Abstract
Ultrasound imaging is a versatile modality frequently used in clinical medicine, most likely due to its low cost, low risk to patients, and the ability to provide images in real time. Ultrasound used typically in clinical settings has frequencies between 2 and 12 MHz. Lower frequencies produce greater resolution but are limited in depth penetration; higher frequencies produce greater resolution, but depth of penetration is limited. High-frequency ultrasound (HFUS) shows promise for detection of certain changes in the skin and this has implications for early detection of changes associated with pressure ulcer formation and wound healing. The purpose of this article was to provide an overview of where HFUS has been used with the skin and provide some discussion on its utility with detecting skin changes related to pressure.
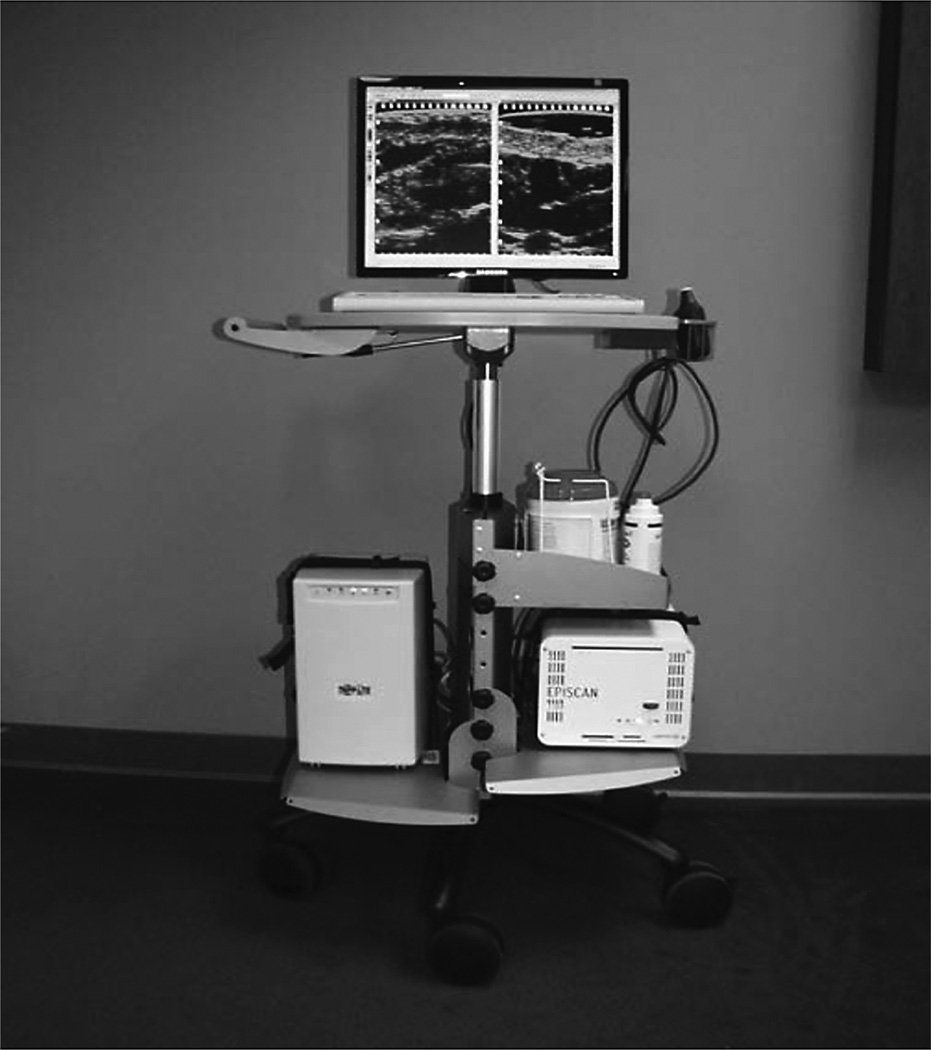
Ultrasound imaging has become one of the most versatile and frequent imaging modalities used in clinical medicine, most likely due to its low cost, unlimited repeatability with high diagnostic value, relative low risk to the patient, and its ability to provide images in real time. Ultrasound units are typically portable and allow for bedside evaluation (Figure 1). Ultrasound imaging is based on sonar principles developed during World War I for use in ships at sea. As sound passes through the body, “echoes” are produced and are picked up by the transducer, which then recognizes the distance, size, and shape of inside structures. Ultrasound waves have varying frequencies. Frequency is the number of vibration cycles that occur in 1 s. Lower frequencies have larger wavelengths, and higher frequencies have smaller wavelengths.
FIGURE 1.
A high-frequency unit on a mobile cart for easy transport throughout patient care areas.
Diagnostic sonography (also ultrasonography or ultrasound echograpy) is an ultrasound-based diagnostic imaging technique used for visualizing subcutaneous body structures to identify possible pathologies. Ultrasound sound waves have frequencies above those audible to the human ear, that is, greater than approximately 20 MHz. Ultrasound typically used in clinical settings has frequencies between 2 and 12 MHz. Lower frequencies produce less resolution but have greater depth of penetration into the body; higher frequencies produce greater resolution but depth of penetration is limited. High-frequency ultrasound (HFUS) with its higher resolution is a useful tool in many specialties, which focus on skin evaluations and skin disorders.
High-frequency ultrasound with frequencies of more than 20 MHz for the examination of human skin is a fully developed technique offering a wide range of possibilities both clinically and experimentally. High-resolution ultrasound imaging systems (ultrasound frequencies above 15–20 MHz) enable ultrasound to differentiate structures of less than 100 microns on the beam axis (depth) and 200 microns on the scan axis (lateral resolution). The purpose of this study is to review the current applications for HFUS as it relates to skin and subcutaneous soft tissue and to discuss its potential applications in the early identification of pressure ulcer (PU) formation.
HFUS AND HUMAN TISSUE
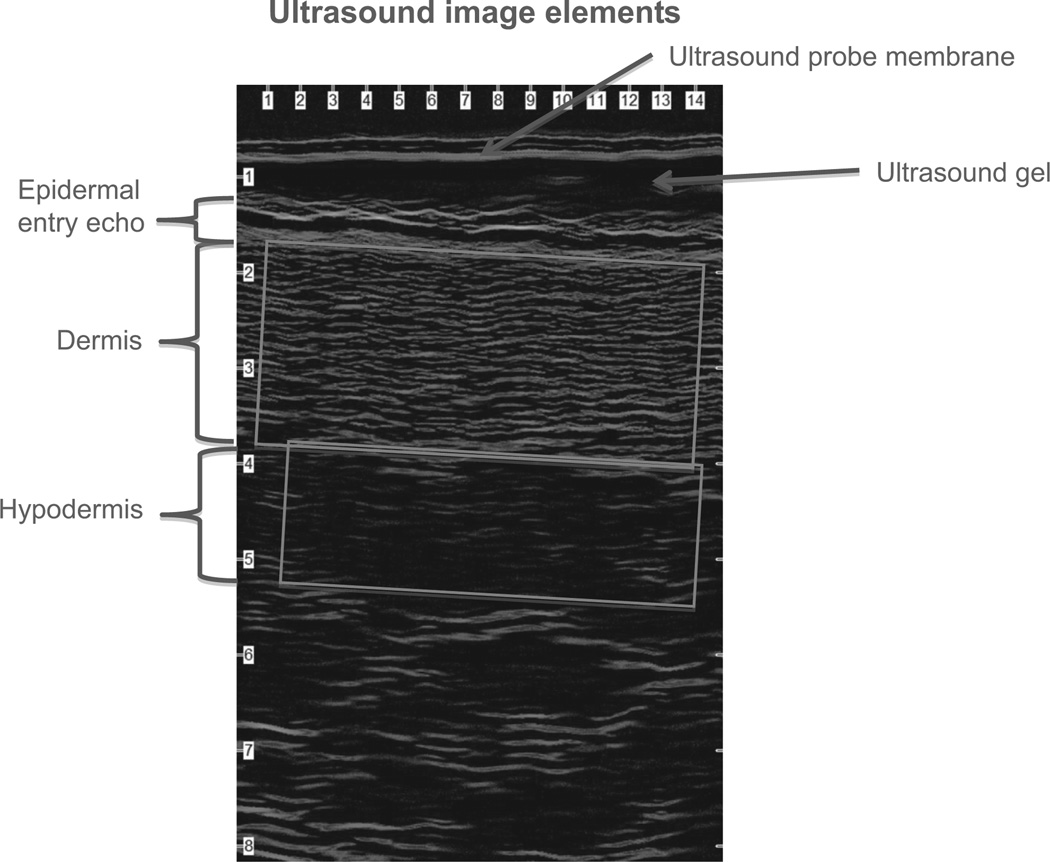
Acoustic energy in the form of ultrasonic sound waves is generated from a transducer as a mechanical vibration. These vibrations are transmitted into the tissues being examined and propagate in the form of an ultrasonic wave that passes easily through fluids and soft tissues. When the wave encounters an interface or a border between two tissues that conduct sound differently, some of the sound waves are reflected back to the transducer, creating an echo (Jasaitiene et al., 2010). Reflected waves captured by the transducer are amplified electronically and displayed on a monitor using shades of gray (from black to white) or colors (spectrum) (Figure 2). Tissue properties play a role in ultrasound processes because sound travels at different speeds through different tissue densities. Ultrasound velocity in human tissues is increased with low fluid content and decreased with higher fluid content-–higher fluid content is associated with a decrease of echogenicity. Therefore, low echogenicity may indicate tissue with higher fluid content reflecting fluid infiltration that may be associated with inflammation.
FIGURE 2.
Typical waveform image generated from a high-frequency ultrasound unit. Note density changes among layers.
HFUS IN DERMATOLOGY, PLASTIC SURGERY, AND WOUND HEALING
The use of HFUS in dermatological practice is well documented. Dermatology residency programs are now incorporating HFUS into physician training. In some parts of the world, physicians cannot become board certified in dermatology unless they have had formal training and have completed the required number of scans on skin lesions (Dill-Muller & Maschke, 2007). In inflammatory or fibrosing diseases, HFUS has been used to determine skin layer morphology including changes in epidermal thickness (Mari & Cachard, 2007).
Preoperative assessment of skin cancers by noninvasive HFUS may assist in determining surgical margins and decrease the need for reexcision and thus may be of benefit in planning surgery by plastic surgeons and dermatologists. Machet et al. (2009) found that HFUS predicted appropriate margins (1, 2, or 3 cm wide) in 26 of 31 subjects with cutaneous melanoma. Desai, Desai, Horowitz, Kartono, and Wahl (2007) showed that HFUS delineated tumor margins in patients with superficial and nodular basal cell carcinomas (BCCs) of various locations. The researchers scanned 50 BCCs preoperatively and found that 45 of the lesions were cleared with the recommended 4-mm margin based on HFUS results. The 5 lesions (10%) that were not clear at the recommended 4-mm margins were located in the high-risk facial “H” zone, the area of the face including the eyes, ears, and nose. Of those 5, HFUS showed one superficial and two nodular BCCs extending past the margins. By adhering to the 3- to 4-mm margin recommendations (Nguyen & Ho, 2002; Thissen, Neumann, & Schouten, 2002), complete excision was not achieved. In addition, the three lesions were more aggressive forms of BCC, morpheaform and infiltrative BCC, extended beyond the margins, and were more ominous on ultrasound. Clinical diagnosis did not correlate with histology (Desai et al., 2007). Using HFUS to guide their treatment plan may have yielded a wider surgical excision or referral to a surgical specialist for more complete excision.
Bessonart, Maredo, and Carmona (2005) used HFUS to determine the features of hypertrophic scars. On the basis of their findings, they were able to develop reliable assessment methods to evaluate the effectiveness of different therapeutic approaches. They found that the scars provided an image that clearly demarcated it from the surrounding normal tissue and concluded that HFUS is a suitable objective and noninvasive method for discriminating healthy skin from pathologic skin in hypertrophic scars and keloids.
Photography, computed tomography, and magnetic resonance imaging have all been used for evaluating dermal pathology and wound healing, but these methods have well-known disadvantages. Photography may not reveal deeper level cutaneous damage. Both computed tomography and magnetic resonance imaging are expensive, and obtaining results in real time is usually not feasible. Other undesirable features include radiation exposure, injected dyes, magnetic fields, and discomfort due to claustrophobia. High-frequency ultrasound may provide a less-invasive means to evaluate dermal anatomy and pathology.
Studies by Kuhn and Angehrn (2009) have supported the use of HFUS for quantitative skin assessment. They demonstrated the utility of using HFUS to obtain noninvasive, objective, quantitative measurements of wound healing instead of relying only on visual inspection in 22 patients with leg ulcers. Fourteen of 18 patients whose ulcers had been evaluated as clinically healed by visual inspection showed large subepidermal deficits of elastic and collagenous fibers using HFUS demonstrating that visual inspection does not fully indicate if normal skin function has been restored within all three layers.
Young, Hampton, and Martin (2013) used HFUS to evaluate periwound edema and granulation tissue formation in PUs and the surrounding tissue. High-frequency ultrasound showed a reduction in periwound edema in all patients after initiating negative pressure wound therapy with levels falling to a mean of 43% after 4 days and by 14 days tissue fluid content was similar to that seen in uninjured tissue within the same patients. Wound healing was measured in the base of the wound, noninvasively measuring the depth of tissue over the fixed bony prominences where the ulcers had originated. A 20% increase in wound bed thickness was observed in as little as 7 days.
Dyson et al. (2003) compared photography and HFUS in human punch biopsy wounds. Wound healing was assessed on several postoperative days by using the scanner’s calibrated linear measurement tool compared with photographs. They concluded that HFUS scanning permitted the quantitative assessment of structural changes deep in the wound in contrast to photography allowing only superficial assessment. In addition, Alexander and Miller (1979) compared radiography to HFUS systems in evaluating full-skin thickness. They found that HFUS echoes could distinguish skin, subcutaneous fat and muscle, and the thickness of the skin could be accurately measured.
A comparison of HFUS assessment of skin and wound tissue with histology was performed by Rippon, Springett, Walmsley, Patrick, and Millson (1998), who visualized structures of skin (human cadaver and porcine) and healing wounds (porcine) using 20 MHz HFUS and compared them with histology from the same site. They found excellent correlation between ultrasound measurements and histology for porcine and human cadaver measurements as well as acute porcine wounds. The study demonstrated that HFUS and histology were comparable in identifying wound depth, eschar/blood clot depth, collagen accumulation, and granulation tissue depth. Rippon, Springett, and Walmsley (1999) compared HFUS to histology in evaluating acute full-thickness leg wounds in patients with amputated lower limb tissue associated with chronic wounds. High-frequency ultrasound allowed visualization of chronic wounds in patients with leg ulcers and showed that differences between scans of healing and nonhealing wounds could be identified. The authors suggested that HFUS could allow investigation of deep tissues without tissue biopsy.
Assessment and comparison of dermal water by HFUS and nuclear magnetic resonance were evaluated by Gniadecka and Quistorff (1996) using both healthy forearm skin on volunteers and acute dermal edema (histamine weals). Dermal echogenicity was determined by counting HFUS image low echogenic pixels as compared with nuclear magnetic resonance spectra using water-fat specific peaks to measure relative water content. They concluded that HFUS is a sensitive method for assessment of changes in dermal hydration and may be important in comparative evaluations of dermal water in skin pathologies associated with edema formation.
High-frequency ultrasound has also been used in obtaining skin thickness measurements, visualizing skin structure, measuring psoriatic plaques, evaluating the effect of drugs, investigating burn depth in animal models, and monitoring healing in acute and chronic wounds (Alexander & Miller, 1979; Brink et al., 1986; Dyson et al., 2003; Hermann, Ellis, Fitting, Ho, & Voorhees, 1988; Milner, Memar, Gherardini, Bennett, & Phillips, 1997; Olsen, Takiwaki, & Serup, 1995; Schiavi, Belletti, & Seidenari, 1996).
HFUS AND PRESSURE ULCERS DETECTION
The use of HFUS in acute care to detect early changes in the epidermis or dermis over a bony prominence has significant clinical relevance. A PU is a localized injury to the skin and/or underlying tissue usually over a bony prominence, as a result of pressure, or pressure in combination with shear (U.S. Agency for Healthcare Research and Quality, 2009). Early identification of a PU and subsequent initiation of evidence-based interventions could halt progression of the injury.
Pressure ulcer prevention and early identification have escalated to become a priority in acute care, long-term care, and home health. In 2006, the President signed the Deficit Reduction Act 2005, requiring Congress to reduce health care costs by identifying conditions that are high costs and/or high volume and could reasonably be prevented through the application of evidence-based guidelines. Stage III and IV PU were identified as one of the initial conditions. As a result, since October 2008, Centers for Medicare & Medicaid Services no longer reimburses health care facilities for any PU identified during the hospital course that was not documented as present on admission. Therefore, acute care facilities have increased vigilance to improve PU prevention.
Estimates of PU prevalence range from 0.4% to 38% in acute care, 2% to 24% in long-term care, and 0% to 17% in home care settings (VanGilder, Amlung, Harrison, & Meyer, 2009). U.S. acute care facilities treat approximately 2.5 million patients with PU per year, costing approximately $11 billion annually (U.S. Agency for Healthcare Research and Quality, 2009; VanGilder et al., 2009). The primary responsibility for PU prevention falls on the bedside clinician. Changes in the dermis, such as collagen fiber flattening, may be a heralding predictor of PU development prior to visual evidence in the epidermis. It is hypothesized that in a deep tissue injury PU, cutaneous manifestation of pressure-related skin injury can occur from 48 hours to 7 days following the actual event that caused the injury. Destruction at the bone muscle interface is not initially visible. The use of a diagnostic tool such as HFUS that detects impaired changes in the dermis and supplements daily visual skin assessments may be beneficial to prevent PU occurrence.
Several studies have evaluated the usefulness of HFUS to detect occult tissue injury. Quintavalle Lyder, Mertz, Phillips-Jones, and Dyson (2006) investigated the utility of using HFUS to describe the pathogenesis of PUs. High-frequency ultrasound images of 119 long-term care facility residents determined to be at risk for PU development (Braden score ≤ 18) were compared with images from 15 healthy volunteers. Images were classified as not readable, normal, or not normal. More than half (53%) of the images obtained from the long-term care residents were different from the control healthy volunteers and these images showed patterns indicative of fluid or edema within the dermal and subdermal tissues. More importantly, the presence of edema in the subdermal tissues, where PUs are believed to begin, was found in 79.3% of individuals before clinical signs were present on the skin.
Helvig and Nichols (2012) evaluated 100 geriatric patients with Braden Scale scores of 10–17 (indicating PU risk) who had remained hospitalized for 28 days or less to compare rates of visualized PUs with the prevalence of occult injury on heels and found that HFUS detected more occult heel injury than visual assessment alone. While ultrasound does appear to reveal changes and possible injury to tissues that cannot be visualized at the bedside, the authors emphasized that the exact meaning of these changes is still difficult to interpret and needs further.
Noninvasive characterization of tissue and wounds is important in clinical practice. Andersen and Karlsmark (2008) examined 15 PUs stages 0–IV, using four different noninvasive techniques, including HFUS. Measurements were made at the ulcer, 5 cm from the ulcer, and at a reference location of skin without ulcers. A hypoechogenic layer believed to represent edema in the tissues was found in or near all the PUs but was not seen in the control reference skin without ulceration. They believed that the subepidermal layer found on ultrasound images might be an indication of the pressure that the skin was subjected to rather than the severity of the actual pressure ulceration and that HFUS might be helpful in predicting whether the skin is at risk of developing PUs.
CONCLUSION
High-frequency ultrasound shows promise in various clinical and research settings, providing a safe, noninvasive, inexpensive, reproducible means to evaluating skin integrity and pathology. However, there is a scarcity of information regarding several aspects of HFUS. The time continuum for deeper cutaneous injury development, variability of quality of images possibly affecting assessment of images, and lack of data with respect to body site hinders more widespread use of this technology. Future studies should explore the possible changes in scans over time to evaluate the progression of tissue injury with regard to pressure and friction.
Some studies have found variability in scan quality and difficulty in interpretation of scans for a variety of reasons (Helvig & Nichols, 2012; Moghimi, Miran Baygi, Torkaman, & Mahloojifar 2010; Quintavalle et al., 2006). More work is needed to evaluate the effect of body site, body mass index, and body position of patients while scanning. Work done by Helvig and Nichols (2012) and Quintavalle et al. (2006) emphasizes that abnormalities noted in high-frequency scans may not necessarily correspond to actual tissue changes or abnormalities. Additional study to obtain baseline normal scans of healthy tissue may help differentiate normal features from tissue with injury (Helvig & Nichols, 2012).
Presently, there are no clinical guidelines for frequency of scanning or recommendations regarding the subset of patients who would benefit. In the acute care setting, time constraints suggest that scanning every patient would not be efficient. However, information concerning the most optimal scanning time frame, setting, and frequency for HFUS evaluation is needed. With additional data, HFUS could become widely used and integral to preventing, identifying, and treating a wide variety of dermatological problems.
Biographies
Valentina S. Lucas, PhD, RN, ANP-BC, is a nurse practitioner in the Division of Plastic and Reconstructive Surgery at Virginia Commonwealth University Health System. She was an investigator for the NIH-funded (R01 NR010381) study “Effect of Backrest Elevation on Skin Integrity in the Critically Ill,” which included the use of high-frequency ultrasound technology.
Ruth S. Burk, PhD, RN, is Assistant Professor at University of Texas School of Nursing at Houston. She was a doctoral research assistant for the NIH-funded (R01 NR010381) study “Effect of Backrest Elevation on Skin Integrity in the Critically Ill,” which used the high-frequency ultrasound technology described here.
Sue Creehan, BSN, RN, CWON, is Certified Wound Ostomy Nurse, the Program Manager for the wound care team at VCU Medical Center, the organizational champion for their interdisciplinary hospital-acquired pressure ulcer reduction program, and chair of the unit-based champions of skin integrity team.
Mary Jo Grap, PhD, RN, FAAN, is the Nursing Alumni Distinguished Professor at Virginia Commonwealth University School of Nursing. She is the Principal Investigator for the NIH-funded (R01 NR010381) study “Effect of Backrest Elevation on Skin Integrity in the Critically Ill” that included the use of the high-frequency ultrasound technology described here.
Footnotes
The authors report no conflicts of interest.
REFERENCES
- Alexander H, Miller DL. Determining skin thickness with pulsed ultra sound. Journal of Investigative Dermatology. 1979;72:17–19. doi: 10.1111/1523-1747.ep12530104. [DOI] [PubMed] [Google Scholar]
- Andersen ES, Karlsmark T. Evaluation of four non-invasive methods for examination and characterization of pressure ulcers. Skin Research and Technology. 2008;14:270–276. doi: 10.1111/j.1600-0846.2008.00290.x. [DOI] [PubMed] [Google Scholar]
- Bessonart MN, Macedo N, Carmona C. High resolution B-scan ultrasound of hypertrophic scars. Skin Research and Technology. 2005;11:185–188. doi: 10.1111/j.1600-0846.2005.00118.x. [DOI] [PubMed] [Google Scholar]
- Brink JA, Sheets PW, Dines KA, Etchison MR, Hanke CW, Sadove AM. Quantitative assessment of burn injury in porcine skin with high-frequency ultrasonic imaging. Investigative Radiology. 1986;21:645–651. doi: 10.1097/00004424-198608000-00008. [DOI] [PubMed] [Google Scholar]
- Desai TD, Desai AD, Horowitz DC, Kartono F, Wahl T. The use of high-frequency ultrasound in the evaluation of superficial and nodular basal cell carcinomas. Dermatologic Surgery. 2007;33:1220–1227. doi: 10.1111/j.1524-4725.2007.33257.x. [DOI] [PubMed] [Google Scholar]
- Dill-Muller D, Maschke J. Ultrasonography in dermatology. Journal der Deutschen Dermatologischen Gesellschaft. 2007;5(8):689–707. doi: 10.1111/j.1610-0387.2007.06453.x. [DOI] [PubMed] [Google Scholar]
- Dyson M, Moodley S, Verjee L, Verling W, Weinman J, Wilson P. Wound healing assessment using 20 MHz ultrasound and photography. Skin Research and Technology. 2003;9:116–121. doi: 10.1034/j.1600-0846.2003.00020.x. [DOI] [PubMed] [Google Scholar]
- Gniadecka M, Quistorff B. Assessment of dermal water by high-frequency ultrasound: Comparative studies with nuclear magnetic resonance. British Journal of Dermatology. 1996;135:218–224. [PubMed] [Google Scholar]
- Helvig EI, Nichols LW. Use of high-frequency ultrasound to detect heel pressure injury in elders. Journal of Wound, Ostomy and Continence Nursing. 2012;39:500–508. doi: 10.1097/WON.0b013e3182652648. [DOI] [PubMed] [Google Scholar]
- Hermann RC, Ellis CN, Fitting DW, Ho VC, Voorhees JJ. Measurement of epidermal thickness in normal skin and psoriasis with high-frequency ultrasound. Skin Pharmacology. 1988;1:128–136. doi: 10.1159/000210760. [DOI] [PubMed] [Google Scholar]
- Jasaitiene D, Valiukeviciene S, Linkeviciute G, Raisutis R, Jasiuniene E, Kazys R. Principles of high-frequency ultrasonography for investigation of skin pathology. Journal of the European Academy of Dermatology and Venereology. 2010;25:375–382. doi: 10.1111/j.1468-3083.2010.03837.x. [DOI] [PubMed] [Google Scholar]
- Kuhn C, Angehrn F. Use of high-resolution ultrasound to monitor the healing of leg ulcers: A prospective single-center study. Skin Research and Technology. 2009;15:161–167. doi: 10.1111/j.1600-0846.2008.00342.x. [DOI] [PubMed] [Google Scholar]
- Machet L, Belot V, Naouri M, Boka M, Mourtada Y, Giraudeau B, Vaillant L. Preoperative measurement of thickness of cutaneous melanoma using high-resolution 20 MHz ultrasound imaging: A monocenter prospective study and systematic review of the literature. Ultrasound in Medicine & Biology. 2009;35:1411–1420. doi: 10.1016/j.ultrasmedbio.2009.03.018. [DOI] [PubMed] [Google Scholar]
- Mari JM, Cachard C. Acquire real time RF digital ultrasound data from a commercial scanner. Electronic Journal “Technical Acoustics”. 2007 http://www.ejta.org [serial online] 2007, 3. [Google Scholar]
- Milner SM, Memar OM, Gherardini G, Bennett JC, Phillips LG. The histological interpretation of high frequency cutaneous ultrasound imaging. Dermatologic Surgery. 1997;23:43–45. doi: 10.1111/j.1524-4725.1997.tb00006.x. [DOI] [PubMed] [Google Scholar]
- Moghimi S, Miran Baygi MH, Torkaman G, Mahloojifar A. Quantitative assessment of pressure sore generation and healing through numerical analysis of high-frequency ultrasound images. Journal of Rehabilitation Research and Development. 2010;47:99–108. doi: 10.1682/jrrd.2009.04.0045. [DOI] [PubMed] [Google Scholar]
- Nguyen TH, Ho DQ. Nonmelanoma skin cancer. Current Treatment Options in Oncology. 2002;3:193–203. doi: 10.1007/s11864-002-0009-0. [DOI] [PubMed] [Google Scholar]
- Olsen LO, Takiwaki H, Serup J. High frequency ultrasound characterisation of normal skin. Skin thickness and echographic density of 22 anatomical sites. Skin Research and Technology. 1995;1:74–80. doi: 10.1111/j.1600-0846.1995.tb00021.x. [DOI] [PubMed] [Google Scholar]
- Quintavalle PR, Lyder CH, Mertz PJ, Phillips-Jones C, Dyson M. Use of high-resolution, high-frequency diagnostic ultrasound to investigate the pathogenesis of pressure ulcer development. Advances in Skin & Wound Care. 2006;19:498–505. doi: 10.1097/00129334-200611000-00010. [DOI] [PubMed] [Google Scholar]
- Rippon MG, Springett K, Walmsley R, Patrick K, Millson S. Ultrasound assessment of skin and wound tissue: Comparison with histology. Skin Research and Technology. 1998;4:147–154. doi: 10.1111/j.1600-0846.1998.tb00101.x. [DOI] [PubMed] [Google Scholar]
- Rippon MG, Springett K, Walmsley R. Ultrasound evaluation of acute experimental and chronic clinical wounds. Skin Research and Technology. 1999;5:228–236. [Google Scholar]
- Schiavi ME, Belletti B, Seidenari S. Ultrasound description and quantification of irritant reactions induced by dithranol at different concentrations. A comparison with visual assessment and colorimetric measurements. Contact Dermatitis. 1996;34:272–277. doi: 10.1111/j.1600-0536.1996.tb02199.x. [DOI] [PubMed] [Google Scholar]
- Thissen MR, Neumann MH, Schouten LJ. A systematic review of treatment modalities for primary basal cell carcinomas. Archives of Dermatology. 1999;135:1177–1183. doi: 10.1001/archderm.135.10.1177. [DOI] [PubMed] [Google Scholar]
- U.S. Agency for Healthcare Research and Quality. Pressure ulcer treatment: Quick reference guide. 2009 Retrieved November 4, 2013, from http://www.npuap.org/Final_Quick_Prevention_for_web.pdf. [Google Scholar]
- VanGilder C, Amlung S, Harrison P, Meyer S. Results of the 2008–2009 International Pressure Ulcer Prevalence Survey and a 3-year, acute care, unit-specific analysis. Ostomy/Wound Management. 2009;55:39–45. [PubMed] [Google Scholar]
- Young SR, Hampton S, Martin R. Non invasive assessment of negative pressure wound therapy using high frequency diagnostic ultrasound: Oedena reduction and new tissue accumulation. International Wound Journal. 2013;10:383–388. doi: 10.1111/j.1742-481X.2012.00994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]