Abstract
The potential for human exposure to engineered nanoparticles due to the use of nanotechnology-based consumer sprays (categorized as such by the Nanotechnology Consumer Products Inventory) is examined along with analogous products, which are not specified as nanotechnology-based (regular products). Photon correlation spectroscopy was used to obtain particle size distributions in the initial liquid products. Transmission electron microscopy was used to determine particle size, shape, and agglomeration of the particles. Realistic application of the spray products near the human breathing zone characterized airborne particles that are released during use of the sprays. Aerosolization of sprays with standard nebulizers was used to determine their potential for inhalation exposure. Electron microscopy detected the presence of nanoparticles in some nanotechnology-based sprays as well as in several regular products, whereas the photon correlation spectroscopy indicated the presence of particles <100 nm in all investigated products. During the use of most nanotechnology-based and regular sprays, particles ranging from 13 nm to 20 μm were released, indicating that they could he inhaled and consequently deposited in all regions of the respiratory system. The results indicate that exposures to nanoparticles as well as micrometer-sized particles can be encountered owing to the use of nanotechnology-based sprays as well as regular spray products.
Keywords: aerosolization, nanoaerosol, emerging contaminants, particulate matter, inhalation exposure, personal exposure
Introduction
The US National Nanotechnology Initiative defines nanotechnology as “the understanding and control of matter at dimensions between approximately 1 and 100 nanometers, where unique phenomena enable novel applications” (National Science and Technology Council, 2011). Given the unique properties of materials at such scale, the development of nanotechnologies and their implementation in consumer products are undergoing rapid growth. Regardless of the public’s perception of nanotechnology as largely a future issue, nanosized ingredients have already been incorporated in an extensive variety of products in the market (Maynard, 2007; Bradford et al., 2009; Woodrow Wilson International Center for Scholars, 2010b). The Project on Emerging nanotechnologies (Woodrow Wilson International Center for Scholars, 2010a) currently lists over 1000 nanotechnology-based consumer products. The development and commercial application of nanotechnologies are progressing in the absence of specific regulations or legal guidelines for labeling (Paull and Lyons, 2008). More importantly, we have a very limited understanding of the potential for exposure to nanoparticles from such products and resulting health effects, which is critical for the development of safety regulations and guidelines (Segal, 2004; Frater et al., 2006; Van Calster, 2006; Drobne, 2007; Warheit et al., 2007a; Schmid et al., 2009).
The concern about exposure to particles in the size range between 1 and 100 nm is based on the fact that the physical and chemical properties of nanosized matter differ substantially from the properties of the same materials in bulk, including their toxicity, biological, and health effects (Maynard et al., 2006). Studies analyzing biological and health effects of nanoparticles have shown a whole array of alarming issues, including incursion, retention, and mobility of nanoparticles within living organisms and tissues (Oberdörster et al., 2005b). For example, 13C-graphite-derived carbon nanoparticles (median diameter =36 nm) were found to translocate from the respiratory system to the olfactory bulb of the rat central nervous system (Oberdörster et al., 2004). The same effect was found for the manganese oxide nanoparticles (median diameter = 30 nm) with resulting inflammatory changes (Elder et al., 2006). Titanium dioxide aerosol particles of 22 nm count median diameter inhaled by rats were later (1 and 24 h) found on the luminal side of airways and alveoli, in all major lung tissue compartments and cells, and within capillaries (Geiser et al., 2005). Size and state of agglomeration of titanium dioxide nanoparticles administered through various routes have been shown to affect inflammatory response in various mice tissues (Grassian et al., 2007a, b; Wang et al., 2007). Differential pulmonary effects following rodent exposure to various nanosized TiO2 particle types (rutile, anatase and their combination) were also documented (Warheit et al., 2007b).
Notably, these toxicological studies investigated only pure nanomaterials. Hagendorfer et al. (2009) investigated the release of nanoparticles from one nanotechnology-based silver spray product. Their approach addressed only one kind of spray product and did not consider a realistic application scenario. It is important to consider the exposure and health effects associated with the use of various available types of nanotechnology-based consumer products. In contrast to pure fresh nanomaterials, consumer product use can lead to the release of both nanoparticles incorporated in a product and the particles from the product’s holding matrix, that is, solvent and other ingredients. Therefore, exposure to nanoparticles from consumer products during their handling, application, and disposal still presents unknown health and environmental risks (Bradford et al., 2009; Keenan et al., 2009; Lioy et al., 2010). Characteristics of nanomaterials incorporated into the consumer products, including their size, surface area and chemistry, solubility, possibly shape, as well as location and concentration of nanoparticles in the product, can affect the potential for exposure by different pathways and the resulting adverse health effects (Shrader-Frechette, 2007). The free nanoparticles that are not fixed within a given material are of special concern in the context of potential exposure through inhalation and dermal routes (Hansen et al., 2008; Shimada et al., 2009). In addition, nanoparticle agglomerates can exhibit different biological effects compared with uniform particles of similar size (Bermudez et al., 2004). Interaction with other ingredients of a nanomaterial-containing product and the form in which it is used by the consumers (liquid, powder, spray, and so on) can lead to chemical modification, agglomeration, and other processes affecting the nanosized and/or nanostructured ingredient(s). This can have a substantial effect on the extent of exposure and health hazard of a given product compared with the pure nanomaterial ingredient(s). Thus, it is very difficult to predict the exposure and health effects of a particular nanotechnology-based consumer product with any certainty solely based on the characteristics of nanosized and/or nano-structured components within such a product (Maynard, 2007; Lioy et al., 2010). Therefore, characterization of nanotechnology-based consumer products in their final form as well as of particle releases during the products’ use is absolutely necessary when performing exposure and health risk assessment. However, there are virtually no studies examining compositions of nanotechnology-based consumer products as well as the exposure of consumers to nanoparticles owing to the use of such products in realistic application scenarios. Given the discussed differences between pure nanoparticles and those incorporated and released from products’ matrices during application, simulation of realistic exposure scenarios is necessary for reducing the uncertainties in exposure characterization and where necessary improve the products in commerce to reduce exposure (Lioy, 2010b).
The route and extent of exposure will depend on the type and application mode of the nanotechnology-based products (Oberdörster et al., 2005a). Application of nanotechnology-based sprays, which are used as cleaners, disinfectants, and cosmetic mists, is likely to result in inhalation exposure as their application yields aerosol emissions in the breathing zone of the user. A report by the Nanomaterial Toxicity Screening Working Group stressed the need to assess exposure to nanoparticles by various exposure routes, including the airborne (inhalation) route (Oberdörster et al., 2005a).
Given the lack of data on exposure to nanoparticles due to the use of consumer products, this study focuses on one product category, namely nanotechnology-based sprays with the following objectives: (1) characterize nanoparticles in several nanotechnology-based consumer spray products currently in the market; (2) characterize potential exposure to airborne nanoparticles due to the use of spray products in a realistic exposure scenario; and (3) study the regular spray products that perform similar functions and compare them with their nanotechnology-based counterparts. The five types of “nanoproduct–regular product” pairs were acquired and tested for potential exposures. They were personal care silver sprays, vitamin-containing facial mists, antioxidant-containing body mists, hair care sprays, and multi-purpose disinfectants. In addition, a cleaning nanoproduct was also tested. Overall, we believe this project responds to the call for independent, conflict of interest-free and open scientific literature published nanomaterial safety research (Maynard, 2007; Maynard and Aitken, 2007; Shrader-Frechette, 2007; Michelson, 2008; Thomas et al., 2009) and attempts to set the ground for more thorough quantitative exposure studies.
Materials and methods
Tested Sprays
Inclusion of the nanotechnology-based consumer sprays in this study was based on the presence of those sprays in the Woodrow Wilson Nanotechnology Consumer Products Inventory (Woodrow Wilson International Center for Scholars, 2010b). It should be noted that inclusion of products in this Inventory is based on the manufacturer’s report regarding the presence of nanocomponents in them. There is no certainty that any given product in the inventory includes nanotechnological components (Hansen et al., 2008; Som et al., 2010).
A common consumer spray, in which the use of nanotechnology has not been specified by the manufacturer (regular product), was selected to match each “nanoproduct” based on the application purpose. The list of both nano and regular products examined in this study along with their compositions and suggested applications as per the manufacturers are presented in Table 1. Owing to high corrosiveness of the Wheel Nanocleaner and high likelihood of its alternative product being corrosive as well, a corresponding alternative regular product was not tested.
Table 1.
Tested consumer spray products.
| Producta | Compositionb | Purposeb |
|---|---|---|
| Silver Nanospray | Silver nanoparticles, purified water | Used topically or internally as a traditional defense against bacteria |
| Regular Silver Spray | 99.99% Pure silver suspended in demineralized water | Used topically to treat burns, rashes, as a nasal spray for hay fever, and deodorant; also for plants and pets |
| Facial Nanospray | Distilled water, vitamin C, nanosize particles of: copper, calcium, magnesium, zinc | Applied topically for younger looking skin |
| Regular Facial Spray | Water, butylene glycol, glycerin, panthenol, tocopheryl acetate, phenoxyethanol, alcohol denat., methylparaben, lecithin, Rosa centifolia (Rose) water, butylparaben, ethylparaben, isobutylparaben, propylparaben | On-call moisture and vitamin E protection for the face skin |
| Hair Nanospray | Alcohol denat., aqua, PVP/VA co-polymer, isopropyl alcohol, mytrimonium bromide, parfum | Used to hold nanofiber hair additions to scalp |
| Regular Hair Spray | SDA alcohol 40-B, water, VA/crotonates/vinyl neodecanoate copolymer, octylacrylamide/acrylates/butylaminoethyl, methacrylate co-polymer, aminomethanol propanol, lauryl pyrrolidone, PEG-75 lanolin, cyclopentasiloxane, fragrance | Hair spray for men |
| Disinfectant Nanospray | Parachlorometaxylenol—0.20%, other ingredients—99.80% | Multi-purpose, ready-to-use disinfectant, sanitizer, and deodorizing cleaner for use on hard surfaces |
| Regular Disinfectant Spray | o-Phenylphenol—0.22%, diisobutylphenoxyethoxy ethyl dimethyl benzyl ammonium chloride monohydrate—0.70%, inert ingredients—99.08% | Multi-purpose, ready-to-use disinfectant for use on hard surfaces |
| Skin Hydrating Nanomist | Purified water, Dimethicone, copolyol, algae extract, mugwort (Artemisia vulgaris) extract, Aloe barbadensis gel, fucogel, plankton extract, lavander (Lavendula angustifolia) oil, calcium PCA, zinc PCA, phenoxyethanol, methylparaben, propylparaben | Face and bodymist that helps reverse UV damage while adding powerful antioxidants and anti-inflammatory properties to sun parched skin |
| Regular Skin Hydrating Mist | Water, glycerin, hyaluronic acid, diazolidinyl urea, polysorbate 80, ergothioneine, Aloe Barbadensis leaf juice, sodium carboxymethyl β-glucan, Camellia sinensis leaf extract, tetrasodium EDTA, allantoin, citrus Aurantium bergamia (Bergamot) fruit oil, citric acid, kinetin, iodopropynyl butylcarbamate | Provides toning action and delivers antioxidant and hydrating benefits to the skin |
| Wheel Nanocleaner | Composition unavailable | Advanced nanotechnology formula quickly penetrates and removes tough brake dust and road grime from all wheel surfaces |
Nanoproduct: as per the Woodrow Wilson Nanotechnology Consumer Products Inventory.
As per the manufacturer.
Analysis of Sprays
As relatively little is known about the size and shape of particles incorporated in the consumer sprays, all the test products were analyzed in liquid state using two different methods described below. In addition, we compared the sizes of particles in liquid state with the sizes of particles from the same products in the airborne state, that is, during simulated application of the products.
Sample Analysis using Transmission Electron Microscopy
Size, shape, and agglomeration of electron-contrast particles (those visible in transmission electron microscopy (TEM)) in the spray products were determined using a TEM 2010F (JEOL, Tokyo, Japan). Small drops of each product were spread on HC300-Cu Holey Carbon Film on 300 Mesh Copper (Electron Microscopy Sciences, Hatfield, PA, USA) and left to dry in the ambient air for at least 1 h before testing. Particle size was measured manually (Matyi et al., 1987) from the resulting micrographs relative to automatically added scale marks. Particles found in both the nano and regular silver sprays were examined under high resolution, so that atomic grid could be seen.
Weak phase objects that have low electron contrast are not visible in TEM images; therefore, only certain types of nanoparticles, for example, certain metal, metal oxide, other inorganic, and some organic nanoparticles, could be seen using the TEM. Another particle feature that could be obtained from the TEM analysis is electron beam sensitivity. It is described as a structural alteration of the tested material owing to radiolysis (Hobbs, 1987; Egerton et al., 2004). Radiolysis can visually be observed during TEM investigations. Electron beam sensitivity results from electron irradiation above a certain magnification setting (Leapman and Sun, 1995; Turgis and Coqueret, 1999; Carlo et al., 2002), which results in a higher electron beam power density per unit area of the sample. As mostly organic nanoparticles tend to be beam sensitive (Egerton et al., 2004), it can be concluded with some degree of certainty about organic or inorganic nature of nanoparticles in the products based on beam sensitivity.
Sample Analysis using Photon Correlation Spectroscopy
Multimodal hydrodynamic particle size distributions in the original concentration of liquid spray products were determined using photon correlation spectroscopy (Bruce and Berne, 2000; Allen, 2003). A ZetaPALS 90 Plus with included Particle Sizing Software (both by Brookhaven Instruments Corporation, Holtsville, NY, USA) was used for this analysis. Hydrodynamic particle diameter includes the electric double layer around the particle and is the diameter of a hypothetical sphere that would diffuse at the same rate as the particle under examination. This diameter may also be called the equivalent sphere diameter (Brookhaven Instruments Corporation, 1995). The software uses Stokes–Einstein equation to transform diffusion coefficients, determined by dynamic light scattering, into hydrodynamic diameters presented as measurement results (Bodycomb, 2009).
Particles of any nature can be registered by this technique as long as their refractive index differs from that of the liquid medium. The Particle Sizing Software calculates multimodal particle size distributions as relative scattering intensity and relative number concentration for each registered hydrodynamic diameter. The highest intensity or the highest number concentration is expressed as 100%. All other intensity or number concentration values are expressed in percentage relative to the corresponding highest values. Thus, the data from these tests provide information used to infer the presence and relative concentration of particles of various hydrodynamic diameters. When discussing the results, the number concentration- and intensity-based hydrodynamic mode diameters have indices “N” and “I”, respectively.
For silver-containing products (Silver Nanospray and Regular Silver Spray), refractive index of 0.18 was used (that of metallic silver). For other products, refractive index of 1.60 was used based on the instrument manual’s (Brookhaven Instruments Corporation, 1995) recommendation for non-absorbing, white, opaque particles in the visible spectral region. Imaginary refractive indices were set to 0.00 (this value assumes absence of light absorption by particles at the used wavelength) for all samples based on the manual’s recommendation as well. The actual refractive indices of different particles within each product are unknown as the composition of most products is rather complex. However, even with these assumed indices, the data are expected to be of very high quality because particle refractive index is not used to calculate any intensity- or number concentration-weighted distributions. Also for particle sizes below ~60 nm, the spherical Mie factors (used by the software for calculating mass and number fractions from the measured intensity fractions) are independent of the particle refractive index (Brookhaven Instruments Corporation, 1995).
Analysis of the Released Particles in the Airborne State
Particle Release During Simulated Use
The first experimental setup shown in Figure 1a was used to measure the airborne particles released during a realistic product application, when a spray product is used near a person’s breathing zone. Here, a spray was applied near a commercially available mannequin’s head (Image Supply House, Endicott, NY, USA) and the inhalation of airborne particles during the spray application was simulated by sampling through two stainless-steel tubes installed in the mannequin’s nostrils. This particular sampling approach mimics the proximity of the spray cone to the breathing zone as it is in the real-life product use. The approach minimizes settling losses of larger droplets that may contain nanoparticles. The two aerosol streams drawn through the mannequin’s nostrils were combined into one at the mannequin’s nape using a stainless-steel Y-connector. The resulting aerosol stream was then split using a flow-splitter (TSI, Shoreview, MN, USA) and drawn by conductive tubing into a Scanning Mobility Particle Sizer (SMPS) consisting of model 3081 Differential Mobility Analyzer and model 3786 Condensation Particle Counter (TSI) and an Aerodynamic Particle Sizer (APS) model 3321 (TSI). The SMPS has an aspiration rate (Qa(SMPS)) of 0.3 l/min. For the APS, Qa(APS) = 1.0 l/min. Thus, the combined aspiration rate through the nostrils of the mannequin was 1.3 l/min (Qa = Qa(SMPS) + Qa(APS)). The SMPS system was used with a 0.0457 cm impactor (D50 = 0.656 μm). For the SMPS, particle density was set as 1.0 g/cm3 as it was impossible to determine the actual density of measured aerosol droplets. In addition, use of 1.0 g/cm3 density allowed easy comparison of the SMPS particle size distribution, expressed as electric mobility diameter, with the APS data expressed as aerodynamic diameter. The plotted range of particle size distributions was between 14.1 and 685.4 nm for SMPS and 723 nm and 19.81 μm for APS. The actual measurement range of the APS was broader: between 542 nm and 19.81 μm.
Figure 1.
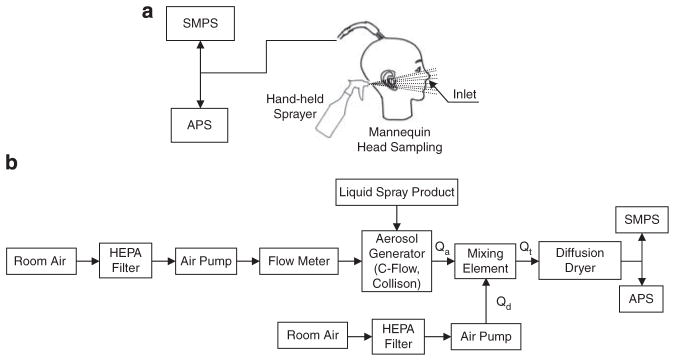
Aerosol generation and analysis experimental setup for simulated application of the spray products (a) and constant output aerosolization (b).
The setup was placed inside a Level II Biosafety cabinet (NuAire, Plymouth, MN, USA) with the mannequin head facing the back of the cabinet, approximately 20 cm from it. The inside dimensions of the cabinet are 178.4 cm width, 71.8 cm height, and 57.2 cm depth. The front of the biosafety cabinet was covered with a near-airtight plastic curtain with installed glove sleeves to handle and operate the sprayers. Before each test, the blower of the biosafety cabinet was operated for 15 min with the curtain open to remove most of the particles inside the cabinet. The background particle concentration was also monitored. Then, the curtain was closed, a spray product was positioned about 10 cm to the right of the mannequin’s head, and operated by hand using a provided sprayer (if available). The spray cone was directed towards the back wall of the cabinet. The lever of a sprayer was fully pressed with a frequency of ~1 s−1 and the spraying lasted for 3 min. Although the duration of a typical application may vary for different products or different users, 3 min is the minimum time needed for the SMPS to measure the entire size spectrum. The concentration and size distribution of aerosolized particles were continuously monitored by the APS and SMPS during the application of each spray. After the end of spraying, the particles inside the biosafety cabinet were removed by turning on the cabinet’s filtration system again.
Although most of the products were used with the provided sprayers, in the case of Silver Nanospray, the sprayer from another randomly selected nanoproduct was used as a sprayer was not supplied by the manufacturer. For the same reason, the sprayer from Disinfectant Nanospray was used with Regular Disinfectant Spray.
Particle Aerosolization using Standard Nebulizers
The concentration and size distribution of aerosolized liquid particles depend on the spraying mechanism and spraying intensity. The same consumer product may be supplied with different spraying mechanisms, thus affecting the size of the resulting particles and consequently exposure parameters. Therefore, we examined the range of particle concentrations and sizes a user could potentially be exposed to by aerosolizing all tested liquid products with two different constant output aerosol generators. The setup for this experimental approach is shown in Figure 1b. The following two constant output nebulizers were used: C-Flow PFA Nebulizer 800-1-020-01-00 (Savillex, Minnetonka, MN, USA) and three-hole Collison Nebulizer. The following accessories facilitating nebulization of small quantities of liquids were used with the Collison Nebulizer: CN41 precious fluids extension sleeve and CN70 polycarbonate precious fluids bottle (BGI, Waltham, MA, USA). The aerosol produced by each nebulizer was diluted with high-efficiency particulate air-filtered air and mixed using a passive box-type mixing element (Han et al., 2007), and then dried using a diffusion dryer model 3062 (TSI) and released into a horizontal test chamber of approximately 10 cm in diameter. The air was isokinetically sampled and measured with the APS and SMPS. The C-Flow PFA Nebulizer was operated in the self-aspirating mode at aerosol flow rate (Qa) of 1.0 l/min at 38 psi air pressure and the dilution air flow rate (Qd) was set to 14 l/min. The three-hole Collison Nebulizer was operated at Qa = 4.8 l/min at 10 psi and the dilution air flow rate (Qd) was set to 10.2 l/min. Thus, in both cases, the total aerosol output flow rate (Qt = Qa + Qd) was 15 l/min. Flow rates were measured with a Mass Flow Meter model 3063 (TSI), which adjusts for standard temperature and pressure.
Background data were obtained before each testing session by operating the C-Flow and Collison Nebulizers without any liquid feed, and then with ultrapure water 18.2 MΩ· cm obtained with Milli-Q Academic System (Millipore, Billerica, MA, USA) feed. The system was placed into a Class II Biosafety Cabinet (NuAire) and the blower of the cabinet was constantly operated throughout the experiments.
Experiments with each product and each test protocol (hand spraying or aerosolization with constant output nebulizers) were repeated at least three times.
Results
Sample Analysis using TEM
A summary of the TEM image analysis results for the spray products is shown in the second column of Table 2. Electron beam sensitivity category was introduced as an additional characteristic of the particles in the products because it was observed when attempting to view samples at higher magnifications (around 40,000 × with dark current of 97 μA). Electron-contrast particles were found in three nanoproducts: Silver Nanospray, Disinfectant Nanospray, and Wheel Nanocleaner and five non-nanoproducts: Regular Silver Spray, Regular Disinfectant Spray, Regular Hair Spray, Regular Skin Hydrating Mist, and Regular Facial Spray.
Table 2.
Mode diameters of particle size distributions and characterization of the tested consumer spray products, obtained using different analysis methods.
| Producta | TEM range of particle diameters, agglomeration, shape, structure, electron beam sensitivity | Hydrodynamic mode diameter(s) <1 μm: number — N, intensity — I | C-Flow Nebulizer used: mode diameter (nm) | Collison Nebulizer used: mode diameter (nm) |
|---|---|---|---|---|
| Silver Nanospray | 3–65 nm, single particles and agglomerates, spheroidal, solid, beam insensitive |
N: 5.6 nm I: 5.6, 17.8, 100 nm |
37 | 30 |
| Regular Silver Spray | <3–435 nm, agglomerate and single particles, various shapes, solid, beam insensitive |
N: 3.2 nm I: 17.8, 100.0 nm |
41 | 41 |
| Facial Nanospray | No particles detected | Unable to measure | 98 | 61 |
| Regular Facial Spray | 82–>6000 nm, single particles and agglomerates, spheroidal and elliptical, beam sensitive |
N: 3.0, 8.6 nm I: 3.0, 9.5, 30.1 nm |
102 | No peak (concentration below water background) |
| Hair Nanospray | No particles detected |
N: 5.3 nm I: 5.6, 24.1 nm |
311 | No data (foaming) |
| Regular Hair Spray | 16.5–683 nm, single particles and agglomerates (two types), spheroidal, solid, beam insensitive |
N: 2.4 nm I: 4.2, 31.6, 749.9 nm |
429 | 334 |
| Disinfectant Nanospray | 71–214 nm, single particles, spheroidal, solid, beam insensitive |
N: 1.0 nm I: 1.2, 3.3, 34.9, 101.2 nm |
85 | No data (foaming) |
| Regular Disinfectant Spray | 3.7–>725 nm, single particles and agglomerates, spheroidal, nanostructured, beam insensitive | Unable to measure | 157 | No data (foaming) |
| Skin Hydrating Nanomist | No particles detected |
N: 8.5 nm I: 17.3, 474.4 nm |
157 | 113 |
| Regular Skin Hydrating Mist | 146–>2500 nm, single particles and agglomerates, spheroidal and elliptical, beam sensitive |
N: 4.4 nm I: 9.5 nm |
102 | No peak (concentration below water background) |
| Wheel Nanocleaner | <20–>1000 nm, single particles and agglomerates, various shapes, beam sensitive | Unable to measure | 181 | No data (foaming) |
Nanoproduct: as per the Woodrow Wilson Nanotechnology Consumer Products Inventory.
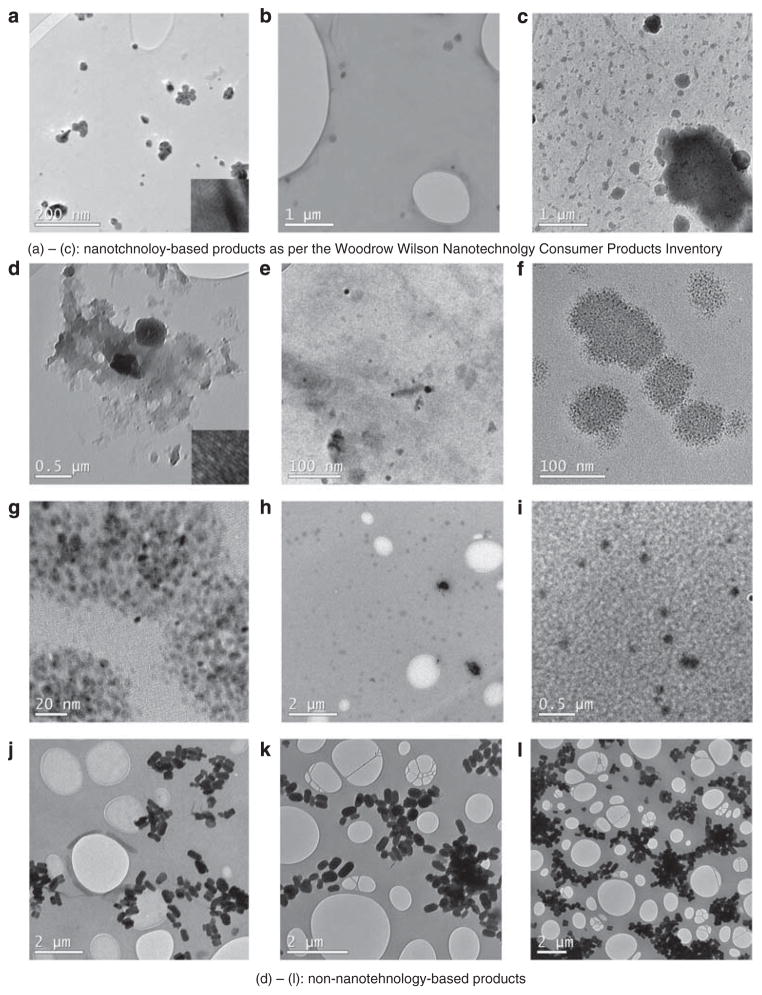
Representative micrographs from a pool of different-magnification micrographs are shown in Figure 2.
Figure 2.
Transmission electron micrographs of Silver Nanospray (a), Disinfectant Nanospray (b), Wheel Nanocleaner (c), Regular Silver Spray (d, e), Regular Disinfectant Spray (f, g), Regular Hair Spray (h, i), Regular Skin Hydrating Mist (j), and Regular Facial Spray (k, l).
Nanotechnology-Based Products
High-contrast single particles and well-defined separated nanoparticle agglomerates were found in the Silver Nanospray sample (Figure 2a). Low-contrast single particles with no agglomerates were found in the Disinfectant Nanospray sample (Figure 2b). While examining the sample of Wheel Nanocleaner at different magnifications, we found a wide size range of slightly electron beam-sensitive nanoparticles and nanoparticle agglomerates ranging from <20 nm to more than 1 μm (Figure 2c). No electron-contrast particles were detected in other nanoproduct samples: Facial Nanospray, Hair Nanospray, and Skin Hydrating Nanomist.
Regular Products
Single particles as well as large agglomerates were found in the Regular Silver Spray sample (Figure 2d and e). Compared with its nanoproduct counterpart, Silver Nanospray, the Regular Silver Spray sample looked much less refined. The sample of Regular Disinfectant Spray contained nanosized particles of approximately 100 nm in diameter (Figure 2f). High-magnification micrographs (Figure 2g) show that these particles are composed of smaller particles of approximately 3–5 nm in diameter. The sample of Regular Hair Spray contained single low-contrast particles with the minimum identified diameter of 16.5 nm and up to approximately 200 nm (Figure 2h and i). Elliptical high-contrast beam-sensitive particles were found in Regular Skin Hydrating Mist (Figure 2j). No particles of less than 100 nm in diameter were present in the sample, but some particles had diameter of less than 200 nm and majority of the particles had lengths between 250 to 600 nm. Most particles were found in a highly agglomerated state. The size and agglomeration, shape and the degree of electron transparency of particles found in Regular Facial Spray (Figure 2k and l) were similar to the particles in Regular Skin Hydrating Mist.
Analysis of Products Using Photon Correlation Spectroscopy
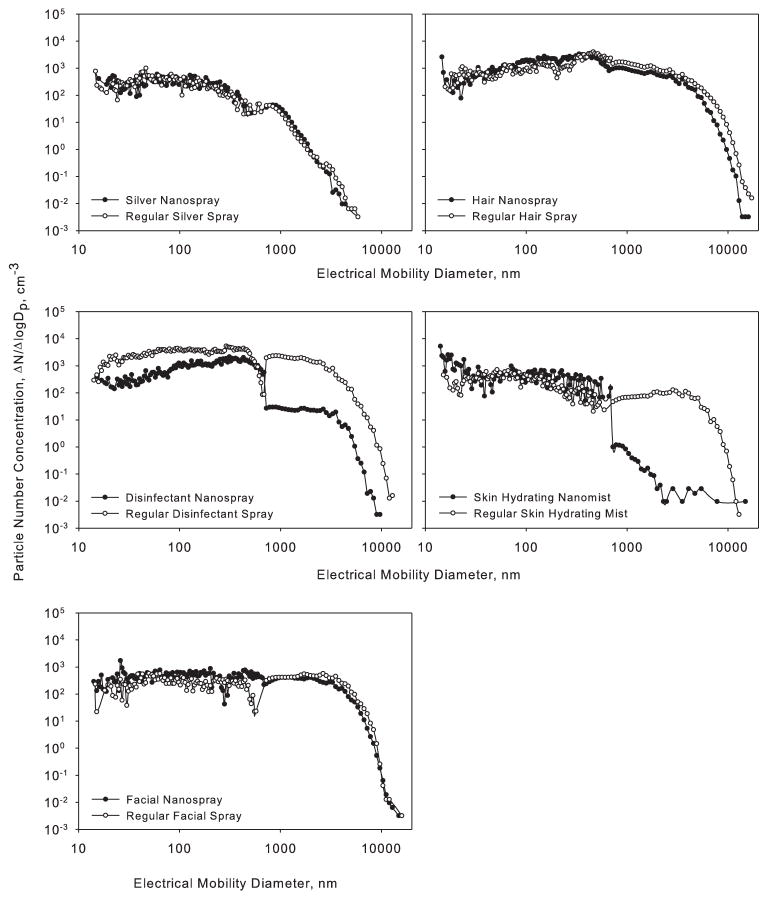
Figure 3 shows multimodal hydrodynamic diameter distributions for eight products (four nanotechnology-based and four regular products). For three other products (Facial Nanospray, Regular Disinfectant Spray, and Wheel Nanocleaner), the instrument could not produce valid data, most likely due to the high levels of large particles that distort the autocorrelation function (Bodycomb, 2009). The hydrodynamic diameter distributions are presented in two ways: as relative scattering intensity (a, c) and as particle number concentration (b, d). As the relative scattering intensity is proportional to particle radius to the sixth power, this method allows detecting the presence of large particles. On the other hand, the particle number size distribution indicates the relative presence of particles as a function of their diameter. The mode diameters of the intensity (I) and number (N) distributions are listed in Table 2. From the data acquired, it can be seen that particles of less than 100 nm in diameter were found in all tested products. Numberwise, the distributions of both nanoproducts and regular products were dominated by particles <20 nm in hydrodynamic diameter. The nanoproduct Disinfectant Nanospray contained particles <5 nm in hydrodynamic diameter. In cases where particles larger than 100 nm were found in the nanotechnology-based products, Skin Hydrating Nanomist had the largest diameters, with peaks observed in the size ranges 0.1–1 μm (100% relative scattering intensity) and 1–5 μm (61% relative scattering intensity) (Figure 3a). Silver Nanospray was observed to have some particles larger than 4 μm. Among the regular products, all were observed to have particles larger than 100 nm, except Regular Facial Spray. Regular Silver Spray had a wide relative scattering intensity peak (max relative scattering intensity) centered on 100 nm with particles as large as 300 nm also present. Regular Hair Spray had a relative scattering intensity peak (maximum relative scattering intensity) around 1 μm. Particles >5 μm were registered in Regular Silver Spray, Regular Hair Spray, and Regular Skin Hydrating Mist.
Figure 3.
Hydrodynamic diameter of nanotechnology-based (a, c) and corresponding regular (b, d) consumer spray products: relative scattering intensity (a, b) and relative number concentration (c, d).
Size Distribution of Airborne Particles Released from Spray Products
Figure 4 shows size distributions of aerosol particles released during the simulated application of spray products when hand-held spraying was used. Each graph contains particle size distributions averaged over three repeats for one nanoproduct and a corresponding regular product with the same application purpose. The SMPS and the APS size ranges are plotted together in the same graphs and presented as a function of the electrical mobility diameter. Although the APS measures the aerodynamic particle diameter, for spherical particles with density of 1.0 g/cm3, it is theoretically equivalent to the electrical mobility diameter. Wheel Nanocleaner and its alternative product were not tested owing to their corrosiveness and potential to damage the equipment.
Figure 4.
Size distributions of spray consumer products aerosolized by hand-held spraying. Nanotechnology-based products are shown in dark symbols, whereas regular products are presented in open symbols. The data present averages of three repeats.
For all but one nanoproduct–regular product pairs, the particle concentrations in the 14–500 nm range were similar within the pairs and ranged from 102 to 103 cm−3 (expressed as ΔN/Δlog Dp, where Dp is particle diameter). The exception was Disinfectant Nanospray–Regular Disinfectant Spray pair where the regular product had the higher particle concentration by about one order of magnitude. As the same sprayer was used for both products (supplied with Disinfectant Nanospray), this concentration difference can be attributed solely to the product properties, including higher concentration of small particles in the liquid. The particle concentration measured by the APS (above 723 nm) was also substantially higher for the regular product. The higher concentration of airborne super-micron particles may be attributed to higher concentration of those particles in the regular product.
A substantial difference in the concentration of airborne super-micron particles was also observed for Skin Hydrating Nanomist–Regular Skin Hydrating Mist product pair. Similar to the product pair mentioned above, the use of regular product resulted in a higher concentration of particles > 1 μm – by several orders of magnitude.
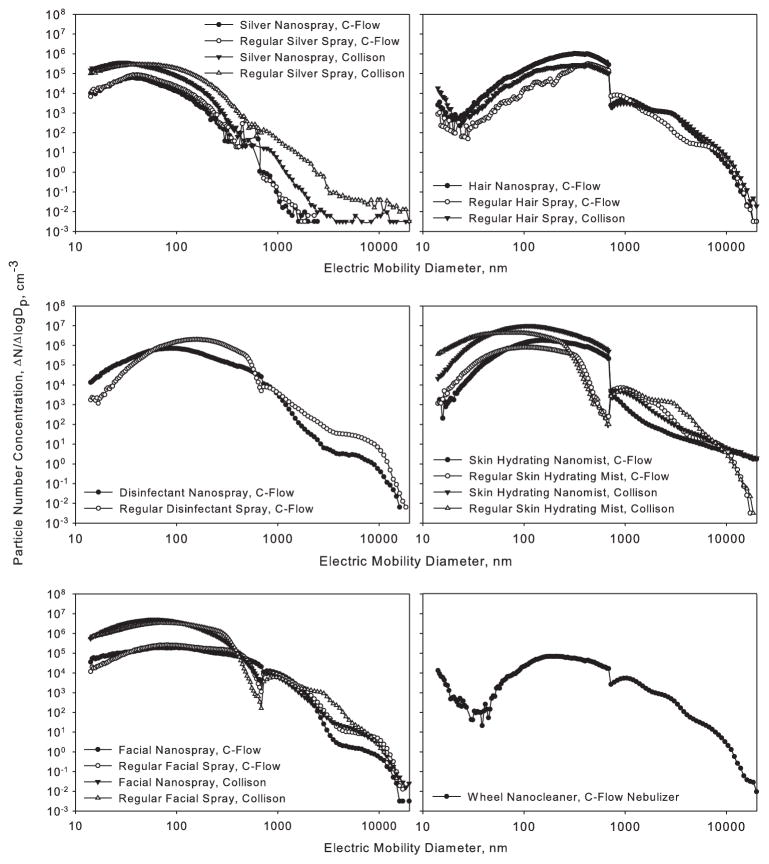
Particle size distributions obtained by aerosolizing spray products using the constant output nebulizers are presented in Figure 5. Each graph contains particle size distributions for one nanotechnology-based product and its corresponding regular product obtained using both constant output atomizers. Consistent with Figure 4, the data are presented as ΔN/ΔlogDp cm−3, where Dp is particle diameter. The mode diameters for all products and both nebulizers are presented in Table 2. The size distributions are averages of three repeats, except for Wheel Nanocleaner and its regular counterpart where only one test was performed due to their high corrosiveness to the APS and SMPS systems. Due to the excessive foaming, the use of the Collison Nebulizer was impossible for Hair Nanospray, Disinfectant Nanospray, and Regular Disinfectant Spray.
Figure 5.
Size distributions of spray consumer products aerosolized by Collison and C-Flow Nebulizers. Nanotechnology-based products are shown in dark symbols, whereas regular products are presented in open symbols. The data present averages of three repeats.
The airborne concentrations of the Silver Nanospray and Regular Silver Spray pair aerosolized by the C-Flow Nebulizer were similar for the entire size range. The difference became more pronounced when these two products were aerosolized using the Collison Nebulizer: the concentration of Regular Silver Spray was higher by 1–2 orders of magnitude for particles of 100 nm and larger. Also, particles as large as 20 μm were detected from both products. For the Hair Nanospray and Regular Hair Spray, the shapes of their size distributions looked very similar with a local minimum around 20–25 nm, a mode diameter between 300–400 nm and a gradual decrease in particle number concentration with increasing particle size. Particles of 20 μm in diameter were detected for both products and both aerosolization techniques. The aerosol from Hair Nanospray had a somewhat higher concentration than its regular product alternative in the diameter range from 20 to 1000 nm.
For the product pair Disinfectant Nanospray and Regular Disinfectant Spray, C-Flow Nebulizer-generated aerosol concentration was higher for Disinfectant Nanospray for particles of 60 nm and less, but generally lower for particles larger than 60 nm. A similar situation was observed for the product pair of Skin Hydrating Nanomist and Regular Skin Hydrating Mist with the latter product’s aerosol having higher concentration than its alternative in the regions below ~43 nm (Collison Nebulizer) and ~66 nm (C-Flow Nebulizer). However, the nanotechnology-based product had much higher concentration of particles in the range from 300 to 700 nm. Interestingly, the nanotechnology-based Skin Hydrating Nanomist had a much higher concentration of particles larger than 10 μm compared to its regular counterpart.
The size distributions of Facial Nanospray and Regular Facial Spray were similar almost over the entire size range. The Collison Nebulizer produced substantially higher concentration in the 15–400 nm range compared to the C-Flow Nebulizer for both these products. The particle size distribution of Wheel Nanocleaner aerosol, generated using only the C-Flow Nebulizer had a local minimum at approximately 35 nm, similarly to the distributions of the ethyl alcohol-containing Hair Nanospray and Regular Hair Spray. Although we did not have information on the solvent used in Wheel Nanocleaner, such a distribution suggests the presence of an organic solvent.
In general, there was a wide particle size distribution with the particle diameters ranging from 14 nm to 20 μm obtained for all products. The only exception was Silver Nanospray, aerosolized with the C-Flow Nebulizer, where the maximum observed diameter was 2 μm. Particle number concentrations in the 14-500 nm size ranged from 103 to 106 ΔN/ΔlogDp cm−3 depending on the product and aerosolization method. Collison Nebulizer typically produced higher particle concentrations compared with the C-Flow Nebulizer for all tested products.
The data for ultrapure water nebulization showed that Collison Nebulizer produced approximately 10-fold higher particle concentrations than C-Flow Nebulizer in almost all particle size channels. For both nebulizers, the airborne particle concentrations were lower compared to those from the aerosolized spray products in all size channels. One exception was silver sprays, where particle concentrations above 1 μm were similar to those for ultrapure water.
Discussion
A very interesting and rather surprising result of the study is the detection of nano-sized (1–100 nm) particles not only in nanotechnology-based, but also in regular (non-nanotechnology) products using different particle analysis techniques.
The data obtained using the TEM and ZetaPALS are important since they describe the particles that are incorporated in spray products. However, during the actual use of those products, droplet formation and dynamics come into play; thus, the size of the particles that a product user could be exposed to will be different than that found in the initial product. Analysis of the aerosol formed during simulated product application showed the presence of nano-sized particles produced from both nano and regular consumer spray products (Table 2, Figures 4 and 5). In addition, coarse (2.5–10 μm) and super-coarse (>10 μm) particles (as defined in Lioy et al., 2002) were released from all products. Since the products were sprayed by hand using the supplied sprayers and the released particles were sampled in a way that simulates particle inhalation by the user, these data show the size distributions and concentrations of particles, to which human exposure would occur during the actual product use. This wide size distribution of aerosolized particles (14 nm–20 μm) indicates that particles would be inhaled and deposit in all regions of the respiratory system: extrathoracic, thoracic and alveolar (Hinds, 1999). The detected large particles are likely to carry material from product matrix and agglomerates of nano-sized particles. Due to the importance of agglomerates in examining biological effects (Wick et al., 2007; Qu et al., 2009; Wirnitzer et al., 2009), subsequent research will need to examine the internal structure and composition of the released super-micron particles including the stability of the nanomaterials in agglomerates at the site of deposition in the lung. Also, testing the effect of spray cone orientation within the breathing zone will be a subject of future studies.
Experiments with two different nebulizers show that the concentration and size distribution of the particles released during product use depend on the spraying technique and thus the exposure of users would be affected by the product packaging and the supplied sprayer.
Use of the constant output nebulizers in conjunction with the diffusion dryer produced lower concentrations of large particles compared to hand-held spraying. The nebulizers produce smaller initial droplets and the diffusion dryer removes most of the solvent from the droplets. Therefore, this method allows simulating cases when a product’s sprayer produces smaller droplets and/or released droplets have a longer residence time, so most of the carrier liquid evaporates. In these cases, the user would be exposed to higher concentrations of smaller particles, which are able to penetrate deeper into the lung.
When looking at the data presented in Figures 4 and 5, one notices a less than smooth transition in the 600–700 nm range where the data from the SMPS and APS overlap. We chose to present the SMPS and APS data in the exact form as they were generated by the instruments without the use of the TSI Data Merge Software Module (Han et al., 2005). For the particle size distribution to achieve a smooth transition from the SMPS measurement range (14–700 nm) to the APS measurement range (0.5–20 μm) using the Merge Software, one needs to know a range of particle parameters (Khlystov et al., 2004), which would be difficult to determine for the diverse range of particle types in the tested spray products. Since the SMPS measurements are based on the electrical properties and APS measurements are based on the aerodynamic properties of particles, the different detection principles can result in different detection efficiency in the transition size range (0.5–0.7 μm) depending on various aerosol characteristics (Hand and Kreidenweis, 2002; Pant et al., 2009). Based on our data, it seems that the extent of this effect depends on the tested spray product and can probably be explained by different properties of carrier liquid and particles, including their density and shape, which are largely unknown. Although these researchers reported undercounting by the APS in the transition range compared to the SMPS, with one product – Regular Skin Hydrating Mist—we observed the opposite in the transition size range.
A major difference was found between the size distributions of particles produced from water-based versus alcohol-containing products using constant output aerosolization techniques (Figure 5). In the case of alcohol-based products, Hair Nanospray and Regular Hair Spray, much lower particle concentrations in 15–100 nm region with a local minimum around 20–25 nm were observed, whereas such a dip was not present in particle size distributions of water-based products. A similar result—a local minimum in the region between 25 and 45 nm—was observed for Wheel Nanocleaner. The composition of this product, including information about main solvent, was not obtainable, but based on similarity of the size distribution to the Hair Nanospray, the data suggest a volatile organic solvent-based solution.
The mode diameters measured by ZetaPALS were smaller compared with the mode diameters of airborne size distributions for all aerosolization methods. For those products where the TEM data indicated prevalence of nanosized particles, the analysis of aerosolized particles did not show the same results. This can be explained by aerosolized particles primarily consisting of larger droplets from the product matrix that contain multiple single particles as well as their agglomerates. The super coarse particles (above 10 μm diameter) could also be a result of particle agglomeration during their release from the sprays. This observation suggests that a comprehensive analysis of nanotechnology-based products should include analysis of particles within a product as well as analysis of particles that are emitted during product use to understand potential exposure (Lioy, 2010b).
On the basis of the obtained data, it is difficult to conclude whether the nanoparticles released during the product use are actually engineered nanoparticles that were incorporated into the product or they are derivatives from natural product ingredients, such as from herbal oil emulsification (Kim et al., 1996; Abismaïl et al., 1999), or they are particles from product carrier liquid. Chemical and/or structural particle analysis could provide only some of that information and only for the particle materials that can be analyzed using specific methods. Therefore, making a definite conclusion about the composition and structure of some released nanoparticles is difficult without information from the manufacturers on the nature and concentration of nanomaterials in their products.
Conclusions
This research examined the potential for nanoparticle exposure due to the use of nanotechnology-based and regular same-purpose consumer spray products.
Electron microscopy showed the presence of free nanoparticles and agglomerates in several examined consumer products, including those that are not designated as nanoproducts. Similarly, simulated use of sprays resulted in the release of nanosized particles in both nano and regular spray products, even though the manufactures do not specify the “nanosize” of ingredients or even may not know that nanoparticles are present or formed during manufacturing of their products. As an example, the manufacturer lists only chemically synthesized ingredients in the composition of Regular Disinfectant Spray—this product is also not reported as containing nanomaterials.
On the whole, use of spray products resulted in the release of particles with a wide size distribution. The toxicological implications of the human respiratory system deposition and possible translocation of these particles are not known.
Experiments with hand spraying and constant output atomizers have shown that the spraying technique affects the concentration and size distribution of the released particles. Thus, the exposure to particles from nanotechnology-based and regular products would be affected by the sprayer type.
Overall, the data suggest that the use of investigated nanotechnology-based as well as regular consumer sprays would result in inhalation exposures to single nanosized particles and multi-sized agglomerates, including complex nanoparticle-containing composites.
Future experiments will examine the structure and composition of the released particles more closely. We will also examine the patterns of particle deposition in the lung owing to short- and long-term product uses. However, the most important conclusion is that controlled human exposure studies and product emission studies are essential for reducing exposures of the general public to nano-based materials, especially those that have shown some mechanistic effects in toxicological studies.
Acknowledgments
This research was supported in part by the NIEHS sponsored UMDNJ Center for Environmental Exposures and Disease, Grant No.: NIEHS P30ES005022. We thank Dr. Satya Seshadri for help in building the experimental system.
References
- Abismaïl B, Canselier JP, Wilhelm AM, Delmas H, Gourdon C. Emulsification by ultrasound: drop size distribution and stability. Ultrason Sonochem. 1999;6(1 and 2):75–83. doi: 10.1016/s1350-4177(98)00027-3. [DOI] [PubMed] [Google Scholar]
- Allen T. Powder Sampling and Particle Size Determination. Elsevier; Amsterdam, the Netherlands: 2003. 1 illustrated edn. [Google Scholar]
- Bermudez E, Mangum JB, Wong BA, Asgharian B, Hext PM, Warheit DB, et al. Pulmonary responses of mice, rats, and hamsters to subchronic inhalation of ultrafine titanium dioxide particles. Toxicol Sci. 2004;77:347–357. doi: 10.1093/toxsci/kfh019. [DOI] [PubMed] [Google Scholar]
- Bodycomb J. Questions and Answers for Driving the Brookhaven 90Plus. Brookhaven Instruments Corporation; Holtsville, NY: 2009. [Google Scholar]
- Bradford A, Handy RD, Readman JW, Atfield A, Mühling M. Impact of silver nanoparticle contamination on the genetic diversity of natural bacterial assemblages in estuarine sediments. Environ Sci Technol. 2009;43(12):4530–4536. doi: 10.1021/es9001949. [DOI] [PubMed] [Google Scholar]
- Brookhaven Instruments Corporation. Instruction Manual for 90Plus/BI-MAS Multi Angle Particle Sizing Option Operation Manual. Brookhaven Instruments Corporation; Holtsville, NY: 1995. [Google Scholar]
- Bruce J, Berne RP. Dynamic Light Scattering: With Applications to Chemistry, Biology, and Physics. Courier Dover Publications; Mineola, NY: 2000. 2 unabridged, illustrated edn. [Google Scholar]
- Carlo SD, El-Bez C, Alvarez-Rua C, Borge J, Dubochet J. Cryo-negative staining reduces electron-beam sensitivity of vitrified biological particles. J Struct Biol. 2002;138:216–226. doi: 10.1016/s1047-8477(02)00035-7. [DOI] [PubMed] [Google Scholar]
- Drobne D. Nanotoxicology for safe and sustainable nanotechnology. Arhiv Higijenu Rada Toksikologiju. 2007;58(4):471–478. doi: 10.2478/v10004-007-0040-4. [DOI] [PubMed] [Google Scholar]
- Egerton RF, Li P, Malac M. Radiation damage in the TEM and SEM. Micron. 2004;35(6):399–409. doi: 10.1016/j.micron.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Elder A, Gelein R, Silva V, Feikert T, Opanashuk L, Carter J, et al. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ Health Perspect. 2006;114(8):1172–1178. doi: 10.1289/ehp.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frater L, Stokes E, Lee R, Oriola T. An Overview of the Framework of Current Regulation affecting the Development and Marketing of Nanomaterials. Cardiff University; Cardiff: 2006. p. 192. [Google Scholar]
- Geiser M, Rothen-Rutishauser B, Kapp N, Schürch S, Kreyling W, Schulz H, et al. Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ Health Perspect. 2005;113(11):1555–1560. doi: 10.1289/ehp.8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassian VH, Adamcakova-Dodd A, Pettibone JM, O’Shaughnessy PT, Thorne PS. Inflammatory response of mice to manufactured titanium dioxide nanoparticles: comparison of size effects through different exposure routes. Nanotoxicology. 2007a;1(3):211–226. [Google Scholar]
- Grassian VH, O’Shaughnessy PT, Adamcakova-Dodd A, Pettibone JM, Thorne PS. Inhalation exposure study of titanium dioxide nanoparticles with a primary particle size of 2 to 5 nm. Environ Health Perspect. 2007b;115(3):397–402. doi: 10.1289/ehp.9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagendorfer H, Lorenz C, Kaegi R, Sinnet B, Gehrig R, Goetz NV, et al. Size-fractionated characterization and quantification of nanoparticle release rates from a consumer spray product containing engineered nanoparticles. J Nanoparticle Res. 2009;12(7):2481–2494. [Google Scholar]
- Han H-S, Whitbey ER, Plate DB, Albertson DP. Combining TSI Scanning Mobility Particle Sizer and Aerodynamic Particle Sizer for Wide Range Particle Size Distribution Measurement. European Aerosol Conference; Ghent, Belgium. 2005. [Google Scholar]
- Han T, O’Neil DL, Ortiz CA. A generic-tee-plenum mixing system for application to single point aerosol sampling in stacks and ducts. Health Phys. 2007;92(1):40–49. doi: 10.1097/01.HP.0000234674.68338.a2. [DOI] [PubMed] [Google Scholar]
- Hand JL, Kreidenweis SM. A new method for retrieving particle refractive index and effective density from aerosol size distribution data. Aerosol Sci Technol. 2002;36:1012–1026. [Google Scholar]
- Hansen SF, Michelson ES, Kamper A, Borling P, Stuer-Lauridsen F, Baun A. Categorization framework to aid exposure assessment of nanomaterials in consumer products. Ecotoxicology. 2008;17:438–447. doi: 10.1007/s10646-008-0210-4. [DOI] [PubMed] [Google Scholar]
- Hinds WC. Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles. Wiley, the University of Michigan; New York: 1999. 2 illustrated edn. [Google Scholar]
- Hobbs LW. Electron-beam sensitivity in inorganic specimens. Ultramicroscopy. 1987;23(3 and 4):339–344. [Google Scholar]
- Keenan CR, Goth-Goldstein R, Lucas D, Sedlak DL. Oxidative Stress Induced by Zero-Valent Iron Nanoparticles and Fe(II) in Human Bronchial Epithelial Cells. Environ Sci Technol. 2009;43(12):4555–4560. doi: 10.1021/es9006383. [DOI] [PubMed] [Google Scholar]
- Khlystov A, Stanier C, Pandis SN. An Algorithm for Combining Electrical Mobility and Aerodynamic Size Distributions Data when Measuring Ambient Aerosol. Aerosol Sci Technol. 2004;38(S1):229–238. [Google Scholar]
- Kim YD, Morr CV, Schenz TW. Microencapsulation Properties of Gum Arabic and Several Food Proteins: Liquid Orange Oil Emulsion Particles. J Agric Food Chem. 1996;44(5):1308–1313. [Google Scholar]
- Leapman RD, Sun S. Cryo-electron energy loss spectroscopy: observations on vitrified hydrated specimens and radiation damage. Ultramicroscopy. 1995;59:71–79. doi: 10.1016/0304-3991(95)00019-w. [DOI] [PubMed] [Google Scholar]
- Lioy PJ, Han TW, Nazarenko Y, Lioy MJ, Mainelis G. Nanotechnology and Exposure Science—What Is Needed To Fill the Research and Data Gaps for Consumer Products. Nanotechnol Expos Assessment (Special issue) 2010;16(4):376–385. doi: 10.1179/107735210799160057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioy PJ, Weisel CP, Millette J, Vallero SED, Offenberg J, Buckley B, et al. Characterization of the dust/smoke aerosol that settled east of the World Trade Center (WTC) in lower Manhattan after the collapse of the WTC September 11, 2001. Environ Health Perspect. 2002;110:703–714. doi: 10.1289/ehp.02110703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioy PJ. Exposure Science: A View of the Past and Major Milestones for the Future. Environ Health Perspect. 2010b;118:1081–1090. doi: 10.1289/ehp.0901634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyi RJ, Schwartz LH, Butt JB. Particle Size, Particle Size Distribution, and Related Measurements of Supported Metal Catalysts. Catalysis Reviews. 1987;29(1):41–99. [Google Scholar]
- Maynard AD, Aitken RJ, Butz T, Colvin C, Donaldson K, Oberdörster G, et al. Safe Handling of Nanotechnology. Nature. 2006;444:267–269. doi: 10.1038/444267a. [DOI] [PubMed] [Google Scholar]
- Maynard AD. Nanotechnology: The Next Big Thing, or Much Ado about Nothing. Ann Occup Hyg. 2007;51(1):1–12. doi: 10.1093/annhyg/mel071. [DOI] [PubMed] [Google Scholar]
- Maynard AD, Aitken RJ. Assessing exposure to airborne nanomaterials: current abilities and future requirements. Nanotoxicology. 2007;1(1):26–41. [Google Scholar]
- Michelson ES. Globalization at the nano frontier: the future of nanotechnology policy in the United States, China, and India. Technol Soc. 2008;30:405–410. [Google Scholar]
- National Science and Technology Council. The National Nanotechnology Initiative Strategic Plan (NNISP) Executive Office of the President, National Science and Technology Council; Washington, DC: 2011. [Google Scholar]
- Oberdörster G, Maynard A, Donaldson K, Castranova V, Fitzpatrick J, Ausman K, et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Particle Fibre Toxicol. 2005a;2(8):1–35. doi: 10.1186/1743-8977-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: An Emerging Discipline Evolving from Studies of Ultrafine Particles. Environ Health Perspect. 2005b;113(7):823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, et al. Translocation of Inhaled Ultrafine Particles to the Brain. Inhal Toxicol. 2004;16(6 and 7):437–445. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- Pant V, Deshpande CG, Kamra AK. The concentration and number size distribution measurements of the Marine Boundary Layer aerosols over the Indian Ocean. Atmos Res. 2009;92(4):381–393. [Google Scholar]
- Paull J, Lyons K. Nanotechnology: the next challenge for organics. J Organic Syst. 2008;3(1):3–22. [Google Scholar]
- Qu G, Bai Y, Zhang Y, Jia Q, Zhang W, Yan B. The effect of multiwalled carbon nanotube agglomeration on their accumulation in and damage to organs in mice. Carbon. 2009;47(8):2060–2069. [Google Scholar]
- Schmid A, Goel S, Wang W, Beiu V, Carrara S, Riediker M. In: Chances and risks of nanomaterials for health and environment. Akan O, Bellavista P, Cao J, Dressler F, Ferrari D, Gerla M, et al., editors. Vol. 20. Nano-NetSpringer; Berlin, Heidelberg: 2009. pp. 128–133. [Google Scholar]
- Segal SH. Environmental Regulation of Nanotechnology: Avoiding Big Mistakes for Small Machines. Nanotechnol Law Business. 2004;1(3):290–304. [Google Scholar]
- Shimada M, Wang W-N, Okuyama K, Myojo T, Oyabu T, Morimoto Y, et al. Development and Evaluation of an Aerosol Generation and Supplying System for Inhalation Experiments of Manufactured Nanoparticles. Environ Sci Technol. 2009;43(14):5529–5534. doi: 10.1021/es9008773. [DOI] [PubMed] [Google Scholar]
- Shrader-Frechette K. Nanotoxicology and Ethical Conditions for Informed Consent. Nanoethics. 2007;1:47–56. [Google Scholar]
- Som C, Berges M, Chaudhry Q, Dusinska M, Fernandes T, Olsen S, et al. The importance of life cycle concepts for the development of safe nanoproducts. Toxicology. 2010;269:160–169. doi: 10.1016/j.tox.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Thomas T, Bahadori T, et al. Moving toward exposure and risk evaluation of nanomaterials: challenges and future directions. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(4):426–433. doi: 10.1002/wnan.34. [DOI] [PubMed] [Google Scholar]
- Turgis J-D, Coqueret X. Electron beam sensitivity of butyl acrylate copolymers: effects of composition on reactivity. Macromol Chem Phys. 1999;200:652–660. [Google Scholar]
- Van Calster G. Regulating Nanotechnology in the European Union. Nanotechnol Law Business. 2006;3(3):359–374. [Google Scholar]
- Wang J, Zhou G, Chen C, Yu H, Wang T, Ma Y, et al. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol Lett. 2007;168(2):176–185. doi: 10.1016/j.toxlet.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Warheit DB, Borm PJA, Hennes C, Lademann J. Testing Strategies to Establish the Safety of Nanomaterials: Conclusions of an ECETOC Workshop. Inhal Toxicol. 2007a;19:631–643. doi: 10.1080/08958370701353080. [DOI] [PubMed] [Google Scholar]
- Warheit DB, Webb TR, Reed KL, Frerichs S, Sayes CM. Pulmonary toxicity study in rats with three forms of ultrafine-TiO2 particles: differential responses related to surface properties. Toxicol Lett. 2007b;230(1):90–104. doi: 10.1016/j.tox.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Wick P, Manser P, Limbach LK, Dettlaff-Weglikowska U, Krumeich F, Roth S, et al. The degree and kind of agglomeration affect carbon nanotube cytotoxicity. Toxicol Lett. 2007;168(2):121–131. doi: 10.1016/j.toxlet.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Wirnitzer U, Herbold B, Voetz M, Ragot J. Studies on the in vitro genotoxicity of baytubes®, agglomerates of engineered multi-walled carbon-nanotubes (MWCNT) Toxicol Lett. 2009;186(3):160–165. doi: 10.1016/j.toxlet.2008.11.024. [DOI] [PubMed] [Google Scholar]
- Woodrow Wilson International Center for Scholars. [accessed 10, June 2010a];The Project on Emerging Nanotechnologies. http://www.nanotechproject.org/
- Woodrow Wilson International Center for Scholars. [accessed 10, June 2010b];Nanotechnology Consumer Products Inventory. http://www.nanotechproject.org/inventories/consumer/