749-6632.2010.05712.x. [DOI] [PubMed] [Google Scholar]
- 54.Liao S, et al. Transgenic LacZ under control of Hec-6st regulatory sequences recapitulates endogenous gene expression on high endothelial venules. Proc Natl Acad Sci U S A. 2007;104(11):4577–82. doi: 10.1073/pnas.0700334104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bentley KL, et al. High endothelial venule reporter mice to probe regulation of lymph node vasculature. In: Wallach D, Kovalenko A, Feldmann M, editors. Advances in TNF Research. Springer Press; New York: 2010. in press. [DOI] [PubMed] [Google Scholar]
- 56.Truman LA, et al. ProxTom lymphatic vessel reporter mice reveal Prox1 expression in the adrenal medulla, megakaryocytes, and platelets. Am J Pathol. 2012;180(4):1715–25. doi: 10.1016/j.ajpath.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Truman LA, et al. Lymphatic vessel function in head and neck inflammation. Lymphat Res Biol. 2013;11(3):187–92. doi: 10.1089/lrb.2013.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Angeli V, et al. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity. 2006;24(2):203–15. doi: 10.1016/j.immuni.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 59.Liao S, Ruddle NH. Synchrony of high endothelial venules and lymphatic vessels revealed by immunization. J Immunol. 2006;177(5):3369–79. doi: 10.4049/jimmunol.177.5.3369. [DOI] [PubMed] [Google Scholar]
- 60.Liao S, et al. Impaired lymphatic contraction associated with immunosuppression. Proc Natl Acad Sci U S A. 2011;108(46):18784–9. doi: 10.1073/pnas.1116152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Knight DM, et al. Construction and initial characterization of a mouse-human chimeric anti-TNF antibody. Mol Immunol. 1993;30(16):1443–53. doi: 10.1016/0161-5890(93)90106-l. [DOI] [PubMed] [Google Scholar]
- 62.Siegel SA, et al. The mouse/human chimeric monoclonal antibody cA2 neutralizes TNF in vitro and protects transgenic mice from cachexia and TNF lethality in vivo. Cytokine. 1995;7(1):15–25. doi: 10.1006/cyto.1995.1003. [DOI] [PubMed] [Google Scholar]
- 63.Ware CF. Protein therapeutics targeted at the TNF superfamily. Adv Pharmacol. 2013;66:51–80. doi: 10.1016/B978-0-12-404717-4.00002-0. [DOI] [PubMed] [Google Scholar]
- 64.van Hensbroek MB, et al. The effect of a monoclonal antibody to tumor necrosis factor on survival from childhood cerebral malaria. J Infect Dis. 1996;174(5):1091–7. doi: 10.1093/infdis/174.5.1091. [DOI] [PubMed] [Google Scholar]
- 65.Ruddle NH, et al. An antibody to lymphotoxin and tumor necrosis factor prevents transfer of experimental allergic encephalomyelitis. J Exp Med. 1990;172(4):1193–200. doi: 10.1084/jem.172.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Santambrogio L, et al. Studies on the mechanisms by which transforming growth factor-beta (TGF-beta) protects against allergic encephalomyelitis. Antagonism between TGF-beta and tumor necrosis factor. J Immunol. 1993;151(2):1116–27. [PubMed] [Google Scholar]
- 67.Brennan FM, et al. Cytokine production in culture by cells isolated from the synovial membrane. J Autoimmun. 1989;2(Suppl):177–86. doi: 10.1016/0896-8411(89)90129-7. [DOI] [PubMed] [Google Scholar]
- 68.Elliott MJ, et al. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet. 1994;344(8930):1105–10. doi: 10.1016/s0140-6736(94)90628-9. [DOI] [PubMed] [Google Scholar]
- 69.Neregard P, et al. Etanercept decreases synovial expression of tumour necrosis factor-alpha and lymphotoxin-alpha in rheumatoid arthritis. Scand J Rheumatol. 2013 doi: 10.3109/03009742.2013.834964. [DOI] [PubMed] [Google Scholar]
- 70.Fava RA, et al. A role for the lymphotoxin/LIGHT axis in the pathogenesis of murine collagen-induced arthritis. J Immunol. 2003;171(1):115–26. doi: 10.4049/jimmunol.171.1.115. [DOI] [PubMed] [Google Scholar]
- 71.Rennert PD, et al. Surface lymphotoxin alpha/beta complex is required for the development of peripheral lymphoid organs. J Exp Med. 1996;184(5):1999–2006. doi: 10.1084/jem.184.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fava RA, et al. LTBR-pathway in Sjogren’s syndrome: CXCL13 levels and B-cell-enriched ectopic lymphoid aggregates in NOD mouse lacrimal glands are dependent on LTBR. Adv Exp Med Biol. 2011;691:383–90. doi: 10.1007/978-1-4419-6612-4_39. [DOI] [PubMed] [Google Scholar]
- 73.Fava RA, et al. Lymphotoxin-beta receptor blockade reduces CXCL13 in lacrimal glands and improves corneal integrity in the NOD model of Sjogren’s syndrome. Arthritis Res Ther. 2011;13(6):R182. doi: 10.1186/ar3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Browning JL. Inhibition of the lymphotoxin pathway as a therapy for autoimmune disease. Immunol Rev. 2008;223:202–20. doi: 10.1111/j.1600-065X.2008.00633.x. [DOI] [PubMed] [Google Scholar]
- 75.Chiang EY, et al. Targeted depletion of lymphotoxin-alpha-expressing TH1 and TH17 cells inhibits autoimmune disease. Nat Med. 2009;15(7):766–73. doi: 10.1038/nm.1984. [DOI] [PubMed] [Google Scholar]
- 76.Emu B, et al. Safety, pharmacokinetics, and biologic activity of pateclizumab, a novel monoclonal antibody targeting lymphotoxin alpha: results of a phase I randomized, placebo-controlled trial. Arthritis Res Ther. 2012;14(1):R6. doi: 10.1186/ar3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arnason BG, Jankovic BD, Waksman BH. Effect of thymectomy on ‘delayed’ hypersensitive reactions. Nature. 1962;194:99–100. doi: 10.1038/194099a0. [DOI] [PubMed] [Google Scholar]
- 78.Arnason BG, et al. Role of the thymus in immune reactions in rats. II. Suppressive effect of thymectomy at birth on reactions of delayed (cellular) hypersensitivity and the circulating small lymphocyte. J Exp Med. 1962;116:177–86. doi: 10.1084/jem.116.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jankovic BD, Waksman BH, Arnason BG. Role of the thymus in immune ractions in rats. I. The immunologic response to bovine serum albumin (antibody formation, Arthus reactivity, and delayed hypersensitivity) in rats thymectomized or splenectomized at various times after birth. J Exp Med. 1962;116:159–76. doi: 10.1084/jem.116.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Waksman BH, Arnason BG, Jankovic BD. Role of the thymus in immune reactions in rats. III. Changes in the lymphoid organs of thymectomized rats. J Exp Med. 1962;116:187–206. doi: 10.1084/jem.116.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gery I, Waksman BH. Role of the thymus in tolerance. V. Suppressive effect of treatment with nonaggregated and aggregated bovine gamma-globulin on specific immune responses in normal adult rats. J Immunol. 1967;98(3):446–60. [PubMed] [Google Scholar]
- 82.Gery I, Gershon RK, Waksman BH. Potentiation of cultured mouse thymocyte responses by factors released by peripheral leucocytes. J Immunol. 1971;107(6):1778–80. [PubMed] [Google Scholar]