Abstract
Rohon-Beard sensory neurons, neural crest cells, and sensory placodes can be distinguished at the boundary of the embryonic epidermis (skin) and the neural plate. The inductive signals at the neural plate border region are likely to involve a gradient of bone morphogenic protein (BMP) in conjunction with FGF and Wnts and other signals. However, how these signals are transduced to produce the final cell fate remains to be determined. Recent evidence from Xenopus and chick suggest that Dlx genes are required for the generation of cell fates at the neural plate border (McLarren, K.W., Litsiou, A., Streit, A., 2003. DLX5 positions the neural crest and preplacode region at the border of the neural plate. Dev. Biol. 259, 34–47; Woda, J.M., Pastagia, J., Mercola, M., Artinger, K.B., 2003. Dlx proteins position the neural plate border and determine adjacent cell fates. Development 130, 331–342). In the present study, we extend these findings to zebrafish, where we unequivocally demonstrate that dlx3b and dlx4b function in a dose-dependent manner to specify cell fates such as Rohon-Beard sensory neurons and trigeminal sensory placodes. dlx function was examined by inhibiting: (1) protein levels with antisense morpholino oligonucleotides (MOs), and (2) activity by repressing the ability of dlx-homeodomain to bind to downstream targets (EnR-dlx3bhd mRNA; dlx3b homeodomain fused to Engrailed transcriptional repressor domain). Inhibition of dlx3b and dlx4b protein and activity resulted in the reduction or complete loss of Rohon-Beard (RB) sensory neurons and trigeminal (TG) sensory placodes. These data suggest that dlx3b and dlx4b function in the specification of RB neurons and trigeminal sensory placodes in zebrafish. Further, we have shown that dlx3b and dlx4b function in a non-cell-autonomous manner for RB neuron development; dlx3b and dlx4b act to regulate bmp2b expression at the non-neural ectodermal border. These data suggest that the contribution of dlx3b and dlx4b to neural plate border formation is partially non-cell-autonomous acting via BMP activity.
Keywords: dlx genes, bmp genes, Neural crest, Rohon-Beard sensory neurons, Sensory placodes, Neural plate
Introduction
In zebrafish and Xenopus laevis, neural crest, Rohon-Beard (RB) neurons, and placodes arise along the neural plate border during neurula stage (Baker and Bronner-Fraser, 2001; Bally-Cuif and Hammerschmidt, 2003). Specification of cells at the neural plate border is thought to be mediated by inductive signals, including BMPs, FGFs, and Wnts, from the adjacent non-neural ectoderm during gastrula and neurula stages (Aybar and Mayor, 2002). In dorsoventral pattern formation of Xenopus gastrulae, Noggin, Chordin, and Follistatin proteins, secreted from the Spemann organizer, bind BMP4 in the dorsal region and prevent it from binding its receptor. Near the organizer, BMP4 is strongly inhibited by Noggin, Chordin, and Follistatin, which causes the specification of the neural plate. Inhibition gradually becomes weaker in the regions more distant from the organizer, such that intermediate levels of BMP signaling specify cell fate at the neural plate border. At a greater distance, there is little or no inhibition, which results in the specification of the epidermis. In zebrafish, the existence of a similar mechanism has been suggested, which was revealed by observations in embryos exhibiting dorsoventral axis defects (Nguyen et al., 1998, 2000).
In Xenopus and chick, Dlx3 and Dlx5, members of distal-less family of homeobox genes, are broadly expressed in the non-neural ectoderm adjacent to the neural plate, where BMP4 also is expressed, between early gastrula stage and neurula stage (Feledy et al., 1999; Pera et al., 1999). From their gene expression patterns, it was suggested that there is a regulatory relationship between Dlx genes and BMP4, and that the Dlx genes can act to promote epidermis and to repress neural plate development. Recent studies concerning Dlx gene function suggest that these transcription factors affect neural crest, RB neuron, and placodal development, as well as neural plate-border positioning (McLarren et al., 2003; Woda et al., 2003). In zebrafish, there are 8 dlx genes (Panganiban and Rubenstein, 2002). Among them, 6 dlx genes constitute three chromosomally linked pairs, and the remaining 2, dlx2b and dlx4a, are not linked to each other. The expression of two of these genes, dlx3b and dlx4b, is in the non-neural ectoderm adjacent to the neural plate at an early embryonic stage, and is similar to those of Xenopus Dlx3 and chick Dlx5. The other dlx genes in zebrafish are not expressed in this region. It has been suggested that dlx3b and dlx4b have cross-regulatory interactions between them and that they function in the development of otic and olfactory placodes (Liu et al., 2003; Solomon and Fritz, 2002). Further, our data from experiments in Xenopus suggest that Dlx genes play a role in specifying neural plate border fates. However, we consider these data equivocal, since we primarily used artificial constructs in which a large Engrailed repressor (EnR) was attached to the homeodomain of Dlx3b. We do not know, for example, if this large repressor domain is interfering with transcription of non-Dlx targets. To determine the role of dlx genes unequivocally, we have turned to a Morpholino-based knockdown in zebrafish and directly compared these data to the EnR–Dlx3b homeodomain injected embryos. Thus, we describe the function of dlx3b and dlx4b in zebrafish neurula, specifically looking at RB neuron, trigeminal placodal development, in addition to neural plate and neural crest development.
Two loss-of-function strategies were employed to investigate dlx3b and dlx4b function in zebrafish: morpholino antisense oligonucleotides to the translation start site (ATG) as well as directed to the exon2/intron2 splice site (E2I2), and a construct where dlx3b homeodomain is fused to Engrailed transcriptional repressor domain. We find that the marker-gene-expression of RB neurons and trigeminal placodes was highly reduced or absent, that of neural crest was slightly reduced, and that of neural plate was slightly broadened, in the embryos injected with MOs or EnR-dlx3bhd mRNA. Further, among bmp genes, a reduction of bmp2b gene-expression occurred at the non-neural ectodermal border in embryos at 90% epiboly, and RB neuron development was affected by non-cell-autonomous action of dlx3b and dlx4b. These findings suggest that dlx3b and dlx4b are required in RB neuron and trigeminal placodal development, and that some of the defects attributable to reduction of these dlx gene products are likely to be caused by the reduction of bmp2b expression.
Materials and methods
Antisense morpholino oligonucleotide
An antisense morpholino oligonucleotide (MO) having the sequence 5′-TATGTCGGTCCACTCATCCTTAATA-3′ was designed to target dlx3b mRNA, and a MO having the sequence 5′ATAGACATCATTAACCGTCAAGTCC-3′ was designed to target dlx4b mRNA (Gene Tools, LLC). These bind the AUG translation initiation site of dlx3b and dlx4b mRNA. Two additional MOs were made to bind the exon2/intron2 splice site (E2I2) of dlx3b and dlx4b nuclear RNA. The dlx3b E2I2 MO sequence was 5′-AGGTGTACCTGTGTCTGTGTGAGCC-3′ and the dlx4b E2I2 MO sequence was 5′-TGATGGATATTTACCTGTGTTTGCG-3′. An MO having the sequence 5′CCTCTTACCTCAGTTACAATTTATA-3′ was used as a control MO. The oligonucleotides were dissolved in distilled water. Eight to 20 ng dlx3b-MO and 8–20 ng dlx4b-MO were simultaneously injected into the yolk of 1- to 8-cell-stage embryos together with 10 kMW lysinated fluorescein dextran (LFD) or 10 kMW lysinated tetramethylrhodamine dextran (LRD) (Molecular Probes) for a total volume of 10–20 nl. Control MO of 40 ng in 10–20 nl was also injected in some experiments.
RT-PCR
Total RNA of wild type or 20 ng dlx3b E2I2 MO/20 ng dlx4b E2I2 MO injected embryos at 90% epiboly or 2–3-somite stage was isolated by using the RNAqueous kit (Ambion). In each case, about 20 embryos were used. First-strand cDNA was synthesized with total RNA, 300 ng of oligo dT primer, 200 units of SuperScriptII Reverse Transcriptase (Invitrogen), 1 mM dNTP, first strand buffer, 0.01 mM DTT, and 40 units of RNase Inhibitor, and incubated at 42°C for 50 min. PCR was performed with a forward primer in exon1 (5′-TGAGTGGACCGACATATGACA-3′) and a reverse primer in exon3 (5′-TTAATACACGGCCCCCACG-3′) of dlx3b, and with a forward primer in exon1 (5′-GTACACGGATTGCACTCA-3′) and a reverse primer in exon3 (5′-CAGTGGCCGTAATTGTTCAT-3′) of dlx4b.
Whole-mount in situ hybridization and immunohistochemistry
The following DIG or fluorescein-labeled antisense RNA probes (Roche Diagnostics) were used for in situ hybridization: axial (Strähle et al., 1993), bmp2b (Kishimoto et al., 1997), bmp4 (Nikaido et al., 1997), bmp7 (Dick et al., 2000), dlx3b (Ekker et al., 1992), gsc (Schulte-Merker et al., 1994a), HuC (Kim et al., 1996), isl-1 (Appel et al., 1995), krx20 (Oxtoby and Jowett, 1993), neuroD (Blader et al., 1997), neurogenin1 (Blader et al., 1997), ntl (Schulte-Merker et al., 1994b), paraxis (Shanmugalingam and Wilson, 1998), pax2a (Krauss et al., 1991), and tlx3a (Langenau et al., 2002). Single color whole-mount in situ hybridization was performed as described by Thisse and Thisse (1998). Two-color whole-mount in situ hybridization was performed essentially as described by Sawada et al. (2000). When MOs were injected into the yolk of the embryos for two-color in situ hybridization, LRD was used as a tracer. First color staining was performed with Fast Red (Roche), and second color staining was with NBT/BCIP (SIGMA), in two-color in situ hybridization.
A polyclonal antibody, anti-Dll (Panganiban et al., 1995) was used at the dilution 1:100. Immunohistochemistry was performed as described by Solnica-Krezel and Driever (1994).
Trypan blue staining
Wild type, 20 ng ATG dlx3b-MO- and 20 ng ATG dlx4b-MO-injected, and 40 ng control MO-injected embryos at 2–3-somite stage were put in 0.2 mg/ml trypan blue (SIGMA) in egg water, and left for 30 min at room temperature. Embryos were then rinsed two times in phosphate-buffered saline (PBS), and fixed in 4% paraformaldehyde (PFA) in PBS overnight. To get blue-stained pattern in embryos as positive control, a tungsten needle or a forcep was used to damage the membranes of cells in wild-type 2-somite-stage embryos, and the embryos were stained.
Constructs and RNA injections
pCS2-EnR-dlx3bhd and pCS2-VP16-dlx3bhd were previously described (Woda et al., 2003). Capped RNAs were produced by using the mMessage mMachine kit (Ambion). EnR-dlx3bhd mRNA (60–200 pg) was injected into the cell of 1-cell-stage embryos together with 10 kMW LFD (Molecular Probes) totally in 3–6 nl. In the rescue experiment, VP16-dlx3bhd mRNA (150 pg) was at first injected into the cell of 1-cell-stage embryos together with 10 kMW LFD in 3–6 nl, and next dlx3b/dlx4b ATG MO was injected into the yolk between 1- and 4-cell stage.
Cell transplantation
Mosaic embryos were generated as described by Schier et al. (1997). At first, the mosaic embryos, where labeled MO-injected cells are in host wild-type embryos, were made. Donor cells were removed from the epiblasts of the donor embryos; mid-blastula embryos which were injected with 20 ng ATG dlx3b-MO/20 ng ATG dlx4b-MO and 5% lysinated fluorescein/biotin dextran (10 kMW, Molecular Probes) into the yolk at 1- to 4-cell stage. Twenty to 40 donor cells were placed into the epiblast of each host embryo, an unlabeled wild-type mid-blastula embryo. The embryos were allowed to develop until 3-somite stage, and fixed in 4% PFA in PBS. Then, whole-mount in situ hybridization was performed using HuC probe, and a peroxidase reaction was done after using the ABC-peroxidase kit (Vector Laboratories, Inc.). Donor cells appeared as brown-stained cells. Next, the mosaic embryos, where labeled wild-type cells are in host MO-injected embryos, were made. Donor cells were removed from the epiblasts of the wild-type mid-blastula embryos, which were injected with fluorescein/biotin dextran into the yolk. The donor cells were put into the epiblasts of MO-injected mid-blastula embryos. Then, analyses were done as in the case with the host wild-type embryos having labeled donor MO-injected cells.
Zebrafish maintenance and strain
The wild-type zebrafish strain used in this study was AB/TL, and maintained according to Westerfield (1993).
Results
dlx3b-MO and dlx4b-MO deplete dlx proteins or alter splicing in MO-injected embryos
To examine the role of dlx3b and dlx4b in the development of the border between neural and non-neural ectoderm in zebrafish neurula, morpholino antisense oligonucleotides, which specifically target transcripts of dlx3b and dlx4b and inhibit their translation or proper splicing were generated (hereafter referred to as dlx3b/dlx4b-ATG-MOs or E2I2-MOs, respectively; Draper et al., 2001; Nasevicius and Ekker, 2000). Morpholinos, as well as a fluorescein dextran (LFD) or a rhodamine dextran (LRD) as a tracer, were injected into the yolk of 1- to 8-cell-stage embryos, and phenotypes were examined at 11 and 12.5 hpf. First of all, to examine whether dlx3b/dlx4b–ATG-MOs deplete dlx3b and dlx4b proteins specifically and effectively in embryos, whole-mount immunostaining with a polyclonal antibody against butterfly Dll homeodomain (Panganiban et al., 1995) was performed on 20 ng dlx3b–ATG-MO- and 20 ng dlx4b–ATG-MO-injected embryos. It was revealed that dlx3b and dlx4b proteins were lost in early stage embryos.
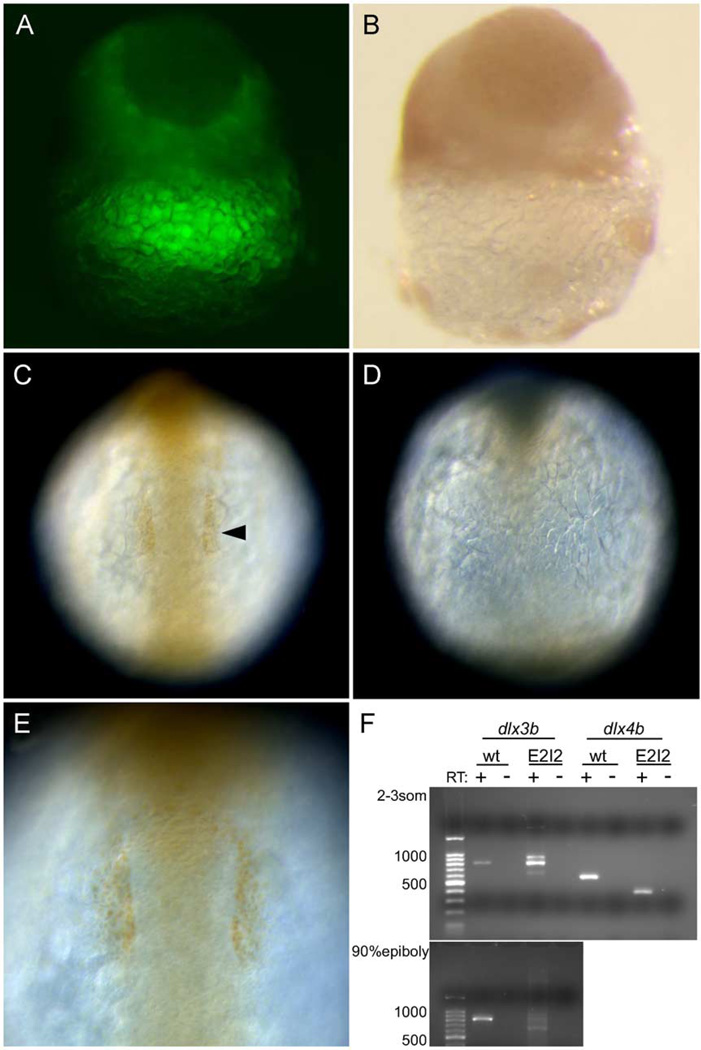
In wild-type embryos at late blastula stage, anti-Dll expression is seen in the entire epiblast (data not shown). We unexpectedly found an embryo that had a partial dispersion of MOs in the epiblast (Fig. 1A). We considered that the dispersion of MOs corresponded to that of the fluorescence (LFD) in the embryo. Fortuitously, the delay of injection (8-cell stage) appears to cause the partial dispersion of MO/LFD, allowing us to visualize where the MO is active. In the epiblast of that embryo, the anti-Dll-stained region and the MO-dispersed region were clearly complementary (Figs. 1A and B). At 6-somite stage, anti-Dll immunoreactivity was compared between wild-type and MO-injected embryos. In wild-type embryos, anti-Dll labeling could be seen in the otic placodal primordium, which is outside the central nervous system at the level of the hindbrain (Figs. 1C and E, arrowhead). In MO-injected embryos, anti-Dll labeling was extremely reduced or absent (Fig. 1D). dlx3b is expressed in the epiblast of late blastulae, and dlx3b and dlx4b are expressed in the otic placodal primordium at 6-somite stage (not shown). These results of late blastula stage and 6-somite stage suggest that dlx3b-MO and dlx4b-MO deplete specifically dlx3b and dlx4b protein, respectively, in embryos. The other zebrafish dlx genes are not expressed in the region of the neural plate border, and thus were not considered in this study.
Fig. 1.
Knockdown of dlx proteins in embryos injected with dlx3b/dlx4b-ATG-MOs and dlx3b/dlx4b-E2I2-MOs. The knockdown by the ATG-MOs was revealed by immunostaining with anti-Dll antibody (A–E). (A and B) Late blastula embryos. (C–E) 6-somite-stage embryos. (C and E) Wild-type embryos. (A, B, D) ATG-MO-injected embryos. (A and B) Same images of a blastula. (A) Fluorescence image revealing MOs + LFD, and (B) bright field. The dispersion of MOs and anti-Dll immunoreactivity are complementary in the epiblast. Lateral views. (C and D) Anti-Dll antibody immunoreactivity in the otic placodal primordium (arrowhead) is absent in MO-injected embryos. Dorsal views, anterior is to the top. (E) The magnification view of the otic placodal primordium in (C). The knockdown by the E2I2-MOs was revealed by RT-PCR followed by gel electrophoresis (F). (F) Wild type (wt) as compared to E2I2 MO (E2I2) dlx3b and dlx4b RT-PCR products. In dlx3b RT-PCR, at 2–3-somite stage, the amplification of cDNA from wild-type embryos produced an 810-bp band, and cDNA from E2I2-MO-injected embryos produced three bands of 630, 810, and 900 -bp in size. At 90% epiboly, in E2I2-MO-injected embryos, the 810-bp band was faint. In dlx4b RT-PCR, at 2- to 3-somite stage, the amplification of cDNA from wild-type embryos produced a 580-bp band, and cDNA from E2I2-MO-injected embryos produced a 390-bp band. Negative-control reactions run in parallel, where reverse transcriptase was omitted in the cDNA synthesis reactions (RT−).
A second set of dlx3b/dlx4b MOs that disrupt splicing in nuclear RNA were also used in this study (now referred to as dlx3b/dlx4b-E2I2-MOs). Splice blocking MOs have been used in zebrafish as an alternative method to knockdown gene function by disrupting proper splicing of RNA (Draper et al., 2001). We designed MOs that disrupt splicing for both dlx3b and dlx4b in the exon2–intron2 boundary of each, which would result in a reduction in size of exon2. This is the exon that contains a large part of the homeodomain, and the splice block MOs at exon2–intron2 would reduce the size of the homeodomain by 72% for dlx3b and by 75% for dlx4b. A reduction in size of 185 bp for dlx3b and 194 bp for dlx4b was predicted. The effect of the 20 ng dlx3b/20 ng dlx4b E2I2-MOs on splicing was observed by isolation of embryos injected with dlx3b/dlx4b-E2I2-MOs at the 1- to 4-cell stage, isolating RNA from non-injected control and dlx3b/dlx4b-E2I2-MO-injected embryos at 2–3-somite stage and 90% epiboly, synthesizing first-strand cDNA, and performing RT-PCR for dlx3b and dlx4b. Embryos injected with the dlx3b/dlx4b-E2I2-MOs had the mis-splicing in dlx3b/dlx4b as compared to a wild-type control (Fig. 1F). In dlx4b, we observed the expected splice variant, where we observed an approximate reduction of 200 bp, suggesting that mis-splicing occurred at the exon2–intron2 boundary. For dlx3b, we observed two forms of mis-spliced mRNA: one form which had approximately 180 bp deleted, as predicted, and another splice variant which had a 100-bp increase in size. Sequence analysis of the latter larger fragment confirmed that it had a 137-bp reduction of the 3′ end of exon2, consistent with the MO binding to this site and interfering with splicing, and in addition had a portion of intron2 attached. Thus, an increase in size of approximately 100 bp was observed in the larger fragment. This suggests that mis-splicing did occur. At 90% epiboly, complete splicing did occur, as shown by the presence of the sharp band at 630 bp (Fig. 1F, 90% epiboly E2I2+RT lane bottom panel). Although there is a wild-type dlx3b mRNA produced at 2- to 3-somite stage, we feel that sufficient mis-splicing has occurred to explain the observed phenotypes, since at around 90% epiboly, we feel dlx3b is acting to pattern the neural plate border. We have used both the ATG and E2I2 dlx3b/dlx4b MOs in this study, with the majority of the analysis with the ATG MOs. The observation that both the dll protein levels and the splicing of dlx3b and dlx4b are defective suggests that the effects on RB neurons and trigeminal placode described below are specific to depletion of dlx3b/dlx4b protein or the creation of a non-functioning protein.
dlx3b and dlx4b are required for Rohon-Beard sensory neuron development
HuC and isl-1 are expressed in all primary neurons at early stages of neural development in zebrafish (Kim et al., 1996; Korzh et al., 1993). In addition, RB neurons at 3-somite stage express a homeobox gene, tlx3a (Langenau et al., 2002). In nrd mutant zebrafish, which lack RB neurons, tlx3a expression is absent (Artinger et al., 1999).
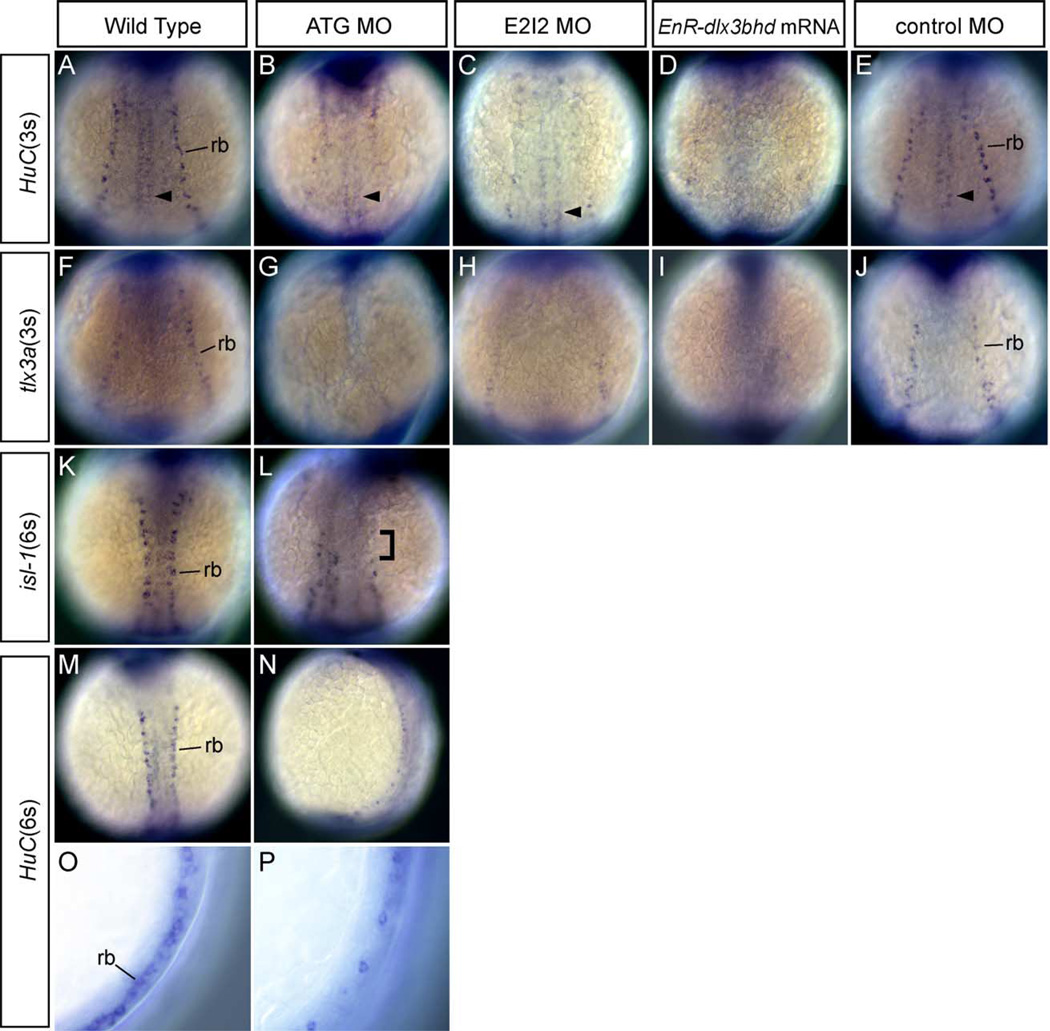
To investigate the role of dlx3b and dlx4b in RB neuron development, the expression of HuC, tlx3a and isl-1 were studied in ATG and E2I2 dlx3b-MO- and dlx4b-MO-injected embryos by whole-mount in situ hybridization at 3- and 6-somite stage. The expression of HuC and tlx3a in RB neurons was significantly reduced or absent as compared to wild-type embryos at 3-somite stage (Figs. 2B, C, G, H compare with wild type in 2A and F; Table 1). To confirm the function of dlx genes which are expressed during early embryonic stages, we also injected approximately 150 pg EnR-dlx3bhd mRNA into the cell of 1-cell-stage embryos. The expression of HuC and tlx3a were examined by in situ hybridization. Phenotypes seen were very similar to those in dlx3b-MO- and dlx4b-MO-injected embryos. In the EnR-dlx3bhd mRNA injected embryos, HuC and tlx3a expression marking RB neurons could not be detected (Figs. 2D and I as compared to wild type in 2A and F). Control MO-injected embryos at the same concentration are also shown for HuC and tlx3a (Figs. 2E and J). These results suggest that dlx activity as mediated through dlx3b and dlx4b is required in RB neuron development.
Fig. 2.
Phenotypes of Rohon-Beard (RB) sensory neurons in MOs and EnR-dlx3bhd mRNA injected embryos at 3- and 6-somite stage. (A–J) Embryos at 3-somite stage. Dorsal views, anterior is to the top. (K–P) Embryos at 6-somite stage. (A, F, K, M, O) Wild-type embryos. (B, G, L, N, P) 20 ng ATG-dlx3b-MO/20 ng ATG-dlx4b-MO-injected embryos. (C and H) 20 ng E2I2-dlx3b-MO/20 ng E2I2-dlx4b-MO-injected embryos. (D and I) One hundred fifty-picogram EnR-dlx3bhd mRNA-injected embryos. (E and J) Forty-nanogram control MO-injected embryos. (A–E, M–P) Expression of HuC. (F–J) Expression of tlx3a. (K and L) Expression of isl-1. (O) High magnification lateral view of a wild-type embryo. (P) High magnification lateral view of (N). In (A), RB neurons (rb) and primary motor neurons (arrowhead) are seen. After injection of dlx3b and dlx4b-MOs or EnR-dlx3bhd mRNA (B–D), primary motor neurons are present, but RB neurons are highly reduced or absent, as seen by the expression of HuC. A control MO injected at the same concentration in (E) has normal RB neurons. In (G–I), RB neurons are highly reduced or absent after injection of dlx3b and dlx4b-MOs or EnR-dlx3bhd mRNA, observed with tlx3a expression. In a control MO injected at the same concentration in (J), no changes are observed. In embryos allowed to develop until the 6-somite stage (L and N), RB neurons continue to show a reduction after injection of dlx3b and dlx4b-MOs. The bracket in (L) shows the region of no RB neurons. In a higher magnification view (O and P), it is easily recognized that RB neurons are highly reduced in MO-injected embryos observed at 6-somite stage.
Table 1.
dlx3b/dlx4b MO effects in zebrafisha
| Injection | Amounts (ng/embryo) |
N | Phenotype (reduced or absent) | Defective gastrulationc |
Deadc | |
|---|---|---|---|---|---|---|
| RBs | TGb | |||||
| Control | 40 | 38 | 0 | 0 | – | – |
| MO | 108 | – | – | 0 | 7 | |
| dlx3bhd– | 0.150–0.200 | 40 | 18 | – | ND | ND |
| EnR | 44 | – | 14 | |||
| dlx3b/dlx4b | 40 | 109 | 52 | – | – | – |
| ATG– | 73 | – | 39 | – | – | |
| MO | 273 | – | – | 17 | 22 | |
| dlx3b/dlx4b | 40 | 56 | 32 | – | – | – |
| E2I2– | 51 | – | 38 | – | – | |
| MO | 97 | – | – | 5 | 11 | |
| dlx3b/dlx4b | 40 | |||||
| ATG– | 30 | 4d | ND | ND | ND | |
| MO+ | 0.150 | |||||
| dlx3bhd– | ||||||
| VP16 | ||||||
ND is not done.
All sets of injection experiments were done at least three times.
RB = Rohon–Beard sensory neuron, TG = Trigeminal ganglia placode.
Checked at the 3-somite stage.
We have defined reduced to have 12 or less RB neurons/per embryo. This is the average number of total RB neurons in the MO-injected embryos (see Table 3).
To determine if the effects are maintained over a longer period of development, we assayed both the expression of HuC and isl-1 at a slightly later stage, 6-somites, in dlx3b-MO- and dlx4b-MO-injected embryos (see Fig. 5 about isl-1 phenotype at 3-somite stage). Consistent with earlier results, we observed a reduction in the number of RB neurons with both isl-1 and HuC (Figs. 2L and N as compared to 2K and M). In a higher magnification view of dlx3b-MO- and dlx4b-MO-injected embryos, the reduction of RB neurons can be easily visualized (Fig. 2P compared with 2O).
Fig. 5.
Double in situ hybridization of HuC/isl-1 and neural/mesodermal markers in MO-injected embryos. (A, C, E, G, I, K, M, O) Wild-type embryos at 3-somite stage. (B, D, F, H, J, L, N, P) 20 ng ATG-dlx3b-MO/20 ng ATG-dlx4b-MO-injected embryos at 3-somite stage. (A and B) Expression of HuC (blue) and krx20/pax2a, a marker of rhombomeres 3 and 5 and midbrain–hindbrain boundary (red). (C and D) Expression of isl-1 (blue) and krx20/pax2a (red). (E and F) Expression of HuC (blue) and axial marking floor plate (red). (G and H) Expression of isl-1 (blue) and axial (red). (I and J) Expression of HuC (blue) and gsc/ntl expressed in the prechordal plate and notochord (red). (K and L) Expression of isl-1 (blue) and gsc/ntl (red). (M and N) Expression of HuC (blue) and paraxis showing expression in the somites (blue). Numbers 1–3 correspond to the somites. (O and P) Expression of isl-1 (blue) and paraxis (blue). Numbers 1– 3 correspond to the somites. In (B, D, J, L), neurons in the trigeminal placodes (arrowhead) are highly reduced or absent, shown by the expression of HuC and isl-1. The expression of HuC and isl-1 in the RB neurons (F, H, N, P; arrow) are highly reduced or absent. However, no changes are observed in neural/mesodermal marker expression in dlx3b and dlx4b MO-injected embryos (B, D, F, H, J, L, N, P).
In addition to RB neurons, other cell types exist at the border of the neural plate, including neural crest cells. Both neural plate and neural crest formation was assayed with kheper and fkd6 (Muraoka et al., 2000; Odenthal and Nüsslein-Volhard, 1998). Slight widening of the neural plate and reduction of neural crest was observed (see wider neural plate in Fig. 2L as compared to 2K; and data not shown). These results suggest that dlx3b and dlx4b are required for RB neuron development, and may play a role in the positioning of other cells at the neural plate border, including neural crest cells.
dlx3b and dlx4b are required for trigeminal placodal development
In the trigeminal placodes of 3-somite-stage zebrafish embryos and Xenopus neurulae, neuroD is expressed (Blader et al., 1997; Schlosser and Northcutt, 2000); in otic placodal primordia at 3-somite stage, pax2a is expressed (Krauss et al., 1991). In zebrafish, neuroD expression is lost in neurogenin1-MO-injected embryos, which develop no trigeminal placodes (Andermann et al., 2002). In Pax2-deficient mice, the development of the inner ear becomes incomplete (Torres et al., 1996).
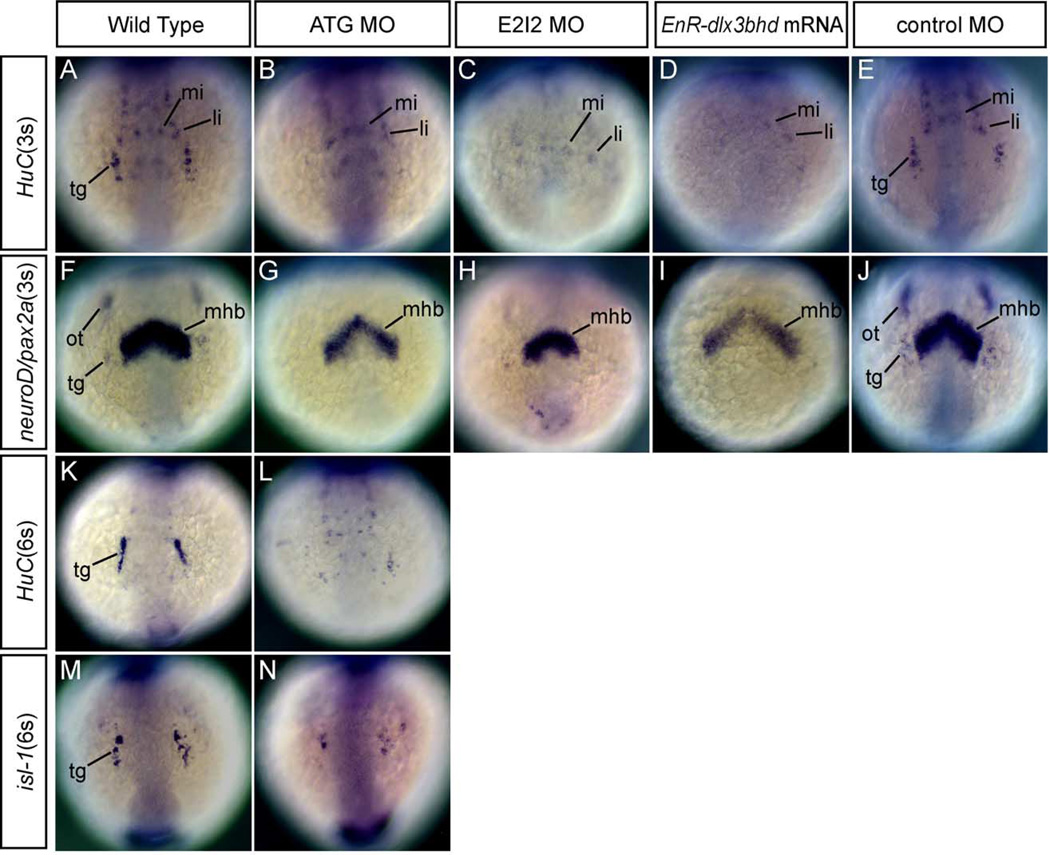
In order to investigate the effects of dlx3b-MO and dlx4b-MO on trigeminal placodal development, we assayed the expression of HuC, neuroD/pax2a, and isl-1 in ATG and E2I2 dlx3b-MO- and dlx4b-MO-injected embryos at 3- and 6-somite stage. Expression of HuC and neuroD/pax2a in the trigeminal placode was extremely reduced or absent as compared to wild-type embryos at 3-somite stage (Figs. 3B and C as compared to 3A and Figs. 3G and H as compared to 3F; Table 1). Consistent with the results reported by Solomon and Fritz (2002), the expression of pax2a marking otic placodal primordia was lost (Figs. 3G and H). neuroD expression, as well as pax2a expression, in the placodal primordial region, which is outside the anterior central nervous system, was extremely reduced or absent (Figs. 3G and H).
Fig. 3.
Phenotypes of neurons in the trigeminal placodes in MOs and EnR-dlx3bhd mRNA injected embryos at 3- and 6-somite stage. (A–J) Embryos at 3-somite stage. Anterior views. (K–N) Embryos at 6-somite stage. Anterior views. (A, F, K, M) Wild-type embryos. (B, G, L, N) 20 ng ATG-dlx3b-MO/20 ng ATG-dlx4b-MO-injected embryos. (C and H) 20 ng E2I2-dlx3b-MO/20 ng E2I2-dlx4b-MO-injected embryos. (D and I) One hundred fifty-picogram EnR-dlx3bhd mRNA-injected embryos. (E and J) Forty-nanogram control MO-injected embryos. (A–E, K, L) Expression of HuC. (F–J) Expression of neuroD/pax2a. (M and N) Expression of isl-1. In a wild-type embryo (A), neurons in the trigeminal placodes (tg) and anterior lateral/medial hindbrain interneurons (li, mi) are observed. After injection of dlx3b and dlx4b-MOs or EnR-dlx3bhd mRNA shown in (B–D), anterior lateral/medial hindbrain interneurons remain, but neurons in the trigeminal placodes are highly reduced or absent as shown by expression of HuC. In a control-MO injected embryo (E), neurons in the trigeminal placodes are seen in the placodal primordial region. neuroD/pax2a expression in (G–I), both are highly reduced or absent in the placodal primordial region following injection of dlx3b and dlx4b-MOs or EnR-dlx3bhd mRNA. Midbrain–hindbrain boundary is seen in the central nervous system (mhb). In a control MO-injected embryos (J), no changes were observed in the trigeminal (tg) and otic placodal primordia (ot). Shown at the 6-somite stage, expression of HuC (L) and isl-1 (N), neurons in the trigeminal placodes remain highly reduced, as compared to wild-type embryos in (K and M), respectively.
To confirm the function of dlx genes on trigeminal placodal development, we also injected approximately 150 pg EnR-dlx3bhd mRNA into the cell of 1-cell-stage embryos. The expression of HuC and neuroD/pax2a at 3-somite stage was examined by in situ hybridization. Phenotypes seen were very similar to those in dlx3b-MO- and dlx4b-MO-injected embryos. In the EnR-dlx3bhd mRNA injected embryos, HuC and neuroD/pax2a expression was highly reduced or absent in the placodal primordial region (Figs. 3D and I as compared to wild type in 3A and F). Control MO-injected embryos at the same concentration are also shown for HuC and neuroD/pax2a (Figs. 3E and J). These results suggest that dlx activity as mediated through dlx3b and dlx4b is required in trigeminal placodal development.
To determine if the effects are maintained over a longer period of development, we assayed both the expression of HuC and isl-1 at a slightly later stage, 6 somites, in dlx3b-MO- and dlx4b-MO-injected embryos (see Fig. 5 about isl-1 phenotype at 3-somite stage). Consistent with earlier results, we observed a reduction in the number of trigeminal placodal neurons with both HuC and isl-1 (Figs. 3L and N as compared to 3K and M).
dlx3b-MO/dlx4b-MO and EnR-dlx3bhd mRNA act in a dose-dependent manner to position cells at the neural plate border
RB neurons, neural crest and placodes develop within the border between neural and non-neural ectoderm of 3-somite-stage zebrafish neurula. Solomon and Fritz (2002) reported that dlx3b and dlx4b are required in otic placodal development of the zebrafish. The expression of pax2a, a marker of otic placode, is lost in about 11.5 hpf embryos injected with 8 ng dlx3b-MO and 8 ng dlx4b-MO (Solomon and Fritz, 2002). To examine whether dlx3b and dlx4b function in the development of RB neurons and trigeminal placodes, expression patterns of marker genes were analyzed in the same concentration (8 ng dlx3b-MO and 8 ng dlx4b-MO) at 3-somite stage by whole-mount in situ hybridization. HuC and tlx3a were used to identify RB neurons and trigeminal ganglia (Kim et al., 1996; Langenau et al., 2002). The expression of HuC and tlx3a were reduced in MO-injected embryos.
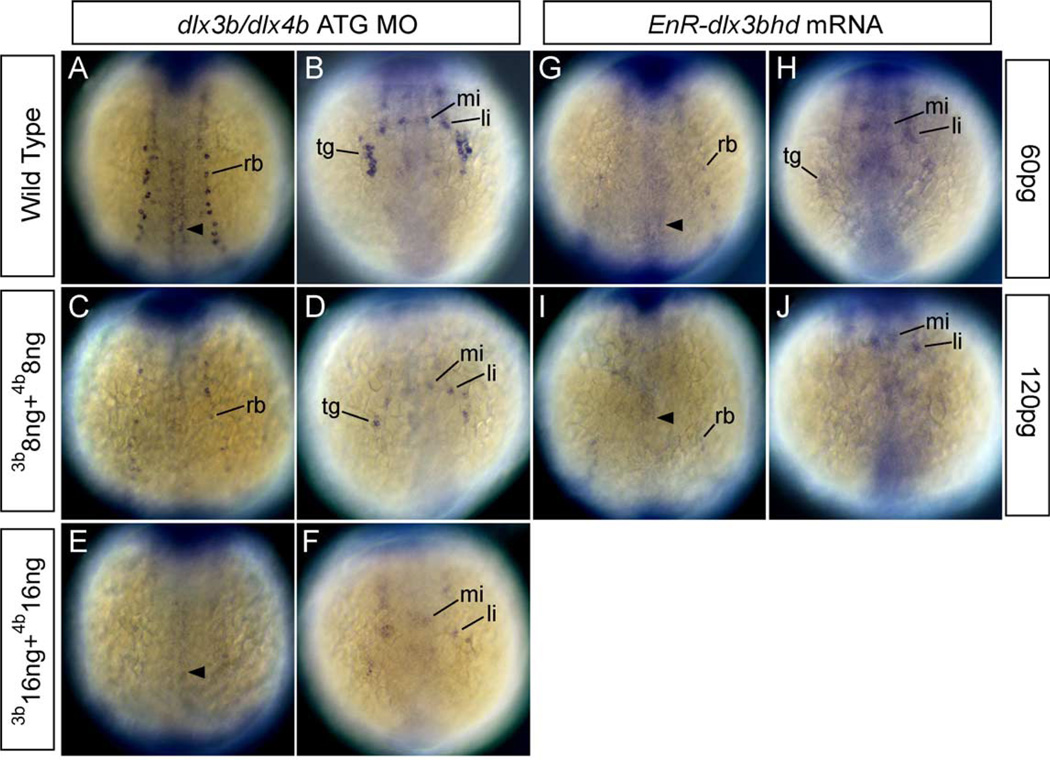
The observation that injection of 8 ng dlx3b-MO and 8 ng dlx4b-MO to embryos caused RB neurons to be reduced lead us to examine the dose-dependency of these morpholinos. HuC was chosen as an indicator of the effect in this experiment. In 8 ng dlx3b-MO- and 8 ng dlx4b-MO-injected embryos, expression of HuC marking RB neurons and neurons in trigeminal placodes was reduced (Figs. 4C and D compare with wild type in 4A and B; Table 2). In 16 ng dlx3b-MO- and 16 ng dlx4b-MO-injected embryos, expression of HuC marking RB neurons and neurons in trigeminal placodes was highly reduced or could not be detected (Figs. 4E and F). In addition, some embryos also displayed a slight expanded neural plate in which the expression of HuC in those neurons was also highly reduced or absent (not shown). These data show that HuC expression in those neurons is sensitive to dlx3b-MO and dlx4b-MO, and that MOs affect embryos in a dose-dependent fashion. Further, these data suggest that more than 16 ng dlx3b-MO and 16 ng dlx4b-MO cause RB neurons and placodes to be lost.
Fig. 4.
HuC expression in embryos injected with dlx3b-ATG-MO and dlx4b-ATG-MO and EnR-dlx3bhd mRNA at 3-somite stage. (A, C, E, G, I) Dorsal views, anterior is to the top. (B, D, F, H, J) Anterior views. (A and B) Wild-type embryos. (C and D) 8 ng dlx3b-MO- and 8 ng dlx4b-MO-injected embryos. (E and F) 16 ng dlx3b-MO- and 16 ng dlx4b-MO-injected embryos. (G and H) 60 pg EnR-dlx3bhd mRNA-injected embryos. (I and J) 120 pg EnR-dlx3bhd mRNA-injected embryos. (A–F) and (G–J) show that the expression of HuC, which is expressed in RB neurons in the trunk (rb) and neurons in trigeminal placodes in the head (tg), is gradually reduced as the concentration of MOs and EnR-dlx3bhd mRNA are increased. In contrast, primary motor neurons (arrowhead) and anterior lateral hindbrain interneurons (li) and anterior medial hindbrain interneurons (mi) are not changed.
Table 2.
Dose effects of dlx3b/dlx4b MOa
| Injection | Amounts (ng/embryo) |
N | Phenotype (reduced or absent) | |
|---|---|---|---|---|
| RBs | TGb | |||
| dlx3b/dlx4b | 8 + 8 | 14 | 7 | 7 |
| ATG-MO | 16 + 16 | 23 | 8 | 10 |
| dlx3bhd-EnR | 0.060 | 20 | 13 | 14 |
| 0.120 | 19 | 15 | 13 | |
All sets of injection experiments were done at least two times.
RB = Rohon-Beard sensory neuron, TG = trigeminal ganglia placode.
In zebrafish, there are 8 dlx genes (Panganiban and Rubenstein, 2002). To confirm that dlx genes other than dlx3b and dlx4b do not function before 3-somite stage, 60 or 120 pg EnR-dlx3bhd mRNA was injected into the cell of 1-cell-stage embryos, and HuC expression was examined at 3-somite stage. The phenotypes of embryos injected with 60 pg EnR-dlx3bhd mRNA were similar to those of embryos injected with 8 ng dlx3b-MO and 8 ng dlx4b-MO. In addition, the phenotypes of embryos injected with 120 pg EnR-dlx3bhd mRNA were similar to those of embryos injected with 16 ng dlx3b-MO and 16 ng dlx4b-MO. In 60 pg EnR-dlx3bhd mRNA-injected embryos, the expression of HuC marking RB neurons and neurons in trigeminal placodes was reduced (Figs. 4G and H as compared to wild type in 4A and B). In 120 pg EnR-dlx3bhd mRNA-injected embryos, the expression of HuC was highly reduced in those neurons (Figs. 4I and J as compared to wild type in 4A and B). The similarity of phenotypes between MO-injected embryos and EnR-dlx3bhd mRNA-injected embryos suggests that only dlx3b and dlx4b function before 3-somite stage in zebrafish.
Effects of the dlx3b/dlx4b MOs on RB neurons and trigeminal placode are specific to these tissues
To confirm that effects of dlx3b/dlx4b MOs are specific to the formation of RB neuron and trigeminal placodal development, we examined the expression of other neural markers, krx20, pax2a, and axial as well as mesodermal markers, gsc, ntl, and paraxis, in dlx3b-MO- and dlx4b-MO-injected embryos. krx20 is expressed in the hindbrain rhombomeres 3 and 5, while pax2a is in the midbrain–hindbrain boundary and the otic placodes (Krauss et al., 1991; Oxtoby and Jowett, 1993). axial is expressed within the floor plate of the ventral neural tube and at lower levels in the notochord (Strähle et al., 1993). Analysis of the expression of the neural markers krx20 and pax2a (red), in double in situ hybridization with HuC and isl-1 to detect the RB neuron and TG phenotype (blue), revealed that it was normal in MO-injected embryos (Figs. 5B and D as compared to wild type in 5A and C). The slight broadening of the neural plate was observed by krx20, consistent with other findings (noticeable in Fig. 5D). The expression of axial (red) in the dlx3b-MO- and dlx4b-MO-injected embryos was also confirmed to be the same as wild type (Figs. 5F and H as compared to wild type in 5E and G), by double in situ hybridization with HuC and isl-1 to detect the RB neuron and TG phenotype (blue). To determine if mesodermal differentiation was affected in dlx3b-MO- and dlx4b-MO-injected embryos, we visualized the pattern of expression of gsc, ntl, and paraxis. gsc is expressed within the anterior midline, prechordal plate, while ntl is primarily expressed within the notochord (Schulte-Merker et al., 1994a,b). paraxis has been shown to be expressed strongly in the somitic mesoderm and faintly in the presomitic mesoderm (Shanmugalingam and Wilson, 1998). Consistent with a specific effect of dlx3b and dlx4b in the specification of RB neurons and trigeminal placodes, no defects on mesodermal marker expression was observed. For gsc/ntl (red), we performed double in situ hybridization with HuC and isl-1 to detect the RB neuron and trigeminal placode phenotype (blue; Figs. 5J and L as compared to wild type in 5I and K). For the paraxis experiment, we used the same color to detect both HuC/isl-1 and paraxis (blue; arrow to RB neurons), demonstrating that in embryos with reduced expression of RB neurons, no difference was observed in paraxis expression (Figs. 5N and P as compared to wild type in Figs. 5M and O).
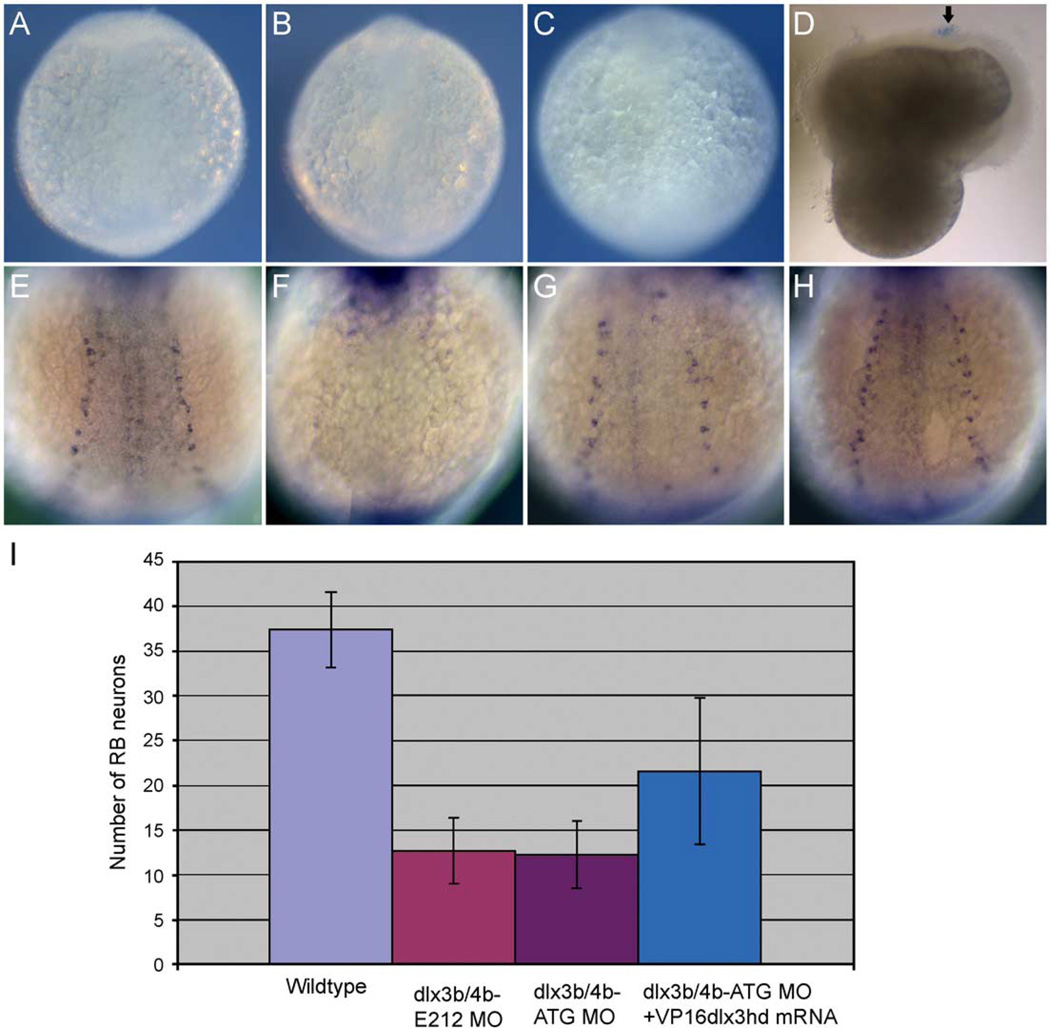
The concentration of MO injected varies for different genes. Some require on 3–5 ng, while others require much more (Agathon et al., 2001; Busch-Nentwich et al., 2004; Lekven et al., 2001), as is the case for dlx3b and dlx4b. Because we are injecting a relatively high concentration of MO to see consistent effects on RB neuron and trigeminal placodal development, we wanted to determine if this caused non-specific necrosis of the embryos (reported by Solomon and Fritz, 2002). In Drosophila, trypan blue is used to determine the extent of cell death caused by necrosis (Krebs and Feder, 1997). We have determined that this technique is useful in visualizing cell necrosis in zebrafish embryos. For our positive control, we purposely damaged an embryo with a needle, and observed positive trypan blue-stained pattern (Fig. 6D, arrow). However, in the highest doses of dlx3b and dlx4b MO, or control MO at the same concentrations, we never observed trypan blue-stained pattern, suggesting that the embryos do not exhibit necrosis at this concentration of MO (Figs. 6B and C as compared to uninjected in 6A or positive control in 6D).
Fig. 6.
Study of necrosis and rescued phenotypes of RB neurons by VP16-dlx3bhd mRNA in dlx3b and dlx4b MO-injected embryos. Trypan blue-stained pattern in 2–3-somite-stage embryos (A–D) and HuC-expressing RB neuron pattern in 3-somite-stage embryos (E–H). (A) A wild-type embryo. (B) A 20-ng ATG-dlx3b-MO/20 ng ATG-dlx4b-MO-injected embryo. (C) A 40-ng control MO-injected embryo. (D) An embryo injured by a tungsten needle. No trypan blue-stained pattern is seen in wild type, 20 ng ATG-dlx3b-MO/20 ng ATG-dlx4b-MO-injected, and 40 ng control MO-injected embryos (A–C). In the injured embryo (D), a blue-stained pattern can be seen (arrow), indicating cell necrosis. (E) A wild-type embryo expressing HuC. (F) An embryo injected with 20 ng ATG-dlx3b-MO/20 ng ATG-dlx4b-MO. (G) A partially rescued embryo in RB neurons by VP16-dlx3bhd mRNA, injected with 20 ng ATG-dlx3b-MO/20 ng ATG-dlx4b-MO. (H) A fully rescued embryo in RB neurons by VP16-dlx3bhd mRNA, injected with 20 ng ATG-dlx3b-MO/20 ng ATG-dlx4b-MO. (I) Numbers of RB neurons are recovered in MO-injected embryos by injection of 150 pg VP16-dlx3bhd mRNA.
In addition to expression analysis, we designed several experiments to ensure specificity: (1) the injection of EnR-dlx3bhd mRNA, (2) the injection of MOs to a different site to block proper splicing of dlx3b/dlx4b (E2I2), and (3) the rescue of the MO phenotype with VP16-dlx3bhd mRNA. As shown above, the effects of injection of 150 pg EnR-dlx3bhd mRNA, examined by the expression of HuC, tlx3a, and neuroD/pax2a at 3-somite stage, showed similar phenotypes of embryos injected with dlx3b/dlx4b-MOs (Figs. 2D, I and 3D, I). These data suggest that the dlx3b/dlx4b-MOs are specific to the knockdown of these proteins. In addition, we have further injected E2I2 splice-blocking dlx3b/dlx4b-MOs to confirm that MOs made to a different region of the gene will have the same effect, assessed by the expression of the same markers. We have shown definitively that the phenotype of the E2I2 MOs is identical to that of the ATG MOs (Figs. 2 and 3). Finally, we have shown that the overexpression of the VP16-dlx3bhd mRNA can rescue the RB neuron phenotype caused by the injection of dlx3b/dlx4b MOs (Figs. 6E–I; Table 3). We have used the VP16-dlx3bhd mRNA for the rescue, since this construct contains the activating domain VP16 attached to the homeodomain region that is conserved between both genes. These results in combination suggest that the effects of knockdown of dlx3b and dlx4b are specific to cells at the neural plate border.
Table 3.
Rescue effect of dlx3bhd-VP16 on the numbers of RB neuronsa
| Injection | Amounts (ng/embryo) |
N | Average number of RB neuronsb | ||
|---|---|---|---|---|---|
| Left | Right | Total | |||
| Uninjected | NA | 31 | 18.5 | 19.0 | 37.5 ± 4.2 |
| dlx3b/dlx4b | 40 | 20 | 6.5 | 6.2 | 12.7 ± 3.7 |
| E2I2-MO | |||||
| dlx3b/dlx4b | 40 | 20 | 6.6 | 5.7 | 12.3 ± 3.7 |
| ATG-MO | |||||
| dlx3b/dlx4b | 40 | ||||
| ATG-MO+ | 30 | 10.3 | 11.3 | 21.6 ± 7.1 | |
| dlx3bhd– | 0.150 | ||||
| VP16 | |||||
All sets of injection experiments were done at least three times.
The number of Rohon-Beard sensory neurons (RB) were counted on each side of a 3-somite-stage embryo, visualized with HuC in situ hybridization.
dlx3b and dlx4b act upstream of neurogenin1 in trigeminal placode
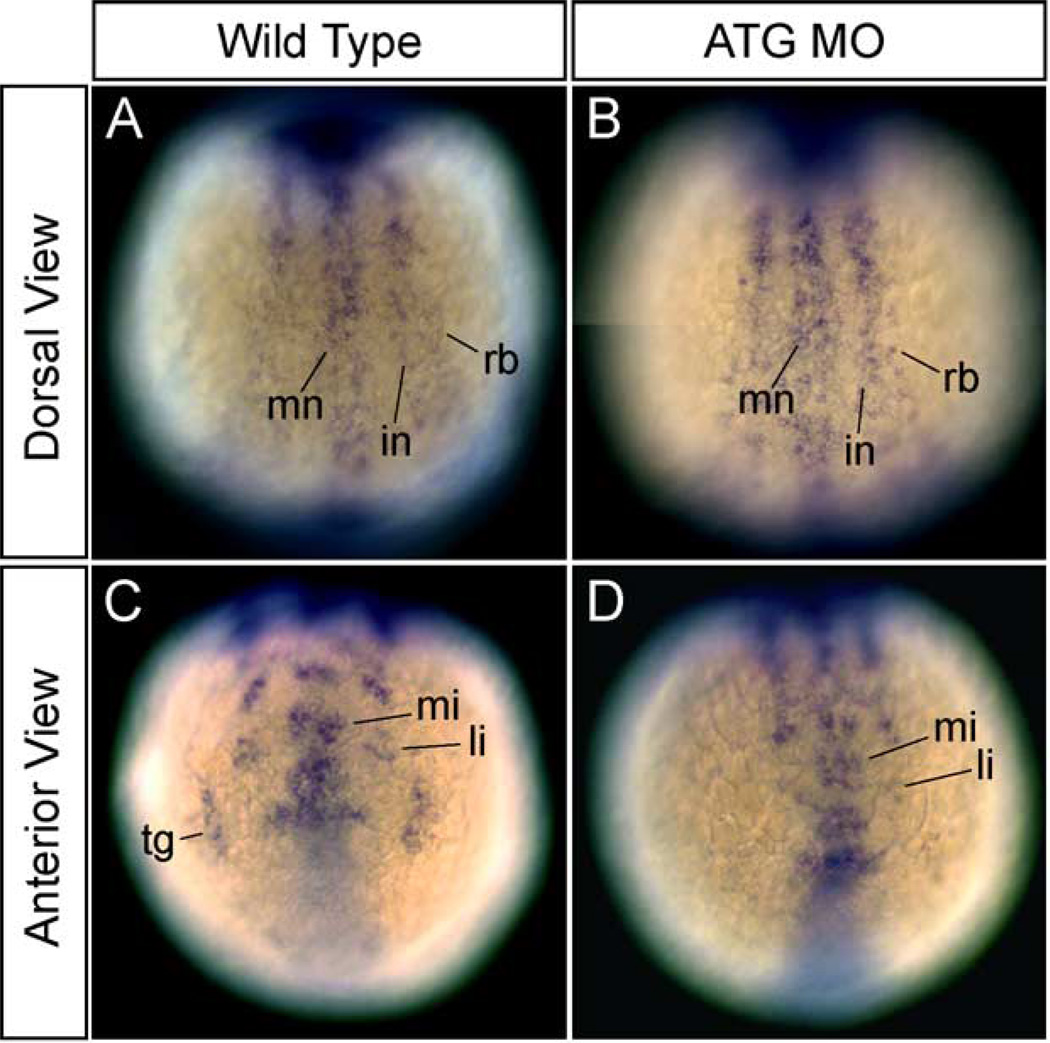
The neurogenin gene family is involved in the specification of sensory neurons, and it is most likely an upstream gene of neuroD marking trigeminal placodes in zebrafish (Andermann et al., 2002). In addition, it is also a probable upstream gene of tlx3a and HuC in RB neuron development (Cornell and Eisen, 2002; Ma et al., 1996). To determine the effects of dlx3b-MO and dlx4b-MO injection on neurogenin expression, we assayed the expression of neruogenin1 at 3-somite stage. The expression of neurogenin1 was not changed in the spinal cord of MO-injected embryos at 3-somite stage (Fig. 7B as compared to wild type in 7A). In contrast, the expression of neurogenin1 in the trigeminal placodes was highly reduced or absent in the placodal primordial region of MO-injected embryos (Fig. 7D as compared to wild type in 7C). These data suggest that dlx3b and dlx4b begin to function to pattern the neural plate border before 90% epiboly, which is the stage that the expression of neurogenin1 is first observed.
Fig. 7.
Expression of the upstream gene, neurogenin1, for HuC, tlx3a, and neuroD marking RB neurons and neurons in the trigeminal placodes, in embryos injected with dlx3b-MO and dlx4b-MO at 3-somite stage. (A and C) Wild-type embryos. (B and D) 20 ng ATG-dlx3b-MO/20 ng ATG-dlx4b-MO-injected embryos. (A and B) The expression of neurogenin1 in RB neuron precursors (rb) is normal in MO-injected embryos. Dorsal views. (C and D) The expression of neurogenin1 in the precursors of neurons in the trigeminal placodes (tg) is absent in MO-injected embryos (D). Anterior views. mn, primary motor neuron precursors; in, primary interneuron precursors; mi, anterior medial hindbrain interneuron precursors; li, anterior lateral hindbrain interneuron precursors.
dlx3b/dlx4b function to regulate the expression of bmp2b at the neural plate border
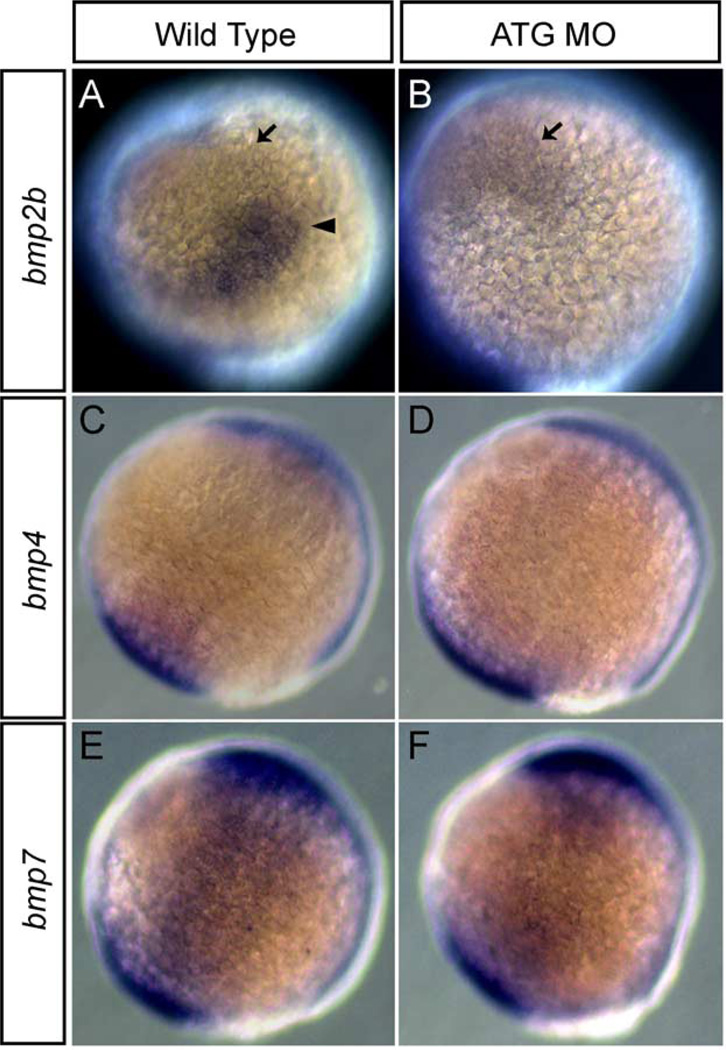
In chick, based upon expression patterns, it is suggested that there is a regulatory relationship between DLX5 and BMP4 (Pera et al., 1999). Similarly, in Xenopus, Xdlx3 regulates Bmp4 expression at neurula stage (Woda, Kaji and Artinger, in preparation). In zebrafish, bmp2b expression was laterally shifted in the non-neural ectoderm of MO-injected embryos at 3-somite stage (not shown). Next, we tested the hypothesis that bmp2b expression was altered by injection of dlx3b and dlx4b MOs at 90% epiboly and at tailbud stage by whole-mount in situ hybridization. bmp2b was expressed in the ventral marginal region and the ventral animal region at 90% epiboly and at tailbud stage in wild type embryos (Fig. 8A). In 90% epiboly wild type embryos, further, bmp2b was strongly expressed in a triangular region at the tip of the non-neural ectodermal border (Fig. 8A, arrowhead). In MO-injected embryos, this strong expression was absent and the entire border expression of bmp2b was reduced, although expression in the ventral region persisted (Fig. 8B; arrow). In tailbud stage wild–type embryos, bmp2b was strongly expressed in the border of the non-neural ectodermal region. In MO-injected embryos, a new region of strong bmp2b expression appeared to form, suggesting the formation of a new border region (data not shown). Interestingly, the position of the border in MO-injected embryos was different from that in wild-type embryos.
Fig. 8.
Expression of bmp2b, 4, and 7 in embryos injected with dlx3b-MO and dlx4b-MO at 90% epiboly. Expression in wild-type embryos of bmp2b (A), bmp4 (C), and bmp7 (E). Expression in 20 ng ATG-dlx3b-MO/20 ng ATG-dlx4b-MO-injected embryos of bmp2b (B), bmp4 (D), and bmp7 (F). (A and B) show that there is a strong triangle of bmp2b expression (arrowhead) at the border of the non-neural ectoderm in the wild-type embryos but this region is lost in MOs-injected embryos. Ventral bmp2b expression persisted in MOs-injected embryos (arrow). No alterations are seen in the expression of bmp4 and bmp7 between wild-type embryos and MOs-injected embryos (C–F). Lateral views, and dorsal is to the right.
bmp4 and bmp7, as well as bmp2b, are also expressed in early stage embryos (Dick et al., 2000; Martínez-Barberá et al., 1997; Nikaido et al., 1997; Schmid et al., 2000). bmp4 was expressed in the ventral marginal region at 90% epiboly and at tailbud stage in wild-type embryos (Fig. 8C). In MO-injected embryos, the expression pattern of bmp4 was unchanged (Fig. 8D), although expression was somewhat reduced at tailbud stage (data not shown). bmp7 was expressed in the ventral marginal region and the ventral animal region at 90% epiboly and at tailbud stage in wild-type embryos (Fig. 8E). In MO-injected embryos, the expression pattern of bmp7 was not changed at either stage (Fig. 8F). These results suggest that only bmp2b expression, and not that of bmp4 or bmp7, is regulated by dlx3b and dlx4b at 90% epiboly and the relationship is limited to the non-neural ectodermal/neural plate border region.
Differential function of dlx3b and dlx4b in RB neuron and trigeminal placodal development
The above analyses indicate that dlx3b and dlx4b are required in RB neuron and trigeminal placodal development, but how dlx3b and dlx4b affect these development is not clear. dlx3b and dlx4b likely function cell-autonomously in placodal development (Liu et al., 2003; Solomon and Fritz, 2002), but whether this function extends to the other cell type of interest is not known. Here, we have studied how dlx3b and dlx4b affect both RB neuron and trigeminal placodal development.
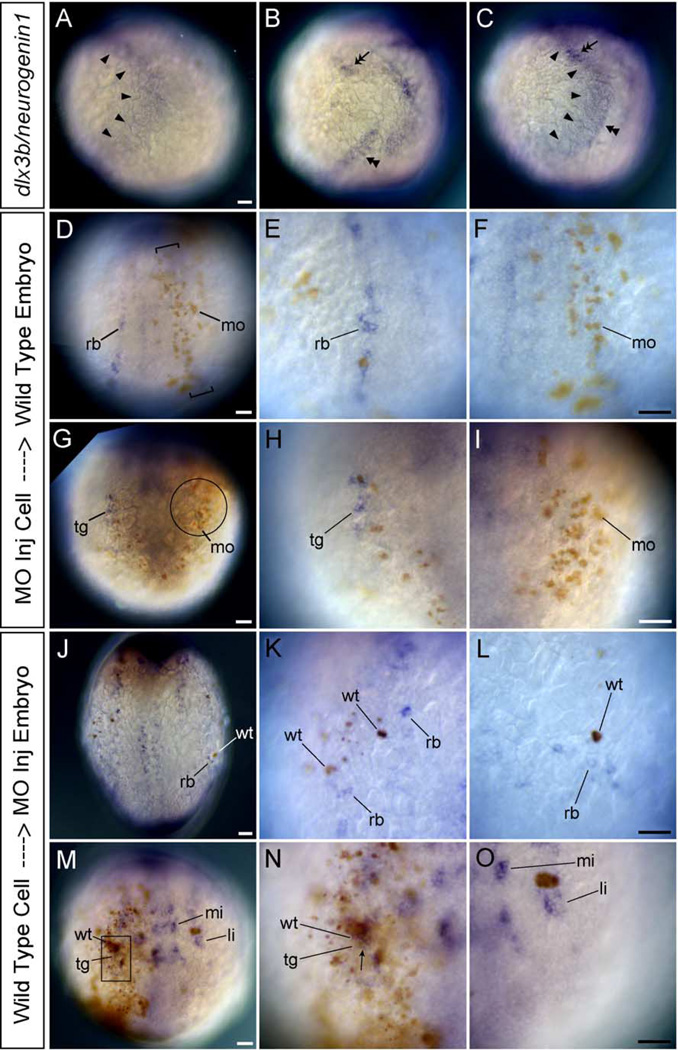
In the future lateral region of the trunk in tailbud stage embryos, the expression of neurogenin1 marks future RB neurons and dlx3b expression was adjacent but not overlapping (Figs. 9A–C; arrowheads, dlx3b and double arrowhead, neurogenin1). This pattern suggests the possibility that dlx3b and dlx4b act non-cell-autonomously in RB neuron development. To test this hypothesis that dlx3b and dlx4b act non-cell-autonomously, we created mosaic embryos between wild-type and MO-injected embryos. MO-injected cells were transplanted into wild-type host embryos as well as wild-type cells transplanted into MO-injected embryos at blastula stage. We examined RB neuron and trigeminal placodal development by whole-mount in situ hybridization with the HuC probe. In a wild-type background, HuC-expressing RB neurons could not be detected in the lateral neural plate when labeled MO-injected cells were situated in the region just outside the neural plate (Figs. 9D–F; rb, RB neurons, mo, MO transplanted cells). In contrast, when labeled wild-type cells expressing normal levels of dlx3b and dlx4b were transplanted into a MO-injected embryo, HuC-expressing RB neurons were seen only around the labeled wild-type transplanted cells (Figs. 9J–L; wt, wild-type transplanted cells). This suggests that a signal from wild-type cells can rescue locally the effects of the dlx3b and dlx4b MO. These data demonstrate that dlx3b and dlx4b function non-cell-autonomously in RB neuron development.
Fig. 9.
HuC expression in mosaic embryos between wild-type and MO-injected embryos. (A–C) Wild-type embryos at tailbud stage. Lateral views, and dorsal is to the right. (D–O) Transplanted embryos at 3-somite stage. (D–I) are the wild-type host embryos. (J–O) are the MOs-injected host embryos. (D and J) Dorsal views. (G and M) Anterior views. (A) Expression pattern of dlx3b (arrowheads). (B) Expression pattern of neurogenin1 (double arrowhead in the trunk and double arrow in the head). (C) Expression pattern of dlx3b and neurogenin1. (D–O) HuC expression in mosaic embryos. (A–C) show that dlx3b expression (arrowheads) and neurogenin1 expression in the lateral trunk (double arrowhead) are adjoining but not overlapping in the trunk. In contrast, dlx3b expression and neurogenin1 expression in the lateral head (double arrow) are overlapping. (D–F) show that RB neurons (rb) do not develop in the neural plate where brown-labeled MOs-injected cells (mo) are in a narrow region (bracket) just outside the neural plate. dlx3b and dlx4b should be expressed in this narrow region under wild-type conditions. (E) A high magnification view of RB neurons (rb) on the left side of embryo in D. (F) A high magnification view of the narrow region defined by brackets on the right side of embryo in D. The high number of MO-injected cells (brown, mo) is able to inhibit HuC expression in neighboring cells. (G–I) show that neurons in the trigeminal placodes (tg) do not develop there when brown-labeled MOs-injected cells occupy the trigeminal placode (circle). dlx3b and dlx4b are expressed in this circle region under wild-type conditions. (H) A high magnification view of neurons in the trigeminal placode (tg) on the right side of embryo in G. (I) A high magnification view of the trigeminal placodal region defined by the circle on the left side of embryo in G. The high number of MO injected cells (brown, mo) is able to inhibit HuC expression in the trigeminal placode. (J–L) In a MO-injected background, RB neurons are seen only around the brown-labeled wild-type cells (wt) in the embryonic epidermis. The host MOs-injected embryos develop no or reduced levels of RB neurons. (K) A high magnification view of RB neurons on the left side of embryo in J. RB neurons can be seen only around the brown wild-type cells (wt). (L) A high magnification view of RB neurons on the right side of embryo in J. RB neurons can be seen only around the brown wild-type cells (wt). (M–O) Neurons in the trigeminal placodes develop when brown-labeled wild-type cells occupy the trigeminal placode (square) in the host MO-injected embryos having no trigeminal placodal neurons. (N) is the high magnification view of the trigeminal placode defined by the square on the right side of embryo in M. The high number of wild-type cells expresses HuC in themselves, so many double-labeled neurons can be seen in the trigeminal placode (arrow). (O) A high magnification view of the trigeminal placodal region on the left side of embryo in M. No neurons in the trigeminal placode are observed where wild-type cells are not present. li, anterior lateral hindbrain interneuron; mi, anterior medial hindbrain interneuron. All scale bars, 50 µm. Scale bar in A applies for A, B, and C. Scale bar in F applies for both E and F, etc.
In the future lateral region of the cranial region of tailbud stage embryos, neurogenin1 is expressed in the future trigeminal placode which overlaps with the expression of dlx3b (Figs. 9A–C; double arrow). This pattern suggests the possibility that dlx3b and dlx4b act cell-autonomously in the development of neurons in trigeminal placodes. To test this hypothesis that dlx3b and dlx4b act cell-autonomously, mosaic embryos were made and analyzed as in the case of RB neuron development. In a wild-type background, HuC-expressing neurons in the trigeminal placode could not be detected there when labeled MO-injected cells occupied the trigeminal placodal region (Figs. 9G–I; tg, neurons in trigeminal placode, mo, MO transplanted cells). In contrast, when labeled wild-type cells expressing normal level of dlx3b and dlx4b were situated in the trigeminal placodal region of a MO-injected embryo, brown and blue double-labeled neurons in the trigeminal placode were seen (Figs. 9M–O; arrow). These data demonstrate that dlx3b and dlx4b function cell-autonomously in the development of neurons in the trigeminal placodes. It remains possible that dlx3b and dlx4b can also act non-cell-autonomously in the trigeminal placode.
Discussion
dlx3b and dlx4b function in RB neuron and trigeminal placodal development in zebrafish
In the present study, using dlx3b-MO and dlx4b-MO and EnR-dlx3bhd mRNA, we have revealed that dlx3b and dlx4b activities are required for RB neuron and trigeminal placodal development. The expression of genes marking RB neurons and trigeminal placodes were absent, those marking neural crest were slightly reduced, and those marking neural plate were slightly broadened in the embryos injected with MOs or EnR-dlx3bhd mRNA (Figs. 2 and 3; and data not shown). In addition, it has been previously reported that dlx3b and dlx4b act in concert to promote zebrafish otic and olfactory placode formation (Liu et al., 2003; Solomon and Fritz, 2002). This suggests that dlx3b and dlx4b play multiple roles in the formation of the neural plate border.
How dlx3b and dlx4b affect the development of RB neurons and trigeminal placodes can be placed in two categories: cell-autonomous and non-cell-autonomous. In the present study, it was revealed that dlx3b and dlx4b act non-cell-autonomously in RB neuron development and cell-autonomously in trigeminal placodal development. In RB neuron development, dlx3b-MO- and dlx4b-MO-injected cells in the embryonic epidermis of a wild-type background cannot form HuC-positive RB neurons in the lateral neural plate. They prevent their neighbors from becoming the RB neuron fate. In addition, in a MO-injected host background, the transplantation of a single wild-type cell in the embryonic epidermis can rescue the HuC expression in RB neurons. This suggests that dlx3b and dlx4b are sufficient to induce the expression of HuC in the lateral neural plate. Since bmp2b expression is partially lost in MO-injected embryos, the non-cell-autonomous function of dlx3b and dlx4b may act via BMP activity. This notion is consistent with many previous reports that BMP actively promotes neural crest and RB neuron development and suppresses neural plate development (Barth et al., 1999; Kishimoto et al., 1997; Nguyen et al., 1998, 2000).
The expression of neurogenin1, an upstream gene of neuroD, was highly reduced in cranial trigeminal placodes in dlx3b-MO- and dlx4b-MO-injected embryos. However, the same gene marking RB neurons was normal in the spinal cord region of MO-injected embryos. The expression of neurogenin1 in the lateral trunk appears at around 90% epiboly (Andermann et al., 2002) in a broad domain at the lateral neural plate. These data in sum suggest the possibility that the non-cell-autonomous function of dlx3b and dlx4b starts relatively late to affect downstream target genes. Alternatively, if the dlx3b and dlx4b function commences early, they may affect only gene expression over a very short range. Our preliminary data that the expression of neuroD marking RB neurons, which is an immediate downstream gene of neurogenin1, was absent in MO-injected embryos would support the latter possibility. dlx3b and dlx4b also function in a cell-autonomous manner. From the expression of neurogenin1 in trigeminal placodal region in MO-injected embryos and that of pax8 in otic placodes in MO-injected embryos (Solomon and Fritz, 2002), it is apparent that this activity begins between 80% and 90% epiboly. In both RB neurons and trigeminal placodes, some common genes are expressed through their development, for example, neurogenin1, neuroD, HuC, and isl-1. However, in RB neuron region, the mediolateral reduction of the width of RB neuron region is observed through development, between neurogenin1 and neuroD/HuC/isl-1 expression (Blader et al., 1997; Korzh et al., 1998; Ma et al., 1996). The non-cell-autonomous function of dlx3b and dlx4b may contribute to the event, as well as competitive lateral inhibition by the activities of multiple delta genes (Haddon et al., 1998).
The data presented here are consistent with our recent report in Xenopus embryos injected with EnR-Dlx3hd mRNA, where downstream targets of Dlx proteins were repressed (Woda et al., 2003). However, the neural crest and neural plate phenotypes differ between zebrafish and Xenopus. In zebrafish embryos injected with dlx3b/dlx4b-MO or EnR-dlx3bhd mRNA, loss of neural crest did not occur, although a reduction in expression of neural crest marker genes could be seen, and the degree of expansion of neural plate was subtle. In Xenopus embryos injected with EnR-Dlx3hd mRNA, loss of neural crest is often seen, and the degree of expansion of neural plate is severe (Woda et al., 2003). These data suggest that there is a compensation mechanism in zebrafish that is not present in Xenopus. Since dlx3b/dlx4b and Xdlx3 are likely to function in a non-cell-autonomous manner for neural crest development in both species, there may be another signal for neural crest development in zebrafish. In fact, in Xenopus, Bmp4 activity is highly reduced in embryos injected with EnR-Dlx3hd mRNA (J.W. and K.B.A. unpublished observation). In contrast, in zebrafish, the reduction of bmp2b expression was seen only at the non-neural ectodermal border at 90% epiboly, and bmp4 and bmp7 expression was almost normal, in MO-injected embryos (Fig. 8). These small reductions of bmp genes might also affect the subtleness of phenotype in neural plate of zebrafish (see below).
In the present study, a relatively high concentration of MO is used. As discussed above, dlx3b and dlx4b may start functioning between 80% and 90% epiboly in the formation of the border between neural and non-neural ectoderm of gastrula and neurula. However, the expression of dlx3b begins at a very early embryonic stage, blastula stage, and it is expressed in the entire of epiblast (Fig. 1B; and data not shown). Because of the early-stage expression of dlx3b, the effect of the injected dlx3b-MO is reduced at the time when it is required for neural plate border formation (90% epiboly; Fig. 1F). In fact, our analysis of the RT-PCR from cDNA isolated at 2–3-somite-stage wild-type and dlx3b/dlx4b-E2I2-MO-injected embryos revealed that by 2–3-somite stage, the reduction in dlx3b-E2I2-MO concentration was unable to knockdown dlx3b fully, while the concentration of dlx4b-E2I2-MO was sufficient. Gastrulation defects occurred in rare cases in 20 ng dlx3b-MO- and 20 ng dlx4b-MO-injected embryos, but it did not occur in 40 ng control MO-injected embryos (Table 1). Thus, the early-stage expression of dlx3b might contribute to process of epiboly.
Two different dlx3b/dlx4b-MOs were used to study the function of these genes. One is dlx3b/dlx4b ATG MO, which binds to the translation initiation site of the mRNA, and the other is dlx3b/dlx4b E2I2 MO, which bind the exon2–intron2 boundary of their nuclear RNA. Phenotypes in RB neurons and trigeminal placodes by dlx3b/dlx4b E2I2 MO were slightly milder than those by dlx3b/dlx4b ATG MO. It is known that some Dlx genes, for example mouse Dlx5 and Dlx4, produce multiple transcripts by alternative splicing (Liu et al., 1997; Nakamura et al., 1996; Yang et al., 1998). From the difference of the degree in phenotypes, there is a possibility of the existence of alternatively spliced forms of dlx3b. In addition, we observed a wild-type form present after the E2I2–dlx3b-MO injection, which could compensate for the function of the alternate spliced form at later stages (2–3-somite stage). However, since the phenotype is consistent with the ATG-MOs and EnR–dlx3bhd, we believe that there is sufficient knockdown of the dlx3b at the appropriate time (90% epiboly) to produce specific phenotypes.
dlx3b and dlx4b may intensify bmp2b activity at the non-neural ectodermal border of gastrula in zebrafish
In zebrafish and Xenopus, the BMP activity gradient model is widely supported as a mechanism of neural crest formation (Aybar and Mayor, 2002; Nguyen et al., 1998, 2000). A BMP activity gradient in the ectoderm of the gastrula stage is established by an interaction between BMPs produced in the ectoderm and BMP inhibitors secreted from the dorsal mesoderm. In the ectoderm, high levels of BMP activity specify the epidermis, and low levels specify the neural plate. Intermediate levels specify the fate of cells at the neural plate border. Our data reveal that there is a region of strong bmp2b expression, at the border of non-neural and neural ectoderm at 90% epiboly, and that this expression of bmp2b is lost in dlx3b-MO- and dlx4b-MO-injected embryos. This result suggests that the specification of cells at the neural plate border in zebrafish may be more complex than simply relying on the BMP activity gradient alone. BMP activity at the border may be slightly higher than is currently suggested. In fact, the neural crest region did not expand laterally and its marker gene expression was slightly reduced in MO-injected embryos, where subtle loss of bmp2b expression is seen. The phenotype is much different from somitabun and snailhouse mutant embryos, which also have low levels of BMP activity and show the phenotype of the expanded neural crest region (Nguyen et al., 1998). dlx3b and dlx4b may function in intensifying BMP activity at the border region, rather than keeping the simple gradient of BMP activity in the ectoderm. The difference of the degree of phenotypes between RB neurons and neural crest in MO-injected embryos may be explained by the fact that RB neuron development is more sensitive to the optimal level of BMP activity than neural crest cells. We did not study the function of dlx3b before 80% epiboly, when the broad homogeneous expression of dlx3b overlaps with bmp2b on the ventral side of the gastrula. Thus, we cannot rule out the possibility that dlx3b regulates bmp2b gene expression during early gastrulation. If there is a role for dlx3b earlier than 80% epiboly, dlx3b function may be separated into two phases: (1) keeping BMP activity in the non-neural ectoderm before 80% epiboly, and (2) intensifying BMP activity in the non-neural ectodermal border at 90% epiboly. This is an interesting issue that remains to be studied in detail.
The loss of the strong bmp2b-expressing triangle region at the tip of the non-neural ectodermal border resulted in a curved bmp2b-expressing non-neural ectodermal region (Fig. 8). In the ectoderm of the gastrula, the bmp2b-expressing region and the neural ectodermal region are always complementary (Muraoka et al., 2000; Nikaido et al., 1999). The loss of bmp2b expression may be involved in the expansion of the neural plate in MO-injected embryos. Interestingly, a new region of intense bmp2b expression appears as a presumably new non-neural ectodermal border forms. This occurs at tailbud stage in the MO-injected embryos (data not shown), and the expression persists at least to 3-somite stage. It appears at a different position, and thus it is clear that it is a different border from the original wild-type border. This suggests that dlx3b and dlx4b may play a role in defining the shape of the non-neural ectodermal border at 90% epiboly.
Acknowledgments
The authors thank Dr. G. Boekhoff-Falk (Panganiban) and Dr. J. Kohtz and Dr. J. Feng for the Dll antibody, Dr. M.C. Mullins and Dr. M. Hammerschmidt for providing bmp7, Dr. M. Ekker for dlx4b, Dr. S. Wilson for paraxis, and others. We would also like to thank Dr. David Stock for providing dlx3b/dlx4b genomic sequence and for discussions; Dr. Linda Barlow for critical reading of the manuscript; Laura Hernandez and Dawn Riedel for excellent technical assistance and Pete Simpson for technical assistance and fish care/maintenance. We gratefully acknowledge the support of NIH/NIDCR (K22DE14200) to KBA.
References
- Agathon A, Thisse B, Thisse C. Morpholino knock-down of antivin1 and antivin2 upregulates nodal signaling. Genesis. 2001;30:178–182. doi: 10.1002/gene.1059. [DOI] [PubMed] [Google Scholar]
- Andermann P, Ungos J, Raible DW. Neurogenin1 defines zebrafish cranial sensory ganglia precursors. Dev. Biol. 2002;251:45–58. doi: 10.1006/dbio.2002.0820. [DOI] [PubMed] [Google Scholar]
- Appel B, Korzh V, Glasgow E, Thor S, Edlund T, Dawid IB, Eisen JS. Motoneuron fate specification revealed by patterned LIM homeobox gene expression in embryonic zebrafish. Development. 1995;121:4117–4125. doi: 10.1242/dev.121.12.4117. [DOI] [PubMed] [Google Scholar]
- Artinger KB, Chitnis AB, Mercola M, Driever W. Zebrafish narrowminded suggests a genetic link between formation of neural crest and primary sensory neurons. Development. 1999;126:3969–3979. doi: 10.1242/dev.126.18.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aybar MJ, Mayor R. Early induction of neural crest cells: lessons learned from frog, fish and chick. Curr. Opin. Genet. Dev. 2002;12:452–458. doi: 10.1016/s0959-437x(02)00325-8. [DOI] [PubMed] [Google Scholar]
- Baker CVH, Bronner-Fraser M. Vertebrate cranial placodes. I. Embryonic induction. Dev. Biol. 2001;232:1–61. doi: 10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- Bally-Cuif L, Hammerschmidt M. Induction and patterning of neuronal development, and its connection to cell cycle control. Curr. Opin. Neurobiol. 2003;13:16–25. doi: 10.1016/s0959-4388(03)00015-1. [DOI] [PubMed] [Google Scholar]
- Barth KA, Kishimoto Y, Rohr KB, Seydler C, Schulte-Merker S, Wilson SW. Bmp activity establishes a gradient of positional information throughout the entire neural plate. Development. 1999;126:4977–4987. doi: 10.1242/dev.126.22.4977. [DOI] [PubMed] [Google Scholar]
- Blader P, Fischer N, Gradwohl G, Guillemot F, Strähle U. The activity of Neurogenin1 is controlled by local cues in the zebrafish embryo. Development. 1997;124:4557–4569. doi: 10.1242/dev.124.22.4557. [DOI] [PubMed] [Google Scholar]
- Busch-Nentwich E, Sollner C, Roehl H, Nicolson T. The deafness gene dfna5 is crucial for ugdh expression and HA production in the developing ear in zebrafish. Development. 2004;131:943–951. doi: 10.1242/dev.00961. [DOI] [PubMed] [Google Scholar]
- Cornell RA, Eisen JS. Delta/Notch signaling promotes formation of zebrafish neural crest by repressing Neurogenin1 function. Development. 2002;129:2639–2648. doi: 10.1242/dev.129.11.2639. [DOI] [PubMed] [Google Scholar]
- Dick A, Hild M, Bauer H, Imai Y, Maifeld H, Schier AF, Talbot WS, Bouwmeester T, Hammerschmidt M. Essential role of Bmp7 (snailhouse) and its prodomain in dorsoventral patterning of the zebrafish embryo. Development. 2000;127:343–354. doi: 10.1242/dev.127.2.343. [DOI] [PubMed] [Google Scholar]
- Draper BW, Morcos PA, Kimmel CB. Inhibition of zebrafish fgf8 pre-mRNA splicing with morpholino oligos: a quantifiable method for gene knockdown. Genesis. 2001;30:154–156. doi: 10.1002/gene.1053. [DOI] [PubMed] [Google Scholar]
- Ekker M, Akimenko MA, Bremiller R, Westerfield M. Regional expression of three homeobox transcripts in the inner ear of zebrafish embryos. Neuron. 1992;9:27–35. doi: 10.1016/0896-6273(92)90217-2. [DOI] [PubMed] [Google Scholar]
- Feledy JA, Beanan MJ, Sandoval JJ, Goodrich JS, Lim JH, Matsuo-Takasaki M, Sato SM, Sargent TD. Inhibitory patterning of the anterior neural plate in Xenopus by homeodomain factors Dlx3 and Msx1. Dev. Biol. 1999;212:455–464. doi: 10.1006/dbio.1999.9374. [DOI] [PubMed] [Google Scholar]
- Haddon C, Smithers L, Schneider-Maunoury S, Coche T, Henrique D, Lewis J. Multiple delta genes and lateral inhibition in zebrafish primary neurogenesis. Development. 1998;125:359–370. doi: 10.1242/dev.125.3.359. [DOI] [PubMed] [Google Scholar]
- Kim C, Ueshima E, Muraoka O, Tanaka H, Yeo S, Huh T, Miki N. Zebrafish elav/HuC homologue as a very early neuronal marker. Neuro. Lett. 1996;216:109–112. doi: 10.1016/0304-3940(96)13021-4. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Lee K, Zon L, Hammerschmidt M, Schulte-Merker S. The molecular nature of zebrafish swirl: BMP2 function is essential during early dorsoventral patterning. Development. 1997;124:4457–4466. doi: 10.1242/dev.124.22.4457. [DOI] [PubMed] [Google Scholar]
- Korzh V, Edlund T, Thor S. Zebrafish primary neurons initiate expression of the LIM homeodomain protein Isl-1 at the end of gastrulation. Development. 1993;118:417–425. doi: 10.1242/dev.118.2.417. [DOI] [PubMed] [Google Scholar]
- Korzh V, Sleptsova I, Liao J, He J, Gong Z. Expression of zebrafish bHLH genes ngn1 and nrd defines distinct stages of neural differentiation. Dev. Dyn. 1998;213:92–104. doi: 10.1002/(SICI)1097-0177(199809)213:1<92::AID-AJA9>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Krauss S, Johansen T, Korzh V, Fjose A. Expression of the zebrafish paired box gene pax[zf-b] during early neurogenesis. Development. 1991;113:1193–1206. doi: 10.1242/dev.113.4.1193. [DOI] [PubMed] [Google Scholar]
- Krebs RA, Feder ME. Tissue-specific variation in Hsp70 expression and thermal damage in Drosophila melanogaster larvae. J. Exp. Biol. 1997;200:2007–2015. doi: 10.1242/jeb.200.14.2007. [DOI] [PubMed] [Google Scholar]
- Langenau DM, Palomero T, Kanki JP, Ferrando AA, Zhou Y, Zon LI, Look AT. Molecular cloning and developmental expression of Tlx (Hox11) genes in zebrafish (Danio rerio) Mech. Dev. 2002;117:243–248. doi: 10.1016/s0925-4773(02)00187-9. [DOI] [PubMed] [Google Scholar]
- Lekven AC, Thorpe CJ, Waxman JS, Moon RT. Zebrafish wnt8 encodes two wnt8 proteins on a bicistronic transcript and is required for mesoderm and neurectoderm patterning. Dev. Cell. 2001;1:103–114. doi: 10.1016/s1534-5807(01)00007-7. [DOI] [PubMed] [Google Scholar]
- Liu JK, Ghattas I, Liu S, Chen S, Rubenstein JLR. Dlx genes encode DNA-binding proteins that are expressed in an overlapping and sequential pattern during basal ganglia differentiation. Dev. Dyn. 1997;210:498–512. doi: 10.1002/(SICI)1097-0177(199712)210:4<498::AID-AJA12>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Liu D, Chu H, Maves L, Yan Y, Morcos PA, Postlethwait JH, Westerfield M. Fgf3 and Fgf8 dependent and independent transcription factors are required for otic placode specification. Development. 2003;130:2213–2224. doi: 10.1242/dev.00445. [DOI] [PubMed] [Google Scholar]
- Ma Q, Kintner C, Anderson DJ. Identification of neurogenin, a vertebrate neuronal determination gene. Cell. 1996;87:43–52. doi: 10.1016/s0092-8674(00)81321-5. [DOI] [PubMed] [Google Scholar]
- Martínez-Barberá JP, Toresson H, Da Rocha S, Krauss S. Cloning and expression of three members of the zebrafish Bmp family: Bmp2a, Bmp2b and Bmp4. Gene. 1997;198:53–59. doi: 10.1016/s0378-1119(97)00292-8. [DOI] [PubMed] [Google Scholar]
- McLarren KW, Litsiou A, Streit A. DLX5 positions the neural crest and preplacode region at the border of the neural plate. Dev. Biol. 2003;259:34–47. doi: 10.1016/s0012-1606(03)00177-5. [DOI] [PubMed] [Google Scholar]
- Muraoka O, Ichikawa H, Shi H, Okumura S, Taira E, Higuchi H, Hirano T, Hibi M, Miki N. Kheper, a novel ZFH/δEF1 family member, regulates the development of the neuroectoderm of zebrafish (Danio rerio) Dev. Biol. 2000;228:29–40. doi: 10.1006/dbio.2000.9909. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Stock DW, Wydner KL, Bollekens JA, Takeshita K, Nagai BM, Chiba S, Kitamura T, Freeland TM, Zhao Z, Minowada J, Lawrence JB, Weiss KM, Ruddle FH. Genomic analysis of a new mammalian Distal-less gene: Dlx7. Genomics. 1996;38:314–324. doi: 10.1006/geno.1996.0634. [DOI] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat. Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Nguyen VH, Schmid B, Trout J, Connors SA, Ekker M, Mullins MC. Ventral and lateral regions of the zebrafish gastrula, including the neural crest progenitors, are established by a bmp2b/swirl pathway of genes. Dev. Biol. 1998;199:93–110. doi: 10.1006/dbio.1998.8927. [DOI] [PubMed] [Google Scholar]
- Nguyen VH, Trout J, Connors SA, Andermann P, Weinberg E, Mullins MC. Dorsal and intermediate neuronal cell types of the spinal cord are established by a BMP signaling pathway. Development. 2000;127:1209–1220. doi: 10.1242/dev.127.6.1209. [DOI] [PubMed] [Google Scholar]
- Nikaido M, Tada M, Saji Y, Ueno N. Conservation of BMP signaling in zebrafish mesoderm patterning. Mech. Dev. 1997;61:75–88. doi: 10.1016/s0925-4773(96)00625-9. [DOI] [PubMed] [Google Scholar]
- Nikaido M, Tada M, Takeda H, Kuroiwa A, Ueno N. In vivo analysis using variants of zebrafish BMPR-IA: range of action and involvement of BMP in ectoderm patterning. Development. 1999;126:181–190. doi: 10.1242/dev.126.1.181. [DOI] [PubMed] [Google Scholar]
- Odenthal J, Nqsslein-Volhard C. Fork head domain genes in zebrafish. Dev. Genes Evol. 1998;208:245–258. doi: 10.1007/s004270050179. [DOI] [PubMed] [Google Scholar]
- Oxtoby E, Jowett T. Cloning of the zebrafish krox-20 gene (krx-20) and its expression during hindbrain development. Nuc. Acid. Res. 1993;21:1087–1095. doi: 10.1093/nar/21.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panganiban G, Rubenstein JLR. Developmental functions of the Distal-less/Dlx homeobox genes. Development. 2002;129:4371–4386. doi: 10.1242/dev.129.19.4371. [DOI] [PubMed] [Google Scholar]
- Panganiban G, Sebring A, Nagy L, Carroll S. The development of crustacean limbs and the evolution of arthropods. Science. 1995;270:1363–1366. doi: 10.1126/science.270.5240.1363. [DOI] [PubMed] [Google Scholar]
- Pera E, Stein S, Kessel M. Ectodermal patterning in the avian embryo: epidermis versus neural plate. Development. 1999;126:63–73. doi: 10.1242/dev.126.1.63. [DOI] [PubMed] [Google Scholar]
- Sawada A, Fritz A, Jiang Y, Yamamoto A, Yamasu K, Kuroiwa A, Saga Y, Takeda H. Zebrafish Mesp family genes, mesp-a and mesp-b are segmentally expressed in the presomitic mesoderm, and Mesp-b confers the anterior identity to the developing somite. Development. 2000;127:1691–1702. doi: 10.1242/dev.127.8.1691. [DOI] [PubMed] [Google Scholar]
- Schier AF, Neuhauss SCF, Helde KA, Talbot WS, Driever W. The one-eyed pinhead gene functions in mesoderm and endoderm formation in zebrafish and interacts with no tail. Development. 1997;124:327–342. doi: 10.1242/dev.124.2.327. [DOI] [PubMed] [Google Scholar]
- Schlosser G, Northcutt RG. Development of neurogenic placodes in Xenopus laevis. J. Comp. Neurol. 2000;418:121–146. [PubMed] [Google Scholar]
- Schmid B, Fürthauer M, Connors SA, Trout J, Thisse B, Thisse C, Mullins MC. Equivalent genetic roles for bmp7/snailhouse and bmp2b/swirl in dorsoventral pattern formation. Development. 2000;127:957–967. doi: 10.1242/dev.127.5.957. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, Hammerschmidt M, Beuchle D, Cho KW, De Robertis EM, Nqsslein-Volhard C. Expression of zebrafish goosecoid and no tail gene products in wild-type and mutant no tail embryos. Development. 1994;120:843–852. doi: 10.1242/dev.120.4.843. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, van Eeden FJM, Halpern ME, Kimmel CB, Nqsslein-Volhard C. No tail (ntl) is the zebrafish homologue of the mouse T (Brachyury) gene. Development. 1994;120:1009–1015. doi: 10.1242/dev.120.4.1009. [DOI] [PubMed] [Google Scholar]
- Shanmugalingam S, Wilson SW. Isolation, expression and regulation of a zebrafish paraxis homologue. Mech. Dev. 1998;78:85–89. doi: 10.1016/s0925-4773(98)00150-6. [DOI] [PubMed] [Google Scholar]
- Solnica-Krezel L, Driever W. Microtubule arrays of the zebrafish yolk cell: organization and function during epiboly. Development. 1994;120:2443–2455. doi: 10.1242/dev.120.9.2443. [DOI] [PubMed] [Google Scholar]
- Solomon KS, Fritz A. Concerted action of two dlx paralogs in sensory placode formation. Development. 2002;129:3127–3136. doi: 10.1242/dev.129.13.3127. [DOI] [PubMed] [Google Scholar]
- Strähle U, Blader P, Henrique D, Ingham PW. Axial, a zebrafish gene expressed along the developing body axis, shows altered expression in cyclops mutant embryos. Genes Dev. 1993;7:1436–1446. doi: 10.1101/gad.7.7b.1436. [DOI] [PubMed] [Google Scholar]
- Thisse C, Thisse B. High resolution whole-mount in situ hybridization. Zebra. Scien. Monit. 1998;15:8–9. [Google Scholar]
- Torres M, Gómez-Pardo E, Gruss P. Pax2 contributes to inner ear patterning and optic nerve trajectory. Development. 1996;122:3381–3391. doi: 10.1242/dev.122.11.3381. [DOI] [PubMed] [Google Scholar]
- Westerfield M. THE ZEBRAFISH BOOK. Eugene: The University of Oregon Press; 1993. [Google Scholar]
- Woda JM, Pastagia J, Mercola M, Artinger KB. Dlx proteins position the neural plate border and determine adjacent cell fates. Development. 2003;130:331–342. doi: 10.1242/dev.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Zhang H, Hu G, Wang H, Abate-Shen C, Shen MM. An early phase of embryonic Dlx5 expression defines the rostral boundary of the neural plate. J. Neurosci. 1998;18:8322–8330. doi: 10.1523/JNEUROSCI.18-20-08322.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]