Summary
Background
Additional treatment with catheter-directed thrombolysis (CDT) has recently been shown to reduce post-thrombotic syndrome (PTS).
Objectives
To estimate the cost effectiveness of additional CDT compared with standard treatment alone.
Methods
Using a Markov decision model, we compared the two treatment strategies in patients with a high proximal deep vein thrombosis (DVT) and a low risk of bleeding. The model captured the development of PTS, recurrent venous thromboembolism and treatment-related adverse events within a lifetime horizon and the perspective of a third-party payer. Uncertainty was assessed with one-way and probabilistic sensitivity analyzes. Model inputs from the CaVenT study included PTS development, major bleeding from CDT and utilities for post DVT states including PTS. The remaining clinical inputs were obtained from the literature. Costs obtained from the CaVenT study, hospital accounts and the literature are expressed in US dollars ($); effects in quality adjusted life years (QALY).
Results
In base case analyzes, additional CDT accumulated 32.31 QALYs compared with 31.68 QALYs after standard treatment alone. Direct medical costs were $64 709 for additional CDT and $51 866 for standard treatment. The incremental cost-effectiveness ratio (ICER) was $20 429/QALY gained. One-way sensitivity analysis showed model sensitivity to the clinical efficacy of both strategies, but the ICER remained < $55 000/QALY over the full range of all parameters. The probability that CDT is cost effective was 82% at a willingness to pay threshold of $50 000/QALY gained.
Conclusions
Additional CDT is likely to be a cost-effective alternative to the standard treatment for patients with a high proximal DVT and a low risk of bleeding.
Keywords: anticoagulation, cost-effectiveness analysis, decision model, post-thrombotic syndrome, thrombolytic therapy, venous thrombosis
Introduction
Anticoagulation effectively prevents thrombus extension, pulmonary embolism, death and recurrence in patients with an acute deep vein thrombosis (DVT) [1]. After a proximal DVT, at least 25% of patients develop venous dysfunction resulting in chronic impairment and a post-thrombotic syndrome (PTS) [2,3]. Extensive thrombosis involving the common femoral and iliac veins increases this risk [4]. PTS is characterized by pain, swelling, heaviness, edema, pigmentation and deterioration of the skin. It is associated with a reduced quality of life and increased economic burden for the individual and society [5–7]. Additional treatment with thrombolysis has been introduced to enhance fibrinolysis and to preserve the deep veins. Targeted administration of the thrombolytic agent with a percutaneous catheter technique requires smaller doses and enhances safety compared with systemic administration. The CaVenT study was the first randomized controlled clinical trial (RCT) to compare the clinical efficacy of additional catheter-directed thrombolysis (CDT) with standard treatment alone. After 2 years 41% in the CDT arm presented with PTS compared with 56% of controls; corresponding to a 26% relative risk reduction [8]. Nevertheless, as additional CDT involves a bleeding risk, lengthens inpatient stay for DVT treatment and consumes resources, the aim of this study was to perform a health economic evaluation using a decision model to assess the cost effectiveness of additional CDT compared with standard treatment alone.
Patients and methods
Decision model
We developed a Markov model [9] with the perspective of a third-party payer and a lifetime horizon to compare two treatment strategies for the prevention of PTS in patients with a high proximal DVT, defined as thrombus above the mid-thigh level. Model uncertainty was assessed with one-way and probabilistic sensitivity analyzes. The two strategies were standard treatment with anticoagulation and elastic compression stockings, and CDT in addition to standard treatment. Our base case was a hypothetical cohort with age 50 years [8]. CDT with alteplase was given for up to 4 days together with heparin and followed by standard treatment [10]. Standard treatment included initial low-molecular-weight heparin (LMWH) followed by 6 months of oral warfarin and knee-high compression stockings class II for 24 months [1]. PTS was defined according to the Villalta scale [11,12]. We expressed the results in monetary costs (April 2012 US $), quality adjusted life years (QALYs) and an incremental cost-effectiveness ratio (ICER). This study was approved by the Regional Committee for Medical and Health Research Ethics and informed consent was obtained from all participants.
We applied a model cycle length of 6 months. All patients entered the model in an index DVT state, that is the first cycle, where, unless experiencing a fatal or disabling CDT-related complication, all patients received 6 months anticoagulation (Fig. 1). The subsequent cycles represented the long-term phase where the mutually exclusive health states were: (i) post DVT, (ii) PTS, (iii) severe PTS, (iv) permanently disabled from an intracranial bleeding and (v) dead (Fig. 1). After a recurrent venous thromboembolism (VTE), the patient would resume standard treatment with long-term anticoagulation for the remaining time in the model or until suffering a disabling intracranial bleeding [13]. States (i–iii) were sub-divided into off anticoagulation and on long-term anticoagulation to reflect their differing risks for recurrence and bleeding (Fig. 1). Major bleeding complications were classified as intracranial or other major. Intracranial bleedings were either fatal, disabling, that is corresponding to a modified Rankin scale (mRS) grade 4–5, or the patient regained their independency, that is a stroke sequela corresponding to a mRS grade 2–3. Other major bleedings could either resolve or result in death. Any non-disabling major bleeding was modeled as a temporary health state for the remainder of the cycle [14]. Additional events that would lead to a transition to another state by the end of each cycle included the development of PTS and severe PTS, and death not related to thrombosis or its treatment.
Fig. 1.
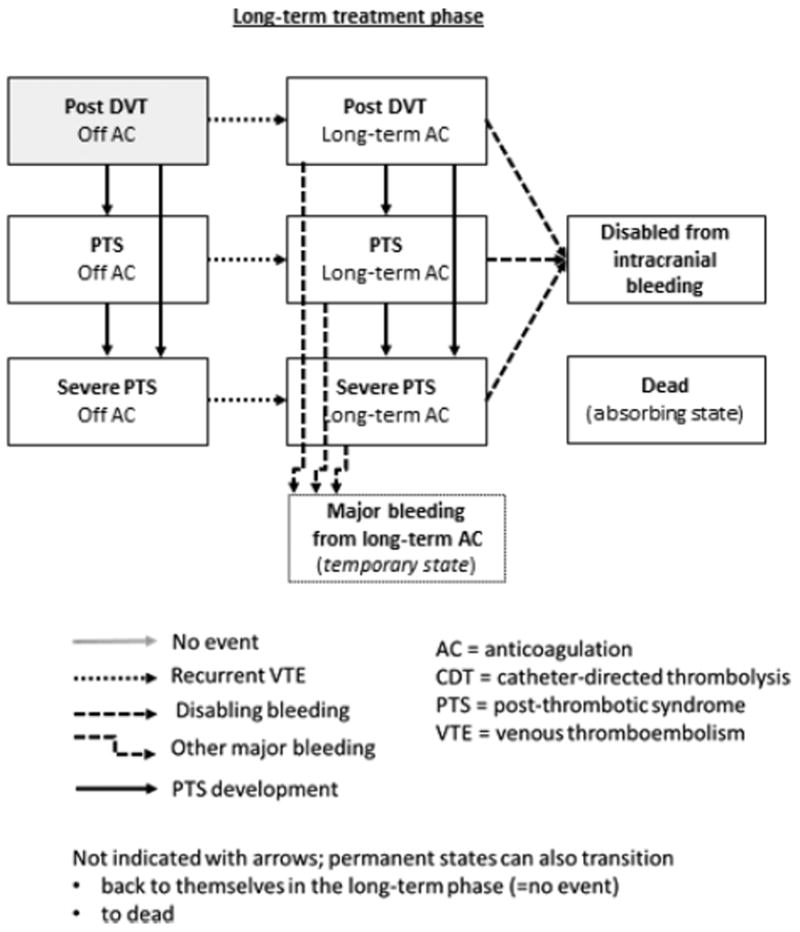
Model states, events and transitions. The boxes represent the different health states of the model. All patients start in an index deep vein thrombosis (DVT) state and, unless an adverse event occurs, transition to a post DVT state in which they remain until an adverse event occurs. The events that can lead to a state transition are represented by the different arrows.
We applied utilities and costs to each event and outcome over their expected durations, and we discounted costs and utilities at 3.0% annually.
The estimated risks for adverse events in the model were derived from the CaVenT study and the scientific literature. For all estimates we used a preference for higher level of evidence, newer meta-analysis where more than one study had been published, studies with validated assessment of PTS and patient characteristics resembling the population of the CaVenT study. We adhered to recommendations for converting between rates and probabilities for the calculation of cycle-specific transition probabilities [15].
In addition to the inherent assumptions within the Markov process [9], we applied the following assumptions: patients who were permanently disabled from an intracranial bleed remained in this state until death; assuming no further anticoagulation or significant impact from PTS and that bleeding or VTE recurrence would be fatal and captured within the background mortality [16]. After a recurrent VTE with resumption or adjustment of anticoagulation, any decrement in utility was assumed to be transient and without significant impact on long-term utility. Hence the utilities for being in health states i–iii did not differ between patients who were on or off anticoagulation [17–20]. Finally, any bleeding while off anticoagulation was assumed to be captured within the background mortality and the utility for the respective health states, and event rates for other conditions not included in our model were assumed to be similar across both strategies.
For model construction and analyzes we used TreeAge Pro Suite 2012 (TreeAge Software, Williamstown, MA, USA) and Microsoft Excel 2010 (Microsoft, Redmond, WA, USA). Model parameters are summarized in Table 1 and described below.
Table 1.
Base-case values and ranges used in sensitivity analyzes
| Base-case value (range) | References | |
|---|---|---|
| Probabilities* | ||
| Clinical efficacy | ||
| PTS 2 years after additional CDT | 0.411 (0.315–0.514) | [8] |
| PTS 2 years after standard treatment alone | 0.556 (0.457–0.650) | [8] |
| Severe PTS 3 years after additional CDT | 0.02 (0.01–0.034) | [22] |
| Severe PTS 2 years after standard treatment alone | 0.06 (0.03–0.08) | [21] |
| CDT related complications | ||
| Major bleeding | 0.033 (0.009–0.088) | [8] |
| Disabling intracranial bleeding | 0.0021 (0.0001–0.010) | [23] |
| Fatal bleeding or pulmonary embolism | 0.0042 (0.0007–0.014) | [23] |
| Anticoagulation related complications | ||
| Major bleeding during 6 months anticoagulation | 0.021 (0.016–0.026) | [24] |
| Major bleeding during long-term anticoagulation | 0.0136 (0.0135–0.0138) | [25] |
| Fraction of the two above being intracranial | 0.087 (0.058–0.125) | [25] |
| Intracranial bleeding case-fatality rate | 0.458 (0.270–0.657) | [25] |
| Independency recovered after intracranial bleeding | 0.26 (0.12–0.39) | [26] |
| Mortality when disabled from intracranial bleeding | 0.1305 (0.0868–0.1855) | [16] |
| Other major bleeding case-fatality rate | 0.091 (0.025–0.217) | [25] |
| Recurrent venous thromboembolism | ||
| Recurrent VTE during 6 months anticoagulation | 0.033 (0.025–0.042) | [24] |
| Case-fatality rate | 0.137 (0.090–0.192) | [24] |
| Recurrent VTE during long-term anticoagulation | 0.0097 (0.0070–0.0129) | [27] |
| Recurrent VTE off anticoagulation | 0.0251 (0.0216–0.0289) | [28] |
| Case-fatality rate | 0.036 (0.019–0.057) | [24], assumption |
| Utilities | ||
| Post DVT† | 0.8628 (0.8225–0.9031) | CaVenT |
| PTS† | 0.7745 (0.7304–0.8186) | CaVenT |
| Severe PTS† | 0.6752 (0.600–0.700) | [31], assumption |
| Major bleeding (1 cycle) | 0.65 (0.49–0.86) | [19], assumption |
| Intracranial bleeding with recovery of independence (1 cycle) | 0.71 (0.68–0.74) | [53,54], assumption |
| Permanently disabled from an intracranial bleeding | 0.32 (0.29–0.34) | [53,54] |
| Dead | 0 (0) | Definition |
| Costs*, $ | ||
| Post DVT and on long-term anticoagulation | 654 (491–818) | CaVenT‡ |
| Cost adverse outcomes and events | ||
| PTS | 611 (87–1310) | [5], CaVenT |
| PTS and long-term anticoagulation | 1265 (578–2128) | [5], CaVenT |
| Severe PTS | 2445 (349–5239) | [34,48], CaVenT |
| Severe PTS and long-term anticoagulation | 3099 (840–6057) | [34,48], CaVenT |
| Recurrent VTE§ | 5399 (4049–6749) | CaVenT |
| Non-disabling intracranial or other major bleeding§ | 19 088 (3334–99 382) | [35,55], assumption |
| Disabling intracranial bleeding§ | 46 501 (2093–148 117) | [35,55] |
| Permanently disabled from intracranial bleeding | 49 766 (37 325–62 208) | [36] |
| Treatment related costs | ||
| Standard treatment strategy | 9780 (5322–14 506) | Hospital accounts, CaVenT |
| Additional CDT treatment strategy | 13 166 (5143–17 960) | Hospital accounts, CaVenT |
| 6 months anticoagulation | 654 (491–818) | CaVenT |
| 24 months use of elastic compression stockings | 931 (698–1164) | CaVenT |
| Discount rate, % | 3.0 (1.0–5.0) | [56,57] |
CDT, catheter-directed thrombolysis; DVT, deep vein thrombosis; PTS, post-thrombotic syndrome; VTE, venous thromboembolism.
Per 6 months cycle unless otherwise noted.
On or off long-term anticoagulation.
The CaVenT study cost estimates are based on study data, hospital accounting, retailers’ bids, market prices, plus Norwegian SPCs and tariffs.
One-time costs.
Probability of adverse outcomes in the decision model
Post thrombotic syndrome
In the base case we assumed that PTS corresponded to the frequency observed in the CaVenT study, that is 0.411 (95% CI 0.315–0.514) in the CDT arm and 0.556 (95% CI 0.457–0.650) after standard treatment alone; severe PTS was not observed in either treatment arm [8,12]. Beyond 24 months less is known about the PTS risk, but based upon the pathophysiologic rationale of early thrombus removal for improved clinical benefit, the great majority of PTS cases are likely to have developed within 2 years, and to avoid favoring the intervention tested, we applied a conservative approach assuming a parallel increase in PTS for the two strategies. We based our estimations on a prospective cohort study where the cumulative incidence after 8 years was 30% for PTS and 8% for severe PTS [21]. Beyond 5 years we modeled no further development of PTS, indicating a negligible risk if previously not established [21]. For the CDT strategy the risk of severe PTS was derived from a retrospective study of 71 patients and a 36.6% cumulative incidence of PTS; hereunder three patients with severe PTS, after a median follow-up of 6 years [22].
Bleeding complications related to treatment
The probability of a CDT-related major bleeding was 0.033 (3/90) as observed in the CaVenT study [8]. The risk of non-fatal intracranial bleeding and death from CDT were 0.0021 (1/473) and 0.0042 (2/473), respectively, as obtained from an American registry study [23].
The probabilities of bleeding complications attributable to oral anticoagulation and the corresponding fatality rates were obtained from two meta-analyzes [24,25]. During the 6 months anticoagulation in either strategy, the risk of a major bleeding was 0.021 with 8.7% being intracranial. The fatality rate was 0.458 for intracranial bleedings and 0.091 for others. The risk of dying after a disabling intracranial bleeding was 0.568 [16], and the probability of recovering from an intracranial bleeding was in the range 0.12–0.39 [26].
Recurrent venous thromboembolism
The risk of recurrence was assumed to be equal between the two strategies [22]. A rate of recurrent VTE during the initial 6 months of anticoagulation of 0.033 with a case-fatality rate of 0.137 derived from a recent meta-analysis [24]. On long-term anticoagulation the annual risk of recurrence was 0.02 [27]. After cessation of anticoagulation the cumulative incidence of recurrence was 40% in a cohort study with 10 years follow-up [28]. The fatality rates of recurrent VTE after and during long-term anticoagulation were assumed to be equal and 0.036 [24].
Death from other causes
The excess mortality of a VTE population is mainly attributable to malignancies [29], but advanced cancer was an exclusion criterion in the CaVenT study and we assumed that cancer-related deaths were captured in the obtained age-adjusted background mortality for the Norwegian population (http://www.ssb.no), and the only excess mortality modeled was fatal recurrences and treatment-related bleedings.
Quality of life estimates
The EQ-5D is a well-established and widely used preference-based instrument for the assessment of quality of life in clinical trials and cost-effectiveness analyzes (http://www.euroqol.org). Completion of the EQ-5D at 24 months follow-up in the CaVenT study assigned the utilities for the health states post DVT and PTS [30]. The utilities for the other adverse events were obtained from the literature (Table 1) and for consistency utilities obtained by the EQ-5D were preferred. Utility for severe PTS was obtained from a British RCT on venous leg ulcers [31]. To calculate QALYs, we multiplied the probabilities of adverse events by utilities and summed over the duration.
Costs
Costs adjusted to April 2012 Norwegian kroner (NOK) by the Norwegian consumer price index (http://www.ssb.no) were converted to April 2012 US dollars ($) (http://www.norges-bank.no) and reflected the perspective of a health care system. Indirect costs were not included in the analysis. Individual level data on costs were partly collected prospectively in the CaVenT Study or obtained retrospectively for a subset of study patients (n = 67). Other estimates for mean hospital service costs were obtained directly from the hospital accounting department. The adjudication of medical costs to be included was based on current recommendations and practice for economic evaluations, and a result of consensus among the authors.
Treatment costs
Initial anticoagulation was according to current recommendations [1]. We assumed LMWH 7500 IU twice daily for 5 days, 3 tablets of 2.5 mg warfarin for 10 days, and four outpatient clinic visits with International Normalized Ratio (INR) monitoring before consecutive individualized follow-up by a general practitioner. The mean days in hospital for patients receiving standard treatment was 2.3 days (standard deviation [SD] 2.7 days) and hospital costs per day were $3429. After discharge, the mean use of warfarin in the CaVenT study was 3.2 tablets per day and the INR was on average monitored every 3rd week. The mean costs for the standard treatment strategy were $10 478, including two pairs of compression stockings replaced every 6 months (market price); the low range value representing outpatient care (Table 1). The additional costs related to the CDT strategy were based on the mean duration of CDT of 2.4 days (SD 1.1 days) and an average hospital stay of 5.3 days (SD 4.2 days) [8]. The mean consumption of other components of the CDT strategy included drugs, angioplasty balloons, stents, other non-reusable equipment in the cath-lab and personnel: one physician and two radiographers per procedure. We assumed 2.0 h of working time in the cath-lab for establishing CDT and 1.5 h for the daily venography. One-third had ambulance transport from a local hospital to an interventional center and when transport costs were included, the mean additional treatment costs for the CDT strategy were $13 166. Drug prices were obtained from the Norwegian summary of product characteristics (SPC, see http://www.felleskatalogen.no), the remaining from hospital accounts and tariffs (Norwegian Medical Association, see http://www.legeforeningen.no).
Complications and adverse events
For recurrent VTE costs we included a 1-day stay in the emergency ward, D-dimer testing and diagnostic ultrasound imaging, obtained from the hospital accounting department. Adjustment or resumption of anticoagulation was assumed to accrue the same costs as the initiation of standard treatment for the index DVT. PTS has been shown to be associated with high and variable direct and indirect costs [32]. We derived a base case estimate for PTS costs by combining the above cost estimate for standard DVT treatment with the findings in a recent Canadian prospective cohort study in which a multivariate regression analysis showed 35% (95% confidence interval [CI] 5–74) higher costs from medical recourse use in patients with PTS compared with those without [5]. At least two-thirds of these costs accrued during the first year. This is in line with previous reports [6,33] and was included in the model. For subsequent years we assumed constant annual costs [5]. The Canadian report did not specify costs for severe PTS, but this has ranged from 2.8–4.9 times higher [33,34]. Hence, we assumed that the costs for severe PTS were four times the costs for non-severe PTS. One-time cost estimates for major bleedings were derived from a Norwegian stroke trial [35] where the mean costs for the 1st year were equivalent to a 2.5-day stay in an intensive care unit (hospital accounts, data not shown). The costs from being permanently disabled, for example in a nursing home, were derived from a Norwegian simulation model of health benefits and cost consequences of cardiovascular interventions [36].
Sensitivity analyzes
To assess the uncertainty of the model one-way sensitivity analyzes were performed for all parameters over their plausible ranges (Table 1).
Probabilistic sensitivity analyzes
To assess the uncertainty and probability of cost-effectiveness a probabilistic sensitivity analysis was performed where second-order Monte Carlo simulations randomly sampled a distribution of all variables 10 000 times and then simulated outcomes. We used beta distributions for events and utilities and gamma and log-normal distributions for costs.
Results
CDT treatment in addition to standard treatment with anticoagulation and compression stockings was associated with an effectiveness of 32.31 QALYs and a lifetime cost of $64 709. Patients receiving standard treatment alone accumulated 31.68 QALYs and a lifetime cost of $51 866. Hence, under base-case conditions the ICER was $20 429/QALY gained and neither strategy was dominant (Table 2).
Table 2.
Cost effectiveness and projected model outputs for the two treatment strategies for a deep vein thrombosis
| Output | Treatment strategy
|
|
|---|---|---|
| Standard treatment* | CDT in addition to standard treatment | |
| Costs, $ | 51 866 | 64 709 |
| QALYs | 31.68 | 32.31 |
| ICER, $/QALY gained | Reference | 20 429 |
| Life expectancy, years | 30.9 | 30.8 |
| Patients who developed any PTS, % | 62.5 | 44.0 |
| Patients who developed severe PTS, % | 7.6 | 3.3 |
| Fatal bleeding, % | 5.5 | 5.9 |
| Fatal recurrent venous thromboembolism, % | 4.2 | 3.9 |
QALY, quality adjusted life year; ICER, incremental cost-effectiveness ratio; CDT, catheter-directed thrombolysis; PTS, post-thrombotic syndrome.
Standard treatment included 6 months of anticoagulation and 24 months of compression therapy.
In a hypothetical cohort of 50 years old with a high proximal DVT followed over their lifetime 62.5% in the standard strategy developed PTS compared with 44.0% in the CDT strategy. For severe PTS, the corresponding numbers were 7.6% and 3.3%. In total, 5.5% and 5.9% suffered a fatal bleeding complication in the standard and CDT strategy, respectively, and correspondingly 4.2% and 3.9% died from a fatal recurrent VTE. The projected life expectancy was 30.9 years after standard treatment and 30.8 years in the CDT strategy, corresponding to a loss of 1.1 months or 32 days from the additional risks associated with CDT (Table 2).
One-way sensitivity analyzes
The cost effectiveness of CDT in our model was sensitive to uncertainty of several key parameters. Variation in the incidence of PTS in the standard treatment strategy had the greatest influence, and was associated with an ICER of $53 268/QALY gained if present in 67% after 2 years. For all other parameters the ICER remained below a willingness-to-pay (WTP) ratio of $50 000/QALY (Fig. 2). Two more clinical efficacy estimates showed a major influence; a 51% incidence of PTS after CDT treatment increased the ICER to $44 534/QALY gained, whereas 8% severe PTS 2 years after standard treatment was associated with $40 709/QALY. Variation in severe PTS after CDT had little influence.
Fig. 2.
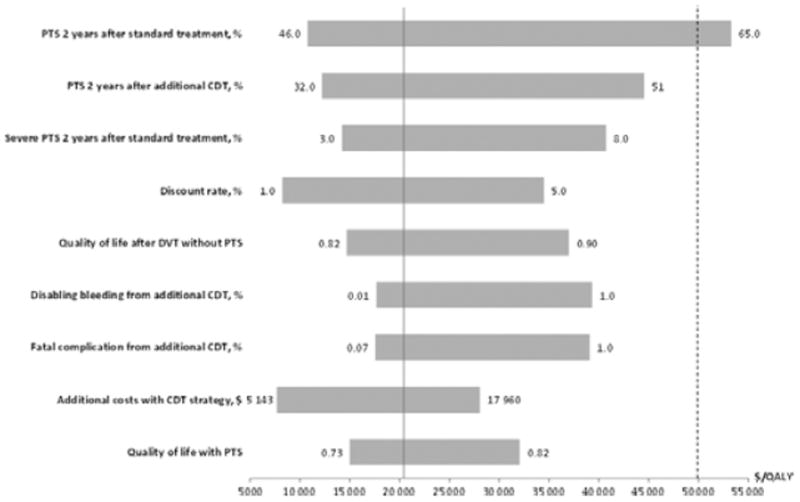
One-way sensitivity analyzes on variables that most influenced the incremental cost effectiveness of additional catheter-directed thrombolysis (CDT) compared with standard treatment alone. Bars indicate the range of incremental cost per additional QALY of additional CDT compared with standard treatment alone as determined in one-way sensitivity analyzes over plausible parameter ranges. Upper and lower value limits evaluated are indicated next to its respective bar. One-way sensitivity analysis was done for all model parameters, and the cost-effectiveness varied the most with the variables shown. The solid vertical axis represents the incremental cost-effectiveness ratio for the base case. The dotted line represents a willingness-to-pay threshold of $50 000 per QALY. All costs are per 6 months unless otherwise stated.
Varying the discount rate between 1.0% and 5.0% influenced the ICER from $8228 to $34 479 per QALY gained.
The additional cost of CDT treatment was the only treatment cost to influence the cost effectiveness and when kept at a minimum, that is corresponding to a 1-day procedure and hospital stay, the ICER was $7667/QALY.
Variation in the risk of CDT-related fatal or disabling complications showed a more moderate influence. If in their upper ranges, i.e. 1.4% suffered a fatal complication from CDT or 1.0% had a disabling bleeding, the ICERs remained < $40 000/QALY gained. Assuming fatal or disabling complications to be negligible (< 0.001), the ICERs were reduced with ~ $2 800/QALY gained compared with the base case. When increasing the risks for severe CDT complications beyond the ranges used in the sensitivity analyzes, a threshold analysis showed that if > 2.4% fatal complications or > 2.3% disabling bleedings, the effectiveness in terms of lifetime accumulated QALYs for the CDT strategy will be lower than for the standard treatment strategy. Finally, a two-way sensitivity analysis combining the risks for fatal and disabling CDT complications showed that the CDT strategy is more attractive in terms of net benefits when the WTP increases (Fig. 3).
Fig. 3.
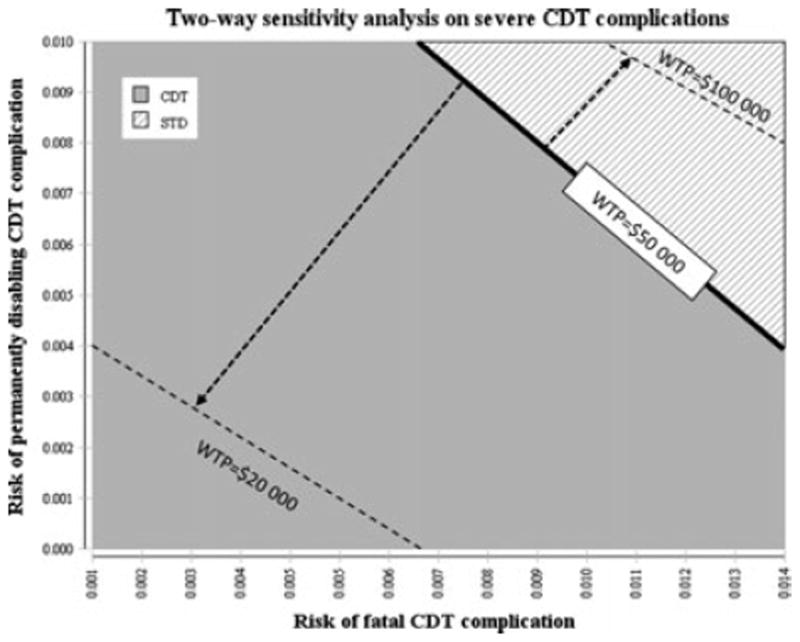
Two-way sensitivity analysis of severe catheter-directed thrombolysis(CDT) complication risks. Two-way sensitivity analysis of the risks of severe CDT complications comparing the net benefits (highest effectiveness and lowest costs) of the CDT strategy with the standard treatment strategy at a willingness-to-pay (WTP) threshold of $50 000/QALY gained. At high risks the standard treatment strategy yields the highest net benefits. At a lower WTP threshold ($20 000) the standard treatment strategy yields the highest net benefits also at low risks, whereas at a high WTP threshold ($100 000) the CDT strategy yields the highest net benefits at most risk values.
The model’s sensitivity to changes in utility was in a similar range; variations in the estimates for the health states post DVT and PTS kept the ICER < $37 000/QALY gained (Fig. 2). When increasing the utility for being in the PTS state beyond the 0.730–0.819 range used in the sensitivity analyzes, a threshold analysis showed that at utilities of 0.846 and 0.871 the CDT strategy would have an ICER of $50 000 and $100 000, respectively. At PTS utilities > 0.896 the CDT strategy would be dominated by the standard treatment strategy. The model was not sensitive to the remaining utilities.
Probabilistic sensitivity analyzes
Monte Carlo simulation of 10 000 samples estimated that the 95% CI for lifetime costs associated with additional CDT was $52 500–82 500 whereas the 95% CI for effectiveness was 25.4–43.4 QALYs. Corresponding intervals for the standard treatment strategy were $37 000–74 500 and 24.9–42.5 QALYs.
Varying all parameters simultaneously, the CDT strategy was cost effective in only 39% of simulations with a WTP of $20 000/QALY gained. For thresholds ≥ $24 500/QALY additional CDT was the strategy more likely to be cost effective (Fig. 4). The probability for the CDT strategy to be cost effective with a WTP of $50 000/QALY gained was 82%, and 93% when increasing the WTP to $100 000/QALY.
Fig. 4.
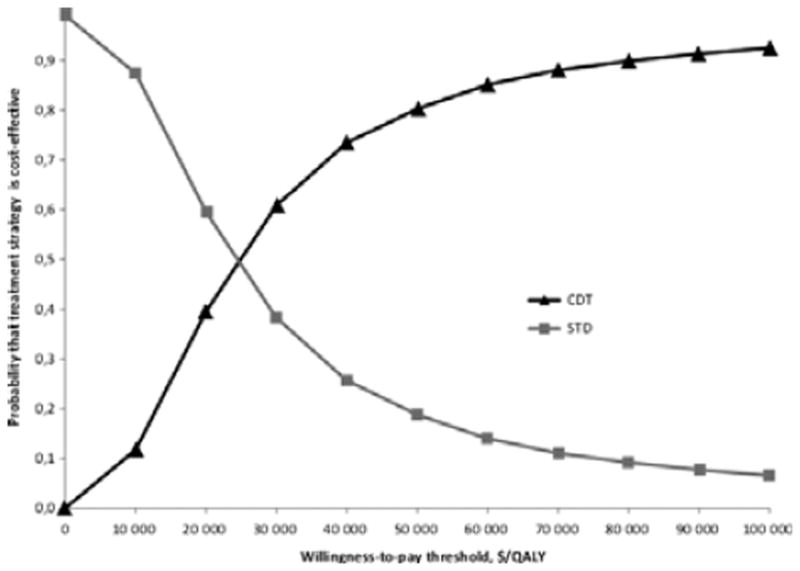
Cost-effectiveness acceptability curves for additional catheter-directed thrombolysis strategy and standard treatment strategy. The graph represents the probability that each treatment strategy is cost effective for a given willingness-to-pay threshold per QALY gained. The curves are based on 10 000 Monte Carlo simulations of the model with simultaneous draws of parameters from their respective probability distributions for each input.
Discussion
We demonstrated that after a high proximal DVT additional treatment with CDT is likely to be a cost-effective alternative to anticoagulation and compression therapy alone when applying a WTP threshold of $24 500 or higher. The base case analysis estimated a cost of $20 429/QALY gained with additional CDT, and this is within what has been considered cost-effective (http://www.euro.who.int) [37]. The cost effectiveness of CDT was sensitive to the variation in clinical efficacy of both treatment strategies, the discount rate, CDT-related costs and complication rates, and the utilities for the two most frequent outcomes: PTS and the asymptomatic post DVT state. However, when varying each variable within their plausible ranges and applying a WTP threshold of $50 000/QALY gained, the ICER remained below the threshold except for the $53 268/QALY associated with a high range incidence of PTS in the standard treatment strategy.
As VTE treatment data to some extent are abundant, some of the variables were modeled with more certainty, for example the estimates of complications related to anticoagulation and their consequences [24,25]. This does not apply to the literature on long-term clinically relevant outcomes such as PTS and severe PTS [38]. A prospective cohort study of 387 patients showed within-patient and between-patient variation in Villalta scores [4]. However, the proportions with mild, moderate and severe PTS remained unchanged during the 2 years of follow-up, and we did not include fluctuations in post-thrombotic symptoms in our model, only progression. To limit uncertainty and avoid possible overestimation from symptoms of the clinical presentation in the sub-acute phase of DVT overlapping the chronic manifestations of PTS, we assumed no PTS before 2 years [8,12,39]. The 5% increase in PTS during 2–8 years after the index DVT was observed in a cohort with mainly less extensive thrombosis and although our rates of PTS intuitively may seem high [40], they may even be under estimated. The model did not take into account that nearly half of recurrences may be ipsilateral and thereby associated with a relative PTS risk of 2.4 [4,21]. This simplification can be justified as the estimated mean PTS risks are likely to reflect the overall risk including any ipsilateral recurrences. A difference in recurrent VTE between the two treatment strategies has not been shown, but cannot be ruled out as residual thrombosis is associated with a reduced risk of recurrence [41,42] and CDT reduces such post-thrombotic changes [43,44]. A time-dependent risk of recurrence and age-dependent risk of bleeding were also left out of the model; however, it is unlikely that this should differ between the two strategies and have an impact on our analyzes. Although ongoing RCTs including the 5 years follow-up of CaVenT will provide further insight on clinical efficacy and may lead to changes in the model, it is unlikely that larger and longer-term trial data will be available in the future (NCT 00790335, NCT00970619, http://www.clinicaltrials.gov). Finally, as more aggressive endovascular approaches, including higher alteplase dose, frequent employment of stents and adjunctive thrombectomy, may improve the clinical efficacy of additional CDT [40], we performed a scenario analysis with a hypothetical improvement in the effect size of another 5% absolute risk reduction, corresponding to 36% PTS 2 years after CDT. This resulted in an ICER of $15 779/QALY gained (data not shown).
The cost data were obtained from various sources including the CaVenT study, hospital accounts, the literature and tariffs. The mean CDT-related costs are likely to be lower compared with contemporary pharmacomechanical CDT methods commonly used in the US and some other countries. Indeed a number of concerns have been identified regarding the transferability of economic evaluations between countries including methodological, healthcare system; hereunder absolute and relative prices, and population characteristics; so far no consensus has been established regarding these issues [45,46]. Our results are likely to be highly generalizable to Norway and countries with similar populations and health care systems, for example Scandinavian countries. While we encourage careful consideration of factors that affect the transferability of CEA results, we believe that the information provided in this analysis is likely to provide useful information for many settings.
Norwegian hospitals are funded by basic allocations (60%) and activity based funding (40%) and there is currently no hospital accounting system enabling the assessment of costs per patient or costs per stay or procedure. Hence, collection of complete individual resource use and medical cost data in our study by use of, for example health care log or patient diaries, would have been of great value. We quantified several types of utilization related to DVT and estimated the cost of these (Table 1). We did not observe total costs for trial patients, so it is possible that there were cost differences for other types of utilization that were not measured. Outpatient care for DVT is acceptable in sufficiently healthy patients and likely to reduce treatment costs, and the lower range for the standard strategy represents the estimated costs for outpatient care [47]. However, in our study setting the patients were admitted for a comprehensive evaluation and start of treatment, and the mean length of hospital stays is likely to reflect the overall severity of a high proximal DVT; on the other hand, it may not represent the most cost-effective standard care. Finally, the exclusion of indirect costs is a limitation to the interpretation and usefulness of our results.
Reports on the annual costs of PTS are restricted and variable [32], and we partly based our estimates on recent data from a Canadian cohort study with validated PTS assessment in a setting that is likely to be transferable because of a similar public health care system and demographics [5]. Our PTS cost estimates were somewhat higher and this may reflect the more extensive venous thrombosis; nonetheless, they were within the range reported in other previous studies [6,48].
Patient values and preferences for antithrombotic therapy after a DVT have been elicited in only a few studies and with different methods [20]. Most data indicate that the disutility from being on anticoagulation is small and may even be unchanged or increased as reflected in reporting of utilities equal to 1.0 and overlapping CIs with results for not taking anticoagulation [19,20]. This can be interpreted as a positive and reassuring effect from being on anticoagulation when you know you are at risk of thrombotic events [19]. To our knowledge, the utility for having PTS while on anticoagulation has not previously been reported. Among the patients in the CaVenT study who had developed PTS after 24 months, the EQ-5D score did not differ with regards to use of anticoagulation (P = 0.50) or the minimal important difference for the instrument [49] (data not shown), supporting our assumption that the same utility could be applied regardless of anticoagulation treatment or not. Correspondingly, the potential disutility from undergoing CDT, possibly combined with an improvement in early symptom release compared with standard treatment, was not assessed or included in the model. However, this is not likely to have a substantial impact on the results as this represents short-term changes in utilities, plus significant fluctuations beyond 6 months are not likely [50].
Finally, the cumulative incidences of approximately 5% fatal bleedings and recurrences, respectively, in this hypothetical cohort may seem high, especially when compared with the < 0.1% annual rates of fatal bleedings observed in the warfarin arms of recent RCTs [51,52]. It is not unlikely that the bleeding rates are lower among the participants in such studies compared with clinical practice, and with the lifetime horizon of our analyzes in mind it can be shown that the 10-years cumulative incidence of recurrent VTE of 40% (Prandoni) combined with the fatality rate of 3.6% extrapolated over four decades results in 6% experiencing a fatal VTE [24,28]. Beyond doubt is the fact that there is a small additional risk associated with thrombolysis in terms of bleeding and, as shown in our model, a small reduction in life expectancy. However this trade-off between clinical benefit and potentially fatal complications was considered in threshold analyses indicating that the CDT strategy can be preferred as long as the risks of fatal or disabling CDT complications are lower than 2.3%; which is well above our risk estimates used in the model. In addition, when further assessing these safety issues of CDT a two-ways sensitivity analysis confirmed that the CDT strategy is more attractive when WTP is higher. While we found that, on average, the risks were worth the improved quality of life, the potentially severe risks of additional CDT must be kept in mind when discussing treatment options with individual patients, and also in a wider setting where an economic evaluation is a part of the grounds for policy making.
In conclusion, we found that additional CDT is likely to be a cost-effective alternative to standard treatment alone. If results from ongoing studies confirm a clinical benefit as observed in the CaVenT study, this is likely to change current recommendations and strengthen our suggestion that CDT should be considered in patients with high proximal DVT and low risk of bleeding.
Acknowledgments
Financial support
South-Eastern Norway Regional Health Authority, Research Council of Norway, University of Oslo and Oslo University Hospital, and the Unger-Vetlesen Medical Fund.
We are grateful to Vinod Kumar and Jack Andersen for the assistance in collection of resource and cost data.
Footnotes
Disclosure of Conflict of Interests
The authors state that they have no conflict of interests.
Addendum
T. Enden wrote the main draft of the manuscript, performed the analyses, interpreted the data, constructed the model, collected the data, obtained funding and designed the original study. S. Resch interpreted the data, constructed the model and critically reviewed the manuscript. C. White interpreted the data, constructed the model and critically reviewed the manuscript. H. S. Wik collected the data and critically reviewed the manuscript. N. E. Kløw collected the data, designed the original study, obtained funding, and critically reviewed the manuscript. P. M. Sandset collected the data, designed the original study, obtained funding, and critically reviewed the manuscript.
References
- 1.Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, Nelson ME, Wells PS, Gould MK, Dentali F, Crowther M, Kahn SR American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e419S–94S. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prandoni P, Lensing AW, Prins MH, Frulla M, Marchiori A, Bernardi E, Tormene D, Mosena L, Pagnan A, Girolami A. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med. 2004;141:249–56. doi: 10.7326/0003-4819-141-4-200408170-00004. [DOI] [PubMed] [Google Scholar]
- 3.Brandjes DP, Büller HR, Heijboer H, Huisman MV, de Rijk M, Jagt H, ten Cate JW. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet. 1997;349:759–62. doi: 10.1016/S0140-6736(96)12215-7. [DOI] [PubMed] [Google Scholar]
- 4.Kahn SR, Shrier I, Julian JA, Ducruet T, Arsenault L, Miron MJ, Roussin A, Desmarais S, Joyal F, Kassis J, Solymoss S, Desjardins L, Lamping DL, Johri M, Ginsberg JS. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008;149:698–707. doi: 10.7326/0003-4819-149-10-200811180-00004. [DOI] [PubMed] [Google Scholar]
- 5.Guanella R, Ducruet T, Johri M, Miron MJ, Roussin A, Desmarais S, Joyal F, Kassis J, Solymoss S, Ginsberg JS, Lamping DL, Shrier I, Kahn SR. Economic burden and cost determinants of deep vein thrombosis during two years following diagnosis: a prospective evaluation. J Thromb Haemost. 2011;9:2397–405. doi: 10.1111/j.1538-7836.2011.04516.x. [DOI] [PubMed] [Google Scholar]
- 6.Bergqvist D, Jendteg S, Johansen L, Persson U, Odegaard K. Cost of long-term complications of deep venous thrombosis of the lower extremities: an analysis of a defined patient population in Sweden. Ann Intern Med. 1997;126:454–7. doi: 10.7326/0003-4819-126-6-199703150-00006. [DOI] [PubMed] [Google Scholar]
- 7.Kahn SR, Hirsch A, Shrier I. Effect of postthrombotic syndrome on health-related quality of life after deep venous thrombosis. Arch Intern Med. 2002;162:1144–8. doi: 10.1001/archinte.162.10.1144. [DOI] [PubMed] [Google Scholar]
- 8.Enden T, Haig Y, Kløw NE, Slagsvold CE, Sandvik L, Ghanima W, Hafsahl G, Holme PA, Holmen LO, Njaastad AM, Sandbæk G, Sandset PM CaVenT Study Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet. 2012;379:31–8. doi: 10.1016/S0140-6736(11)61753-4. [DOI] [PubMed] [Google Scholar]
- 9.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13:322–38. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 10.Enden T, Sandvik L, Kløw NE, Hafsahl G, Holme PA, Holmen LO, Ghanima W, Njaastad AM, Sandbaek G, Slagsvold CE, Sandset PM. Catheter-directed venous thrombolysis in acute iliofemoral vein thrombosis-the CaVenT Study: rationale and design of a multicenter, randomized, controlled, clinical trial ( NCT00251771) Am Heart J. 2007;154:808–14. doi: 10.1016/j.ahj.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Villalta S, Bagatella P, Piccioli A, Lensing AW, Prins MH, Prandoni P. Assessment of validity and reproducibility of a clinical scale for the postthrombotic syndrome. Haemostasis. 1994;24 (Suppl 1):157. [Google Scholar]
- 12.Kahn SR, Partsch H, Vedantham S, Prandoni P, Kearon C. Definition of post-thrombotic syndrome of the leg for use in clinical investigations: a recommendation for standardization. J Thromb Haemost. 2009;7:879–83. doi: 10.1111/j.1538-7836.2009.03294.x. [DOI] [PubMed] [Google Scholar]
- 13.de Jong PG, Coppens M, Middeldorp S. Duration of anticoagulant therapy for venous thromboembolism: balancing benefits and harms on the long term. Br J Haematol. 2012;158:433–41. doi: 10.1111/j.1365-2141.2012.09196.x. [DOI] [PubMed] [Google Scholar]
- 14.Freeman JV, Zhu RP, Owens DK, Garber AM, Hutton DW, Go AS, Wang PJ, Turakhia MP. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med. 2011;154:1–11. doi: 10.7326/0003-4819-154-1-201101040-00289. [DOI] [PubMed] [Google Scholar]
- 15.Fleurence RL, Hollenbeak CS. Rates and probabilities in economic modelling: transformation, translation and appropriate application. Pharmacoeconomics. 2007;25:3–6. doi: 10.2165/00019053-200725010-00002. [DOI] [PubMed] [Google Scholar]
- 16.Zia E, Engstrom G, Svensson PJ, Norrving B, Pessah-Rasmussen H. Three-year survival and stroke recurrence rates in patients with primary intracerebral hemorrhage. Stroke. 2009;40:3567–73. doi: 10.1161/STROKEAHA.109.556324. [DOI] [PubMed] [Google Scholar]
- 17.Marchetti M, Pistorio A, Barone M, Serafini S, Barosi G. Low-molecular-weight heparin versus warfarin for secondary prophylaxis of venous thromboembolism: a cost-effectiveness analysis. Am J Med. 2001;111:130–9. doi: 10.1016/s0002-9343(01)00793-8. [DOI] [PubMed] [Google Scholar]
- 18.Forgie MA, Wells P. Thesis/Dissertation. University of Ottawa Canada; 2001. Duration of oral anticoagulation in first-episode idiopathic deep vein thrombosis: A Markov decision analysis. [Google Scholar]
- 19.Locadia M, Bossuyt PM, Stalmeier PF, Sprangers MA, van Dongen CJ, Middeldorp S, Bank I, van der Meer J, Hamulyák K, Prins MH. Treatment of venous thromboembolism with vitamin K antagonists: patients’ health state valuations and treatment preferences. Thromb Haemost. 2004;92:1336–41. doi: 10.1160/TH04-02-0075. [DOI] [PubMed] [Google Scholar]
- 20.MacLean S, Mulla S, Akl EA, Jankowski M, Vandvik PO, Ebrahim S, McLeod S, Bhatnagar N, Guyatt GH American College of Chest Physicians. Patient values and preferences in decision making for antithrombotic therapy: a systematic review: Anti-thrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e1S–23S. doi: 10.1378/chest.11-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prandoni P, Villalta S, Bagatella P, Rossi L, Marchiori A, Piccioli A, Bernardi E, Girolami B, Simioni P, Girolami A. The clinical course of deep vein thrombosis. Prospective long-term follow-up of 528 symptomatic patients. Haematologica. 1997;82:423–8. [PubMed] [Google Scholar]
- 22.Ghanima W, Kleven IW, Enden T, Rosales A, Wik HS, Pederstad L, Holme PA, Sandset PM. Recurrent venous thrombosis, post-thrombotic syndrome and quality of life after catheter-directed thrombolysis in severe proximal deep vein thrombosis. J Thromb Haemost. 2011;9:1261–3. doi: 10.1111/j.1538-7836.2011.04298.x. [DOI] [PubMed] [Google Scholar]
- 23.Mewissen MW, Seabrook GR, Meissner MH, Cynamon J, Labropoulos N, Haughton SH. Catheter-directed thrombolysis for lower extremity deep venous thrombosis: report of a national multicenter registry. Radiology. 1999;211:39–49. doi: 10.1148/radiology.211.1.r99ap4739. [DOI] [PubMed] [Google Scholar]
- 24.Carrier M, le Gal, Wells PS, Rodger MA. Systematic review: case-fatality rates of recurrent venous thromboembolism and major bleeding events among patients treated for venous thromboembolism. Ann Intern Med. 2010;152:578–89. doi: 10.7326/0003-4819-152-9-201005040-00008. [DOI] [PubMed] [Google Scholar]
- 25.Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Ann Intern Med. 2003;139:893–900. doi: 10.7326/0003-4819-139-11-200312020-00007. [DOI] [PubMed] [Google Scholar]
- 26.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–76. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- 27.Ost D, Tepper J, Mihara H, Lander O, Heinzer R, Fein A. Duration of anticoagulation following venous thromboembolism: a meta-analysis. JAMA. 2005;294:706–15. doi: 10.1001/jama.294.6.706. [DOI] [PubMed] [Google Scholar]
- 28.Prandoni P, Noventa F, Ghirarduzzi A, Pengo V, Bernardi E, Pesavento R, Iotti M, Tormene D, Simioni P, Pagnan A. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica. 2007;92:199–205. doi: 10.3324/haematol.10516. [DOI] [PubMed] [Google Scholar]
- 29.Flinterman LE, van Hylckama Vlieg A, Cannegieter SC, Rosendaal FR. Long-term survival in a large cohort of patients with venous thrombosis: incidence and predictors. PLoS Med. 2012;9:e1001155. doi: 10.1371/journal.pmed.1001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wittrup-Jensen KU, Lauridsen J, Gudex C, Pedersen KM. Generation of a Danish TTO value set for EQ-5D health states. Scand J Public Health. 2009;37:459–66. doi: 10.1177/1403494809105287. [DOI] [PubMed] [Google Scholar]
- 31.Michaels JA, Campbell WB, King BM, Macintyre J, Palfreyman SJ, Shackley P, Stevenson MD. A prospective randomised controlled trial and economic modelling of antimicrobial silver dressings versus non-adherent control dressings for venous leg ulcers: the VULCAN trial. Health Technol Assess. 2009;13:1–114. iii. doi: 10.3310/hta13560. [DOI] [PubMed] [Google Scholar]
- 32.Prandoni P. Healthcare burden associated with the post-thrombotic syndrome and potential impact of the new oral anticoagulants. Eur J Haematol. 2012;88:185–94. doi: 10.1111/j.1600-0609.2011.01733.x. [DOI] [PubMed] [Google Scholar]
- 33.Caprini JA, Botteman MF, Stephens JM, Nadipelli V, Ewing MM, Brandt S, Pashos CL, Cohen AT. Economic burden of long-term complications of deep vein thrombosis after total hip replacement surgery in the United States. Value Health. 2003;6:59–74. doi: 10.1046/j.1524-4733.2003.00204.x. [DOI] [PubMed] [Google Scholar]
- 34.Ramacciotti E, Gomes M, de Aguiar ET, Caiafa JS, de Moura LK, Araújo GR, Truzzi A, Dietrich-Neto F CLE-PTS Investigators. A cost analysis of the treatment of patients with post-thrombotic syndrome in Brazil. Thromb Res. 2006;118:699–704. doi: 10.1016/j.thromres.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Fjaertoft H, Indredavik B, Magnussen J, Johnsen R. Early supported discharge for stroke patients improves clinical outcome. Does it also reduce use of health services and costs? One-year follow-up of a randomized controlled trial. Cerebrovasc Dis. 2005;19:376–83. doi: 10.1159/000085543. [DOI] [PubMed] [Google Scholar]
- 36.Wisløff T, Selmer RM, Halvorsen S, Kristiansen IS. Norwegian Cardiovascular Disease Model (NorCaD) - a simulation model for estimating health benefits and cost consequences of cardiovascular interventions. Vol. 23. The Norwegian Knowledge Centre for the Health Services; Oslo: 2008. [Google Scholar]
- 37.Weinstein MC. How much are Americans willing to pay for a quality-adjusted life year? Med Care. 2008;46:343–5. doi: 10.1097/MLR.0b013e31816a7144. [DOI] [PubMed] [Google Scholar]
- 38.Schulman S. Getting intimate with the venous thrombus. J Thromb Haemost. 2009;7:1266–7. doi: 10.1111/j.1538-7836.2009.03484.x. [DOI] [PubMed] [Google Scholar]
- 39.Vedantham S, Grassi CJ, Ferral H, Patel NH, Thorpe PE, Antonacci VP, Janne d’Othée BM, Hofmann LV, Cardella JF, Kundu S, Lewis CA, Schwartzberg MS, Min RJ, Sacks D Technology Assessment Committee of the Society of Interventional Radiology. Reporting standards for endovascular treatment of lower extremity deep vein thrombosis. J Vasc Interv Radiol. 2006;17:417–34. doi: 10.1097/01.RVI.0000197359.26571.c2. [DOI] [PubMed] [Google Scholar]
- 40.Hofmann LV, Kuo WT. Catheter-directed thrombolysis for acute DVT. Lancet. 2012;379:3–4. doi: 10.1016/S0140-6736(11)61875-8. [DOI] [PubMed] [Google Scholar]
- 41.Prandoni P, Lensing AW, Prins MH, Bernardi E, Marchiori A, Bagatella P, Frulla M, Mosena L, Tormene D, Piccioli A, Simioni P, Girolami A. Residual venous thrombosis as a predictive factor of recurrent venous thromboembolism. Ann Intern Med. 2002;137:955–60. doi: 10.7326/0003-4819-137-12-200212170-00008. [DOI] [PubMed] [Google Scholar]
- 42.Prandoni P, Prins MH, Lensing AW, Ghirarduzzi A, Ageno W, Imberti D, Scannapieco G, Ambrosio GB, Pesavento R, Cuppini S, Quintavalla R, Agnelli G AESOPUS Investigators. Residual thrombosis on ultrasonography to guide the duration of anticoagulation in patients with deep venous thrombosis: a randomized trial. Ann Intern Med. 2009;150:577–85. doi: 10.7326/0003-4819-150-9-200905050-00003. [DOI] [PubMed] [Google Scholar]
- 43.Elsharawy M, Elzayat E. Early results of thrombolysis vs anticoagulation in iliofemoral venous thrombosis. A randomised clinical trial. Eur J Vasc Endovasc Surg. 2002;24:209–14. doi: 10.1053/ejvs.2002.1665. [DOI] [PubMed] [Google Scholar]
- 44.Enden T, Kløw NE, Sandvik L, Slagsvold CE, Ghanima W, Hafsahl G, Holme PA, Holmen LO, Njaastad AM, Sandbaek G, Sandset PM CaVenT study group. Catheter-directed thrombolysis vs. anticoagulant therapy alone in deep vein thrombosis: results of an open randomized, controlled trial reporting on short-term patency. J Thromb Haemost. 2009;7:1268–75. doi: 10.1111/j.1538-7836.2009.03464.x. [DOI] [PubMed] [Google Scholar]
- 45.Welte R, Feenstra T, Jager H, Leidl R. A decision chart for assessing and improving the transferability of economic evaluation results between countries. Pharmacoeconomics. 2004;22:857–76. doi: 10.2165/00019053-200422130-00004. [DOI] [PubMed] [Google Scholar]
- 46.Goeree R, He J, O’Reilly D, Tarride JE, Xie F, Lim M, Burke N. Transferability of health technology assessments and economic evaluations: a systematic review of approaches for assessment and application. Clinicoecon Outcomes Res. 2011;3:89–104. doi: 10.2147/CEOR.S14404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holbrook A, Schulman S, Witt DM, Vandvik PO, Fish J, Kovacs MJ, Svensson PJ, Veenstra DL, Crowther M, Guyatt GH American College of Chest Physicians. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e152S–84S. doi: 10.1378/chest.11-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MacDougall DA, Feliu AL, Boccuzzi SJ, Lin J. Economic burden of deep vein thrombosis, pulmonary embolism, and post-thrombotic syndrome. Am J Health Syst Pharm. 2006;63:S5–15. doi: 10.2146/ajhp060388. [DOI] [PubMed] [Google Scholar]
- 49.Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res. 2005;14:1523–32. doi: 10.1007/s11136-004-7713-0. [DOI] [PubMed] [Google Scholar]
- 50.Kahn SR, Shbaklo H, Lamping DL, Holcroft CA, Shrier I, Miron MJ, Roussin A, Desmarais S, Joyal F, Kassis J, Solymoss S, Desjardins L, Johri M, Ginsberg JS. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost. 2008;6:1105–12. doi: 10.1111/j.1538-7836.2008.03002.x. [DOI] [PubMed] [Google Scholar]
- 51.Büller HR, Prins MH, Lensin AW, Decousus H, Jacobson BF, Minar E, Chlumsky J, Verhamme P, Wells P, Agnelli G, Cohen A, Berkowitz SD, Bounameaux H, Davidson BL, Misselwitz F, Gallus AS, Raskob GE, Schellong S, Segers A EINSTEIN–PE Investigators. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287–97. doi: 10.1056/NEJMoa1113572. [DOI] [PubMed] [Google Scholar]
- 52.Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, Lensing AW, Misselwitz F, Prins MH, Raskob GE, Segers A, Verhamme P, Wells P, Agnelli G, Bounameaux H, Cohen A, Davidson BL, Piovella F, Schellong S EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- 53.Post PN, Stiggelbout AM, Wakker PP. The utility of health states after stroke: a systematic review of the literature. Stroke. 2001;32:1425–9. doi: 10.1161/01.str.32.6.1425. [DOI] [PubMed] [Google Scholar]
- 54.Dorman P, Dennis M, Sandercock P. Are the modified “simple questions” a valid and reliable measure of health related quality of life after stroke? United Kingdom Collaborators in the International Stroke Trial. J Neurol Neurosurg Psychiatry. 2000;69:487–93. doi: 10.1136/jnnp.69.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fjaertoft H, Indredavik B. Cost-estimates for stroke. Tidsskr Nor Laegeforen. 2007;127:744–7. [PubMed] [Google Scholar]
- 56.Tan-Torres Edejer T. Making Choices in Health: WHO Guide to Cost-effectiveness Analysis. Geneva: World Health Organization; 2003. [Google Scholar]
- 57.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]


