Abstract
The currently available therapies for Alzheimer’s disease (AD) and related forms of dementia are limited by modest efficacy, adverse side effects, and the fact that they do not prevent the relentless progression of the illness. The purpose of the studies described here was to investigate the neuroprotective effects of the nicotine metabolite cotinine as well as a small series of cotinine and nicotine analogs (including stereoisomers) and to compare their effects to the four clinically prescribed AD therapies.
Keywords: Neuroprotection, nicotinic, multi-target-directed ligands, multifunctional compounds, amyloid, glutamate, excitotoxicity, neurodegeneration, dementia, disease modifying
Alzheimer’s disease (AD) is the most common neurodegenerative disease in the elderly and its prevalence is expected to rise sharply in the next several decades.1 Unfortunately, the currently available therapies (acetylcholinesterase inhibitors and the glutamate, NMDA antagonist, memantine) are limited by modest efficacy, adverse side effects, and the fact that they do not prevent or even significantly delay the relentless progression of the illness. The varied symptoms of AD which include cognitive deficits, non-cognitive behavioral symptoms (e.g., agitation, hallucinations), and the complex pathophysiology (amyloid-β neurotoxicity, tau hyperphosphorylation, glutamate excitotoxicity etc.) support the argument that novel compounds that affect multiple drug targets (i.e., multi-target-directed ligands” or MTDLs) or that have multifunctional properties (e.g., pro-cognitive and neuroprotective, pro-cognitive and antipsychotic actions) are needed for more optimal therapeutic interventions.2–5
Interestingly, the tobacco alkaloid nicotine has been shown to possess multifunctional properties including pro-cognitive effects in humans, rodents, and non-human primates6–7 and neuroprotective activities in a variety of model systems.8 The use of nicotine as a therapeutic agent, however, is clearly limited by its short half-life, abuse potential, and cardiovascular side effects.9 An increasing body of evidence suggests that the most predominant metabolite of nicotine in mammalian species, cotinine, might retain the positive features of nicotine while exhibiting fewer limitations. In vitro, cotinine protects against toxic insults in PC12 cells with potency similar to that of nicotine10, suppresses the release of oxygen free radicals from neutrophils11, augments PI3K-dependent anti-inflammatory pathways in human monocytes12, protects against 6-OHDA-toxicity in SH-SY5Y cells13, and reduces death induced by Aβ neurotoxicity in primary cortical neurons.14 In vivo, cotinine has been observed to prevent memory loss in transgenic (Tg) 6799 Alzheimer’s disease mice as well as to stimulate the Akt/GSK3β pathway and reduce Aβ aggregation in their brains.15 Cotinine has also been evaluated across a variety of additional behavioral assays in rodents and non-human primates for potential effects on information processing and cognition. In monkeys cotinine elicited dose-dependent improvements of a delayed match to sample (DMTS) task as well as a modified version of the task (DMTS-D) where randomly-presented (task-relevant) distractors were presented.16 Cotinine also attenuated deficits of DMTS in monkeys produced by the glutamate NMDA receptor antagonist ketamine17 and it attenuated the deficits of sustained attention in rats induced by the NMDA receptor antagonist MK-801.18 Cotinine also improved prepulse inhibition (PPI) of the acoustic startle response in pharmacological impairment models19, a property that may predict the efficacy of compounds as antipsychotic agents as well as cognitive enhancers.
Collectively, the results described above indicate that cotinine has neuroprotective properties and that it improves information processing, attention, and memory-related task performance in model systems that have relevance to both AD and other neuropsychiatric disorders such as schizophrenia. Given the much longer half-life of cotinine compared to nicotine, its considerably lower toxicity20, and apparent lack of abuse potential9, it may serve as a superior prototypical therapeutic agent for neuropsychiatric disorders.
The purpose of the studies described here was to further investigate the neuroprotective potential of cotinine (and nicotine) as well as a small series of their analogs (including stereoisomers) which are commercially available (see Fig 1) and to compare their effects to the four clinically prescribed AD therapies. The purpose of evaluating the analogs was to establish a preliminary structure–activity relationship (SAR) and define the features of the molecules that might be optimal for neuroprotective activity. We focused on neuroprotection against amyloid β (Aβ) and glutamate-mediated toxicity which are well established as major contributing factors to the neurodegeneration of AD.21,22 The neuroprotection assays are based on methods described previously23,24 with modifications.25–26
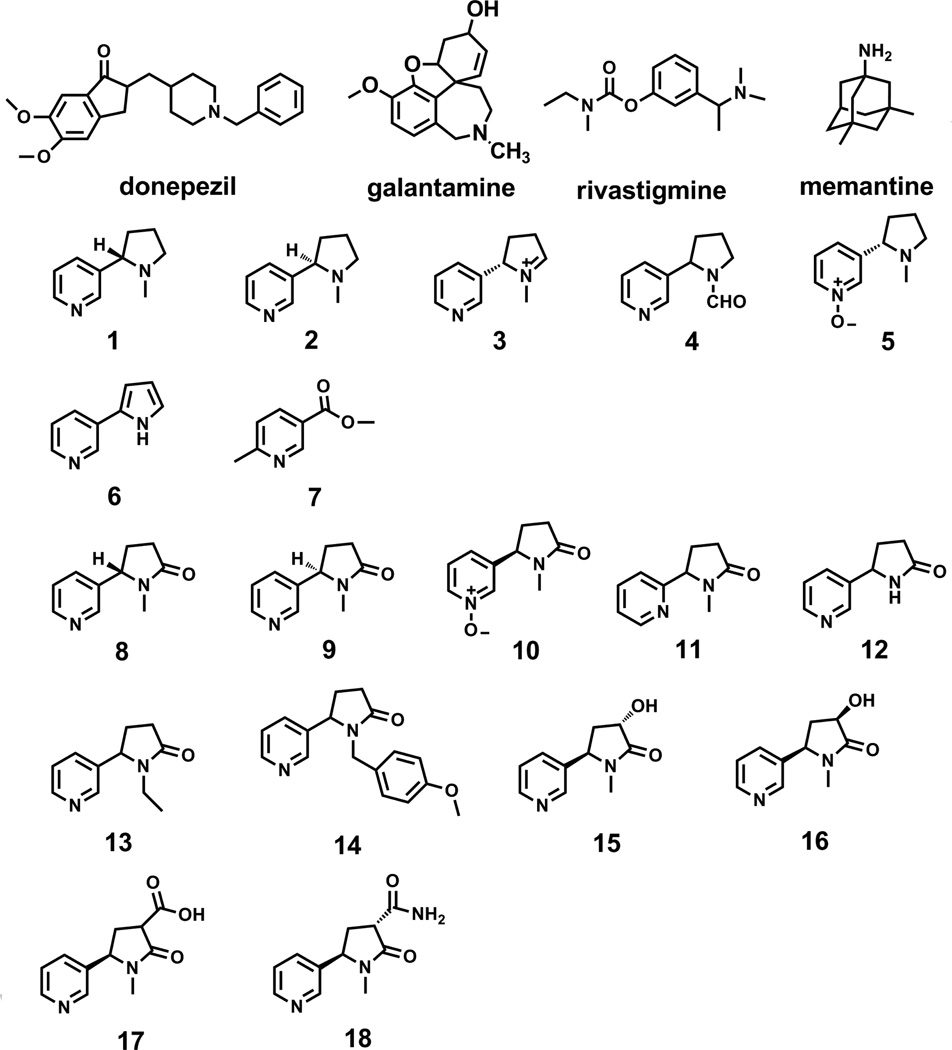
Fig 1.
Chemical Structures of the currently prescribed AD therapeutic agents, commercially available nicotine analogs (compounds 1–7), and commercially available cotinine analogs (compounds 8–18).
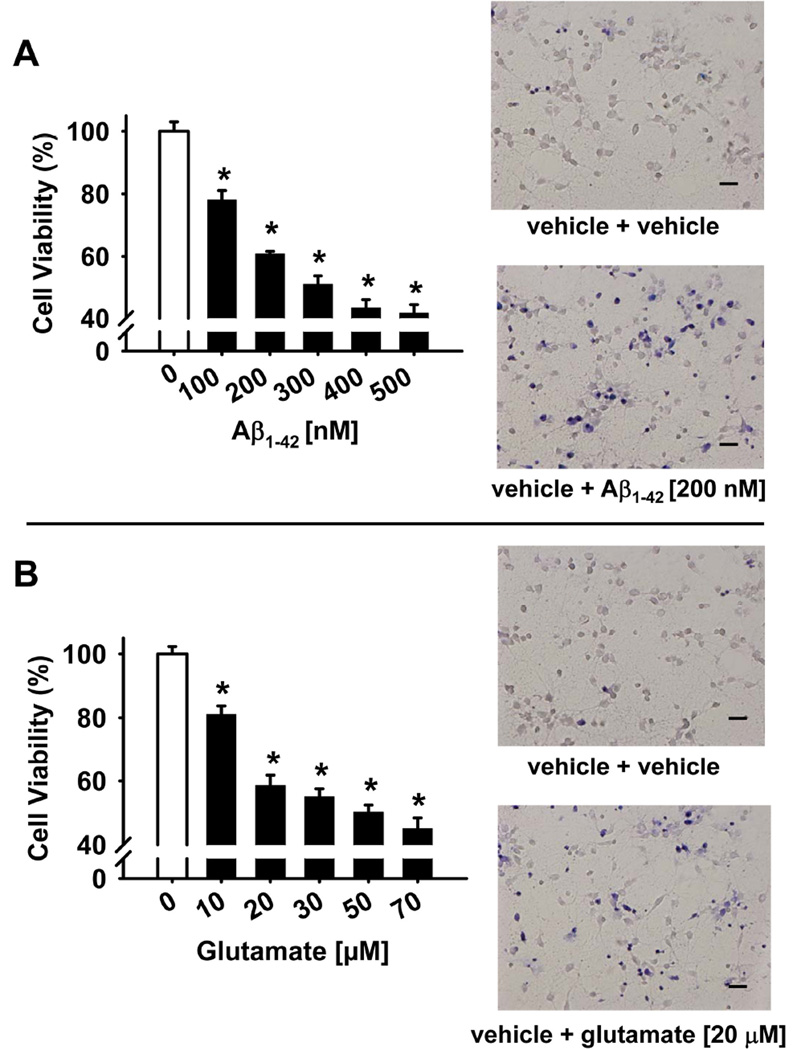
Concentration-effect relationships for Aβ1–42 and glutamate treatment on the viability of rat primary cortical neurons are illustrated in Fig 2A and 2B, respectively. As illustrated, after exposure to either the Aβ1–42 peptide or glutamate for 24 hours, there was a concentration-dependent decrease in cell viability as indicated by the MTT assay. From these concentration-response curves, Aβ1–42 [200 nM] and glutamate [20µM] were selected for subsequent neuroprotection evaluations with each compound reducing cell viability to approximately 60% of control (specifically, 60.8 ± 2.4% for Aβ1–42 exposure and 58.6± 3.2% for glutamate exposure when compared with the vehicle-treated sample). In a second set of (confirmatory) experiments, these selected concentrations of Aβ1–42 and glutamate produced a similar decrease in cell viability as indicated by the Trypan blue exclusion method. Note the increase in nonviable cells in the representative photomicrographs in the neurotoxin treated cultures (compared to vehicle-treated controls) which are membrane-porous and stain blue, whereas the viable cells exclude trypan blue stain due to their intact cell membranes.
Fig 2.
Concentration-effect relationships for Aβ1–42 and glutamate treatment on cell viability in primary cultures of rat cortical neurons. Cultures were exposed to various concentrations of the Aβ1–42 peptide (A) or glutamate (B) for 24 hours and cell viability was determined in an MTT assay (see Materials and Methods), calculated as percentage survival rate, and compared to a negative control (i.e., cultures without the Aβ1–42 peptide). Each bar represents the mean ± S.E.M (derived from 2–4 independent experiments with 7 replicates per drug concentration). *p < 0.05 compared to wells with no Aβ1–42 peptide. The effects of the selected concentrations of the Aβ1–42 peptide and glutamate to be used in subsequent neuroprotection experiments were confirmed via a Trypan Blue exclusion assay (see Materials and Methods) and are illustrated in representative photomicrographs. Scale bar = 100 µm.
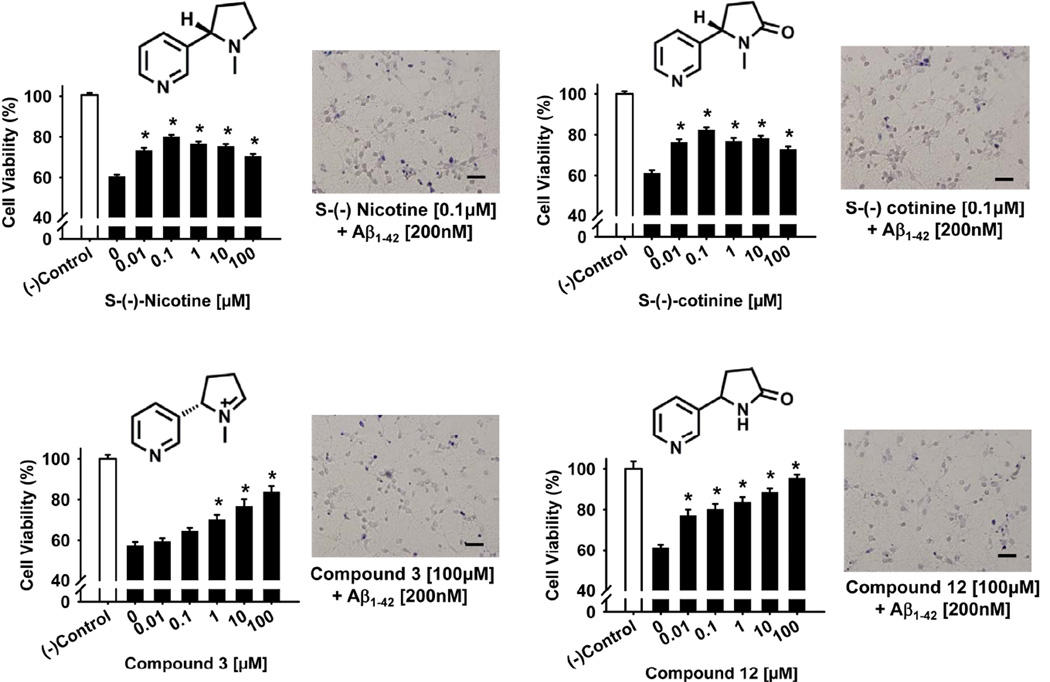
The results of experiments designed to assess the potential neuroprotective effects of nicotine, cotinine and structural analogs against the compromised neuronal viability induced by the Aβ1–42 peptide are illustrated in Fig 3 and Table 1. In Fig 3, concentration-effect relationships for the most effective compounds (in the MTT assay) are illustrated in the bar plots and the effects of optimal concentrations (confirmed by the trypan blue exclusion method) are illustrated in the representative photomicrographs. As shown, 24 hr incubation with the Aβ1–42 peptide [200 nM] decreased cell survival by about 40% in each series of experiments. (−)-Nicotine (compound 1), (−)-cotinine (compound 8) and compounds 3 and 12 significantly protected against Aβ-induced neurotoxicity. In fact, all of the concentrations of nicotine, cotinine and compounds 12 evaluated (10.0 nM to 100 µM) offered some degree of protection (p<0.05) while the highest 3 concentrations of compound 3 afforded significant protection. Compound 12 appeared to offer the greatest degree of protection with the 100 µM concentration producing cell viability greater than 90% of control values. As indicated in Table 1, nine of the experimental compounds evaluated ((−)-nicotine, (−)-cotinine and their analogs) offered some degree of neuroprotection, while none of the currently prescribed AD therapies (donepezil, galantamine, rivastigmine, or memantine) were effective. The highest dose of donepezil (100 µM) was, in fact, associated with an increase in neurotoxicity compared to Aβ1–42 peptide exposure alone. It is also important to note that while the (+) isomers of nicotine (compound 2) and cotinine (compound 9) offered some degree of neuroprotection in these experiments, they were considerably less effective that the (−) isomers.
Fig 3.
Neuroprotective effects of nicotine, cotinine, and compounds 3 and 12 against the Aβ1–42 peptide as determined in a cell viability assay in primary cultures of rat cortical neurons. Pretreatment of the cultures with various concentrations of nicotine, cotinine, and compounds 3 and 12 for 24 hours was followed by exposure to the Aβ1–42 peptide (200 nM) for another 24 hours. Cell viability for each treatment was determined in an MTT assay (see Materials and Methods), calculated as percentage survival rate, and compared to a negative control (i.e., cultures without the Aβ1–42 peptide or test compound). Each bar represents the mean ± S.E.M (derived from 2–4 independent experiments with 7 replicates per drug concentration). *p<0.05 compared to wells with the Aβ1–42 peptide added, but no test compound. The effects of optimal concentrations of each compound were confirmed via a Trypan Blue exclusion assay (see Materials and Methods) and are illustrated in representative photomicrographs. Scale bar = 100 µm.
Table 1.
Protective effect of test compounds against the decreases in cell viability induced by Aβ1–42 [200nM] in primary cortical neuronal cultures.
| Compound | Cell Viability (% of Control) | ||||
|---|---|---|---|---|---|
| 10nM | 100nM | 1uM | 10uM | 100uM | |
| Donepezil | 67.0 ± 2.1% | 64.2 ± 2.5% | 63.2 ± 3.0% | 58.3± 2.3% | 48.7 ± 1.8%# |
| Galantamine | 66.5 ± 2.0% | 65.4 ± 1.7% | 65.4 ± 1.9% | 65.3 ± 2.0% | 65.9 ± 2.5% |
| Rivastigmine | 63.4 ± 1.3% | 64.5 ± 1.8% | 64.5 ± 1.1% | 60.9 ± 1.2% | 64.5 ± 1.2% |
| Memantine | 63.3 ± 1.8% | 63.9 ± 1.7% | 62.2± 1.6% | 63.2± 2.2% | 60.2± 1.9% |
| 1 | 73.3±1.4%* | 80.0 ± 1.0%* | 76.4 ± 1.3%* | 75.3 ± 1.1%* | 70.5 ± 1.1%* |
| 2 | 63.8 ± 1.3% | 64.6 ± 1.2% | 68.1 ± 1.6% | 65.8 ± 1.4% | 66.5 ± 1.7% |
| 3 | 62.0 ± 1.7% | 67.4 ± 1.5% | 73.3 ± 2.3%* | 80.1 ± 3.5%* | 87.5 ± 2.9%* |
| 4 | 69.1 ± 1.9%* | 71.0 ± 2.4%* | 76.3 ± 3.7%* | 80.8 ± 2.9%* | 93.2 ± 2.8%* |
| 5 | 66.6 ± 2.2% | 71.9 ± 2.3%* | 70.3 ± 2.2%* | 64.6 ± 1.8% | 64.4 ± 2.4% |
| 6 | 60.0 ± 2.5% | 60.5 ± 2.8% | 62.1 ± 2.4% | 63.3 ± 2.6% | 65.7 ± 3.5% |
| 7 | 61.4 ± 4.0% | 61.7 ± 3.7% | 62.2 ± 3.7% | 64.5 ± 3.5% | 64.2 ± 3.4% |
| 8 | 76.3 ± 1.4%* | 82.3 ± 1.2%* | 76.8 ± 1.7%* | 78.1 ± 1.4%* | 72.8 ± 1.4%* |
| 9 | 63.1 ± 1.6% | 63.5 ± 1.7% | 66.2 ± 1.6% | 66.2 ± 1.5% | 69.9 ± 1.5% |
| 10 | 74.1 ± 2.9%* | 74.9 ± 3.1%* | 73.3 ± 3.8%* | 76.9 ± 3.2%* | 87.0 ± 2.9%* |
| 11 | 64.7 ± 3.1% | 66.6 ± 2.9% | 62.7 ± 3.1% | 67.1 ± 4.8% | 58.5 ± 4.1% |
| 12 | 75.5 ± 3.0%* | 78.6 ± 2.6%* | 82.0 ± 2.6%* | 86.8 ± 1.8%* | 93.6 ± 1.7%* |
| 13 | 63.6 ± 2.0% | 69.5 ± 1.8%* | 72.6 ± 1.9%* | 75.5 ± 2.8%* | 82.4 ± 2.5%* |
| 14 | 63.9 ± 3.6% | 68.9 ± 2.8% | 67.9 ± 2.3% | 66.3 ± 1.3% | 65.3 ± 2.5% |
| 15 | 69.2 ± 2.8% | 67.4 ± 6.0% | 67.3 ± 3.5% | 68.4 ± 3.4% | 74.3 ± 4.6% |
| 16 | 62.3 ± 3.1% | 66.0 ± 3.7% | 68.8 ± 1.9% | 69.4 ± 2.7% | 58.5 ± 2.3% |
| 17 | 70.1 ± 1.6%* | 73.1 ± 1.6%* | 77.0 ± 1.3%* | 78.9 ± 2.2%* | 86.0 ± 1.8%* |
| 18 | 57.4 ± 2.1% | 58.3 ± 2.0% | 57.0 ± 1.9% | 59.1 ± 1.4% | 58.9 ± 1.8% |
P < 0.05, compared with Aβ1–42 treatment only.
Data represent the mean ± S.E.M. derived from 2–4 independent experiments with 7 replicates per drug concentration.
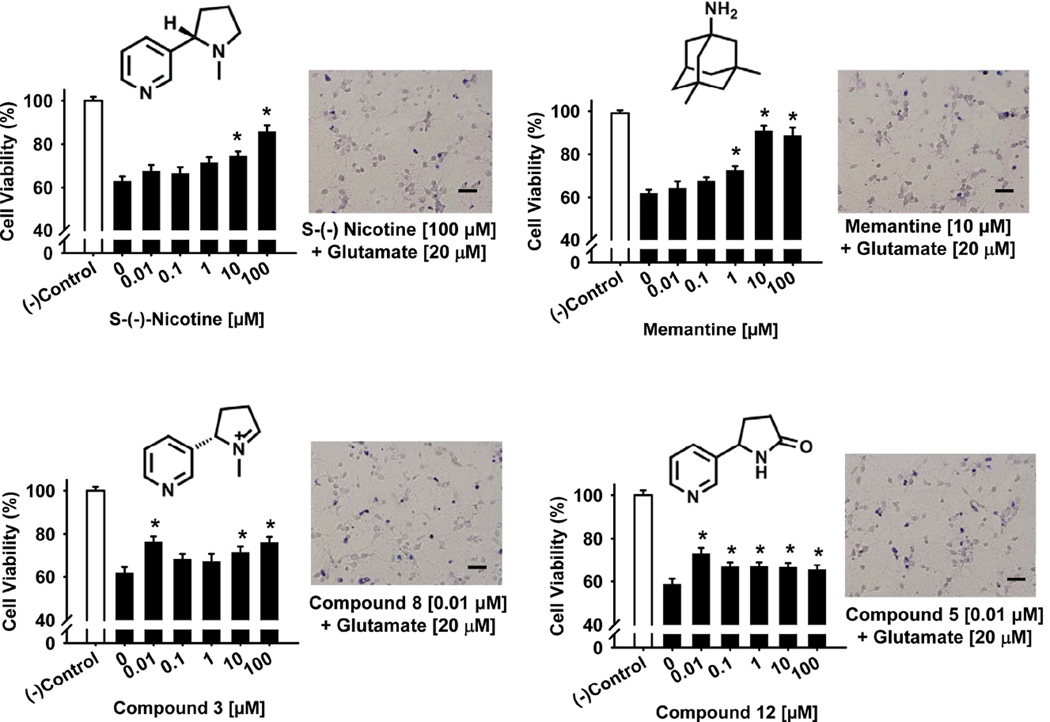
The results of experiments designed to assess the potential neuroprotective effects of nicotine, cotinine and structural analogs against glutamate neurotoxicity are illustrated in Fig 4 and Table 2. Similar to the toxicity associated with the Aβ1–42 peptide, 24 hr incubation with glutamate (20 µM) decreased cell survival by about 40% in each series of experiments. Based on the number of concentrations that afforded significant protection against glutamate neurotoxicity, (−)-nicotine, memantine, and compounds 3 and 12 were most effective. In Fig 4, concentration-effect relationships for these compounds (using the MTT assay) are illustrated in the bar plots and the effects of optimal concentrations (confirmed by the trypan blue exclusion method) are illustrated in the representative photomicrographs. In these experiments, (−)-nicotine (compound 1) and memantine were clearly the most effective compounds with their highest concentrations (100 µM) improving cell viability to over 85% of control. The (+) isomer of nicotine (compound 2) did not retain the neuroprotective activity of the (−) isomer. There were a few other instances where some level of neuroprotection was afforded against glutamate neurotoxicity depending on the compound and drug concentration evaluated. For example, two concentrations of donepezil (1.0 and 10.0 µM) improved cell viability; while (similar to the case of Aβ1–42 toxicity) the highest concentration (100 µM) appeared to increase glutamate toxicity. One concentration of galantamine (10 µM) and one concentration of compound 14 (100 µM) also improved cell viability.
Fig 4.
Neuroprotective effects of nicotine, memantine, and compounds 3 and 12 against the glutamate toxicity as determined in a cell viability assay in primary cultures of rat cortical neurons. Pretreatment of the cultures with various concentrations of nicotine, memantine, and compounds 3 and 12 for 24 hours was followed by exposure to glutamate (20 µM) for another 24 hours. Cell viability for each treatment was determined in an MTT assay (see Materials and Methods), calculated as percentage survival rate, and compared to a negative control (i.e., cultures without glutamate or test compound). Each bar represents the mean ± S.E.M (derived from 2–4 independent experiments with 7 replicates per drug concentration). *p < 0.05 compared to wells with glutamate added, but no test compound. The effects of optimal concentrations of each compound were confirmed via a Trypan Blue exclusion assay (see Materials and Methods) and illustrated in representative photomicrographs. Scale bar = 100 µm.
Table 2.
Protective effect of test compounds against the decreases in cell viability induced by glutamate [20µM] in primary cortical neuronal cultures
| Compound | Cell Viability (% of control) | ||||
|---|---|---|---|---|---|
| 10nM | 100nM | 1uM | 10uM | 100uM | |
| Donepezil | 63.2 ± 1.3% | 65.3 ± 1.5% | 69.5 ± 1.4%* | 70.0± 2.0%* | 43.6 ± 1.8%# |
| Galantamine | 54.4 ± 3.8% | 57.1 ± 3.3% | 64.1 ± 2.5% | 71.7 ± 3.5%* | 63.5 ± 2.4% |
| Rivastigmine | 60.0 ± 1.8% | 66.7 ± 1.1% | 60.0 ± 2.5% | 67.4 ± 1.5% | 70.6 ± 2.6% |
| Memantine | 62.1 ± 3.3% | 65.4 ± 1.7% | 70.3± 1.9%* | 88.0± 2.4%* | 85.8 ± 3.8%* |
| 1 | 65.2± 2.8% | 63.6 ± 2.8% | 67.0 ± 3.1% | 72.0 ± 2.2%* | 85.8 ± 2.9%* |
| 2 | 57.3 ± 2.2% | 61.5 ± 1.8% | 65.4 ± 1.4% | 66.4 ± 2.2% | 67.1 ± 1.3% |
| 3 | 74.3 ± 2.6%* | 66.6 ± 2.3% | 65.5 ± 3.5% | 69.7 ± 2.7%* | 74.0 ± 2.6%* |
| 4 | 59.4 ± 2.2% | 59.5 ± 2.0% | 56.9 ± 1.7% | 55.7 ± 2.6% | 54.8 ± 2.4% |
| 5 | 63.2 ± 2.0% | 58.5 ± 2.7% | 56.7 ± 2.2% | 58.9 ± 3.7% | 66.3 ± 2.8% |
| 6 | 69.2 ± 3.4% | 64.1 ± 2.7% | 67.6 ± 2.2% | 67.3 ± 3.2% | 68.9 ± 3.1% |
| 7 | 55.2 ± 2.7% | 58.7 ± 1.9% | 59.1 ± 1.9% | 60.4 ± 2.8% | 60.4 ± 3.4% |
| 8 | 60.5 ± 1.9% | 61.7 ± 1.9% | 63.8 ± 1.7% | 61.6 ± 1.7% | 66.5 ± 2.0% |
| 9 | 60.5 ± 1.7% | 60.7 ± 1.6% | 62.6 ± 1.8% | 61.9 ± 1.9% | 64.3 ± 2.0% |
| 10 | 62.6 ± 4.7% | 65.7 ± 3.3% | 68.6 ± 2.8% | 65.6 ± 3.2% | 67.2 ± 4.5% |
| 11 | 60.5 ± 1.1% | 62.9 ± 2.9% | 66.2 ± 3.6% | 65.6 ± 4.6% | 64.9 ± 4.1% |
| 12 | 74.5 ± 2.7%* | 68.3 ± 2.2%* | 68.3 ± 2.2%* | 68.0 ± 2.2%* | 66.9 ± 2.2%* |
| 13 | 66.6 ± 2.6% | 62.6 ± 2.5% | 60.0 ± 3.3% | 63.9 ± 2.8% | 66.3 ± 3.0% |
| 14 | 68.0 ± 4.5% | 66.4 ± 2.8% | 64.4 ± 2.8% | 66.8 ± 2.6% | 83.9 ± 2.7%* |
| 15 | 61.8 ± 3.0% | 60.3 ± 3.1% | 57.9 ± 2.5% | 57.3 ± 2.0% | 59.2 ± 2.2% |
| 16 | 66.8 ± 2.9% | 62.9 ± 2.9% | 59.6 ± 2.8% | 58.3 ± 3.8% | 56.8 ± 3.2% |
| 17 | 54.9 ± 3.5% | 61.5 ± 2.4% | 61.0 ± 3.5% | 63.4 ± 4.5% | 63.7 ± 4.1% |
| 18 | 63.6 ± 1.5% | 63.8 ± 1.5% | 61.6 ± 2.5% | 59.4 ± 2.5% | 59.2 ± 2.3% |
P < 0.05, compared with glutamate treatment only.
Data represent the mean ± S.E.M. derived from 2–4 independent experiments with 7 replicates per drug concentration.
The data obtained in the experiments described in this manuscript provide: 1) confirmatory evidence that (−)-nicotine and its most predominant metabolite (−)-cotinine have neuroprotective properties in vitro, 2) that the protective effect of the (−) isomers of nicotine and cotinine is significantly reduced or lost in the (+) isomers, 3) that some of commercially available analogs of nicotine and cotinine also possess neuroprotective activity in vitro: 4) and that (−)-nicotine and at least two of the nicotine/cotinine analogs (by exhibiting efficacy in two neurotoxicity models) appear to be superior as neuroprotective agents when compared to the currently prescribed AD therapeutic agents.
In the Aβ1–42 neurotoxicity model, the (−)-nicotine and (−)-cotinine analogs could be categorized into two main groups: those affording protection similar to or better than their parent compounds (e.g. Compounds 4 and 12) and those that showed complete loss of activity (e.g. compound 11 and 15). These results allowed for an initial prediction of the molecular features that might underlie nicotine/cotinine’s protective activity. First, oxidation of the nitrogen in the pyridine ring with a positive charged cation (compound 5 and 10) preserved neuroprotective activity of the parent compounds. However, compound 11, where the substituted position on the pyridine ring was switched from meta to ortho, lost the protective activity. Second, when the pyrrolidine ring is reduced to an aromatic pyrrole ring (compound 6) or is replaced by a chain ester substituent (compound 7), the protective activities were also reduced. However, compound 3, where the pyrrolidine ring is replaced with a 3,4-dihydro-2H-pyrrol-1-ium, retained neuroprotective activity. These data suggest that the flexibility of this ring system might be essential for optimum neuroprotective activity, given that the aromatization of the pyrrolidine introduced conformational changes in the structure and restricted the carbon positions in the ring. Third, a small substituent on the nitrogen of the pyrrolidine appears to be important for neuroprotective activity (in the Aβ1–42 neurotoxicity model) since the effect was lost by the addition of a para-methoxylmethylbenzyl group as observed in compound 14, while compound 12 and 13 without any substituent or with a small ethyl group, exhibited comparable activities to the parent compounds. Fourth, the substituted groups on the pyrrolidine ring (except for the nitrogen) might also be critical based on the mild decrease in activity in the compounds with the hydroxyl substituent (compounds 15 and 16) and complete loss of activity in the compound with an amide substituent (compound 18). However, compound 17 with the carboxylic group retained activity which suggested that a strong electronegative group might be favorable for neuroprotective activity.
In the glutamate neurotoxicity model, the low number of effective nicotine and cotinine analogs prevented any clear predictions as to the optimal structural features for neuroprotection. The fact that compound 3 (a nicotine analog) and 12 (a cotinine analog) each afforded significant neuroprotection in both the Aβ1–42 and the glutamate neurotoxicity model suggests that the extra carbonyl group in the cotinine structure may (alone) have little influence on neuroprotective activity. The observation that compound 14 with a bulky substituent on the pyrrolidine ring did not exhibit protective activity in the Aβ1–42 neurotoxicity model, whereas it exhibited a strong neuroprotective effect (83.9 ± 2.7% of control cell viability) in the glutamate neurotoxicity model (albeit at a single concentration), further suggests that the substituent size of the nitrogen in the pyrrolidine ring might be an important target for structural modifications. The fact that memantine (a glutamate NMDA antagonist) was effective in the glutamate neurotoxicity model was not surprising and it effectively served as a positive control for the later series of experiments described in this manuscript. There may be features of this molecule that could be combined with the structure of nicotine or cotinine to enhance activity against glutamate neurotoxicity.
The mechanisms of the neuroprotective effects of the various compounds observed in this study are unclear. It has been reported that the neuroprotective effects of nicotine and acetylcholinesterase inhibitors (AChEIs) observed previously in Aβ1–42 and glutamate neurotoxicity models is related to direct (nicotine) and indirect (AChEIs) effects at α4β2 and α7 nicotinic acetylcholine receptors (nAChRs) as well as effects on the PI3K-Akt pathway, activation of calcineurin, and L-type calcium channels.27–30 In older nAChR binding assays, cotinine was found to be approximately 100–1000 fold less potent than nicotine at displacing radiolabeled nAChR ligands31–34, therefore, it appears unlikely that the neuroprotective effects of cotinine observed in the Aβ1–42 neurotoxicity assay (i.e., at similar concentrations to nicotine) could be fully explained by direct effects at nAChRs. Interestingly, effectiveness of nicotine and cotinine and some other compounds (e.g., choline analogs) in memory-related behavioral tasks has been correlated with their effectiveness in producing nAChR desensitization.35 It would, therefore, be interesting to determine if such a relationship could be made between nAChR desensitization and neuroprotective activity. To our knowledge the nicotine and cotinine analogs evaluated in the current studies have not been assessed in nAChR binding or functional assays. The neuroprotective effects of some of the compounds evaluated in this study might also be related to effects on growth factors (i.e., neurotrophins) and/or their receptors. Interestingly, nicotine has been shown in culture systems (SH-SY5Y cells) to increase the release of Brain-Derived Neurotrophic Factor (BDNF) and to increase the cell surface expression of TrkB receptors.36 Likewise, nicotine, in primary cultures of rat basal forebrain neurons, was found to increase the release of nerve growth factor (NGF) and to increase TrkA receptors.37 Such effects on neurotrophin-related proteins might be especially relevant to the observations in the current study given that the test compounds (i.e., including nicotine) were administered first then washed out of the culture medium prior to toxin exposure (i.e., indicative of a prolonged neuroprotective effect). It is important to note that (to date) the effects described above have only be shown with nicotine, therefore, future experiments will be required to determine if such effects occur after exposure to the analogs of nicotine, cotinine, and cotinine analogs.
In conclusion, the results of this study indicated that S-(−)-nicotine, S-(−)-cotinine, and nine of their analogs (especially compounds 3 and 12) exhibited neuroprotective activities against amyloid-β neurotoxicity while only four of the compounds evaluated, nicotine, compounds 3 and 12, and the clinically prescribed NMDA antagonist, memantine exhibited significant protective effects against glutamate-mediated toxicity. The results with the analogs also indicated that the substituent size of the nitrogen in the pyrrolidine portion of these compounds is critical for neuroprotective activity and that the extra carbonyl group in the cotinine structure has little influence on this activity. The efficacy of (−)-nicotine and compounds 3 and 12 in both neuroprotection models used in these experiments suggest superior potential as disease-modifying agents when compared to the available prescription therapies (acetylcholinesterase inhibitors and the glutamate, NMDA antagonist, memantine). Given the limitations of nicotine as a potential therapeutic agent (e.g., cardiovascular side effects, abuse potential), compounds 3 and 12 may serve as superior prototypical compounds for the treatment of neurodegenerative conditions such as AD. Further, their structural features may aid in future rational drug design approaches.
Acknowledgements
This work was supported by grants from the National Health Institutes of Health: AG032140 and DA029127.
List of abbreviations
- Aβ
amyloid β
- AChEI
acetylcholinesterase inhibitor
- AD
Alzheimer’s disease
- nAChR
nicotinic acetylcholine receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Palmer AM. Neuroprotective therapeutics for Alzheimer's disease: progress and prospects. Trends Pharmacol Sci. 2011;32(3):141–147. doi: 10.1016/j.tips.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Youdim MB, Buccafusco JJ. CNS Targets for multi-functional drugs in the treatment of Alzheimer's and Parkinson's diseases. J Neural Transm. 2005 Apr;112(4):519–537. doi: 10.1007/s00702-004-0214-z. [DOI] [PubMed] [Google Scholar]
- 3.Buccafusco JJ. Multifunctional receptor-directed drugs for disorders of the central nervous system. Neurotherapeutics. 2009 Jan;6(1):4–13. doi: 10.1016/j.nurt.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavalli A, Bolognesi ML, Minarini A, Rosini M, Tumiatti V, Recanatini M, Melchiorre C. Multi-target-directed ligands to combat neurodegenerative diseases. J Med Chem. 2008;51(3):347–372. doi: 10.1021/jm7009364. [DOI] [PubMed] [Google Scholar]
- 5.León R, Garcia AG, Marco-Contelles J. Recent advances in the multitarget-directed ligands approach for the treatment of Alzheimer's disease. Med Res Rev. 2013 Jan;33(1):139–189. doi: 10.1002/med.20248. [DOI] [PubMed] [Google Scholar]
- 6.Buccafusco JJ, Jackson WJ, Jonnala RR, Terry AV., Jr Differential improvement in memory-related task performance with nicotine by aged male and female rhesus monkeys. Behav Pharmacol. 1999 Nov;10(6–7):681–690. doi: 10.1097/00008877-199911000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Rezvani AH, Levin ED. Cognitive effects of nicotine. Biol Psychiatry. 2001 Feb 1;49(3):258–267. doi: 10.1016/s0006-3223(00)01094-5. [DOI] [PubMed] [Google Scholar]
- 8.Picciotto MR, Zoli M. Neuroprotection via nAChRs: the role of nAChRs in neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease. Front Biosci. 2008;13:492–504. doi: 10.2741/2695. [DOI] [PubMed] [Google Scholar]
- 9.Martin LF, Freedman R. Schizophrenia and the α7 Nicotinic Acetylcholine Receptor. International Review of Neurobiology. 2007;78:225–246. doi: 10.1016/S0074-7742(06)78008-4. [DOI] [PubMed] [Google Scholar]
- 10.Buccafusco JJ, Terry AV. The potential role of cotinine in the cognitive and neuroprotective actions of nicotine. Life Sci. 2003;72(26):2931–2942. doi: 10.1016/s0024-3205(03)00226-1. [DOI] [PubMed] [Google Scholar]
- 11.Srivastava ED, Hallett MB, Rhodes J. Effect of nicotine and cotinine on the production of oxygen free radicals by neutrophils in smokers and non-smokers. Hum Toxicol. 1989 Nov;8(6):461–463. doi: 10.1177/096032718900800605. [DOI] [PubMed] [Google Scholar]
- 12.Rehani K, Scott DA, Renaud D, Hamza H, Williams LR, Wang H, Martin M. Cotinine-induced convergence of the cholinergic and PI3 kinase-dependent anti-inflammatory pathways in innate immune cells. Biochim Biophys Acta. 2008 Mar;1783(3):375–382. doi: 10.1016/j.bbamcr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Riveles K, Huang LZ, Quik M. Cigarette smoke, nicotine and cotinine protect against 6-hydroxydopamine-induced toxicity in SH-SY5Y cells. Neurotoxicology. 2008 May;29(3):421–427. doi: 10.1016/j.neuro.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burgess Sarah, Zeitlin Ross, Echeverria Valentina. Cotinine Inhibits Amyloid-β Peptide Neurotoxicity and Oligomerization. J Clinic Toxicol. 2012;S6 [Google Scholar]
- 15.Echeverria V, Zeitlin R, Burgess S, Patel S, Barman A, Thakur G, Mamcarz M, Wang L, Sattelle DB, Kirschner DA, Mori T, Leblanc RM, Prabhakar R, Arendash GW. Cotinine reduces amyloid-β aggregation and improves memory in Alzheimer's disease mice. J Alzheimers Dis. 2011;24(4):817–835. doi: 10.3233/JAD-2011-102136. [DOI] [PubMed] [Google Scholar]
- 16.Terry AV, Jr, Hernandez CM, Hohnadel EJ, Bouchard KP, Buccafusco JJ. Cotinine, a neuroactive metabolite of nicotine: potential for treating disorders of impaired cognition. CNS Drug Rev. 2005;11:229–252. doi: 10.1111/j.1527-3458.2005.tb00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buccafusco JJ, Terry AV. A reversible model of the cognitive impairment associated with schizophrenia in monkeys: potential therapeutic effects of two nicotinic acetylcholine receptor agonists. Biochem Pharmacol. 2009;78(7):852–862. doi: 10.1016/j.bcp.2009.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terry AV, Jr, Buccafusco JJ, Schade RF, Vandenhuerk L, Callahan PM, Beck WD, Hutchings EJ, Chapman JM, Li P, Bartlett MG. The nicotine metabolite, cotinine, attenuates glutamate (NMDA) antagonist-related effects on the performance of the five choice serial reaction time task (5C-SRTT) in rats. Biochem Pharmacol. 2012 Apr 1;83(7):941–951. doi: 10.1016/j.bcp.2011.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terry AV, Jr, Hernandez CM, Hohnadel EJ, Bouchard KP, Buccafusco JJ. Cotinine, a neuroactive metabolite of nicotine: potential for treating disorders of impaired cognition. CNS Drug Rev. 2005 Autumn;11(3):229–252. doi: 10.1111/j.1527-3458.2005.tb00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatsukami DK, Grillo M, Pentel PR, Oncken C, Bliss R. Safety of cotinine in humans: physiologic, subjective, and cognitive effects. Pharmacol Biochem Behav. 1997 Aug;57(4):643–650. doi: 10.1016/s0091-3057(97)80001-9. [DOI] [PubMed] [Google Scholar]
- 21.Hynd MR, Scott HL, Dodd PR. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer's disease. Neurochem Int. 2004;45(5):583–595. doi: 10.1016/j.neuint.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Bredesen DE. Neurodegeneration in Alzheimer's disease: caspases and synaptic element interdependence. Mol Neurodegener. 2009;4:27. doi: 10.1186/1750-1326-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keowkase R, Aboukhatwa M, Adam B-L, Beach W, Terry A, Jr, Buccafussco J, Luo Y. Neuroprotective effects and mechanism of cognitive-enhancing choline analogs JWB 1-84-1 and JAY 2-22-33 in neuronal culture and Caenorhabditis elegans. Molecular Neurodegeneration. 2010;5:59. doi: 10.1186/1750-1326-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taguchi R, Shirakawa H, Yamaguchi T, Kume T, Katsuki H, Akaike A. Nitric oxide-mediated effect of nipradilol, an α- and β-adrenergic blocker on glutamate neurotoxicity in rat cortical cultures. European Journal of Pharmacology. 2006;535:86–94. doi: 10.1016/j.ejphar.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 25.Chemicals and suppliers-The source of drugs and materials used are as follows: Cell culture materials (Gibco, Grand Island, NY, USA); Invitrogen Vybrant® MTT Cell Proliferation Assay Kit (Molecular probes, Eugene, OR, USA), Aβ1–42 (American Peptide, Sunnyvale, CA, USA), R-(+)-Nicotine, S-(−)-Cotinine, R-(+)-Cotinine, (+/−)-ortho-Cotinine Perchlorate, trans-3’-Hydroxy Cotinine (10mg), cis-3’-Hydroxy Cotinine, (S)-Cotinine N-Oxide, (2’S)-Nicotine 1-Oxide, N-(4-Methoxybenzyl)cotinine, trans-Cotinine Amide, N-Formylnornicotine, S-(−)-Nicotine-Δ1’(5’)-iminium Diperchlorate Salt, (R,S)-norcotinine, β-Nornicotyrine, (R,S)-N-Ethylnorcotinine (Toronto Research Chemicals, Toronto, ON, Canada), Methyl 6-methylnicotinate (Alfa Aesar, Ward Hill, MA, USA), trans-1-Methyl-4-carboxy-5-(3-pyridyl)-2-pyrrolidinone (TCI America, Portland, OR, USA), (−)-Nicotine, Memantine hydrochloride, MK-801, (±)-Nornicotine, L-glutamic acid monosodium salt hydrate (Sigma, St. Louis, MO, USA), Donepezil, Galantamine (A&A Pharmachem, Shenzhen, China).
- 26.Neuroprotection Assays-Cortical neuronal cultures were derived from the cerebral cortex of Sprague–Dawley rat embryos (E17–18) as described previously [32]. Briefly, cells dissociated from the cerebral cortex of embryos were seeded at a density of 5×105 cells/mL onto poly-D-lysine pre-coated 96-well plates for neuronal cytotoxicity assay. For image analysis, cells were seeded at a density of 2×105 cells/mL onto poly-L-lysine pre-coated 12mm glass coverslips in 24-well plates (Corning, Corning, NY, USA). Cultures were incubated in neurobasal medium supplemented with 2% B27, 0.5M L-glutamax, 100 U/mL penicillin and streptomycin and maintained at 37°C in a 5% CO2 humidified atmosphere. Experiments were performed at 37°C on the culture day 7–8. In order to determine concentration-effect relationships for the neurotoxic effects of the Aβ1–42 peptide or glutamic acid, cultured neurons were exposed to Aβ1–42 (100, 200, 400, 800 and 1000nM) or glutamate (10, 20, 40, 80 and 100uM) for 24 h. To determine the neuroprotective effects of the test compounds against Aβ- and glutamate-induced neurotoxicity, neurons were pre-treated with test compounds alone (10nM, 100nM, 1uM, 10uM and 100uM) for 24 hours. The cells were washed and then challenged with 200nM Aβ or 20uM glutamate for another 24 hours. For each condition described above, a total of 2–4 independent experiments were performed with 7 replicates per drug concentration evaluated. Cell viability was determined using a commercially available MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay (Invitrogen Vybrant® MTT Cell Proliferation Assay Kit). Briefly, after exposures to neurotoxins and/or test compounds, primary cortical neurons were treated with 0.5 mg/ml MTT for 4 h at 37°C. 100 µl of the SDS-HCl solution were added to each well and mixed thoroughly and incubated for another 14 hours. The absorbance was measured at 570 nm. As a secondary method of confirming the results obtained with the MTT assay, cell viability was confirmed via the Trypan blue exclusion method. Briefly, cultured cells were incubated in 1.5% trypan blue PBS solution for 10 minutes at room temperature and fixed in 4% paraformaldehyde (pH 7.2, 2–4°C), and then rinsed with PBS. Culture fields were photographed with a Zeiss Axioplan 2 Microscope with AxioCam camera. Results are expressed as percentage of control values obtained from cultures not exposed to glutamate or the Aβ1–42 peptide. Differences were analyzed for statistical significance using one-way ANOVA, followed by Holm-Sidak post hoc comparison method. Significance was set at p < 0.05.
- 27.Stevens TR, Krueger SR, Fitzsimonds RM, Picciotto MR. Neuroprotection by nicotine in mouse primary cortical cultures involves activation of calcineurin and L-type calcium channel inactivation. J Neurosci. 2003;23(31):10093–10099. doi: 10.1523/JNEUROSCI.23-31-10093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Picciotto MR, Zoli M. Neuroprotection via nAChRs: the role of nAChRs in neurodegenerative disorders such as Alzheimer's and Parkinson's disease. Front Biosci. 2008 Jan 1;13:492–504. doi: 10.2741/2695. [DOI] [PubMed] [Google Scholar]
- 29.Akaike A, Takada-Takatori Y, Kume T, Izumi Y. Mechanisms of neuroprotective effects of nicotine and acetylcholinesterase inhibitors: role of alpha4 and alpha7 receptors in neuroprotection. J Mol Neurosci. 2010;40(1–2):211–216. doi: 10.1007/s12031-009-9236-1. [DOI] [PubMed] [Google Scholar]
- 30.Yu W, Mechawar N, Krantic S, Quirion R. α7 Nicotinic receptor activation reduces β-amyloid-induced apoptosis by inhibiting caspase-independent death through phosphatidylinositol 3-kinase signaling. J Neurochem. 2011;119(4):848–858. doi: 10.1111/j.1471-4159.2011.07466.x. [DOI] [PubMed] [Google Scholar]
- 31.Abood LG, Reynolds DT, Booth H, Bidlack JM. Sites and mechanisms for nicotine's action in the brain. Neurosci Biobehav Rev. 1981 Winter;5(4):479–486. doi: 10.1016/0149-7634(81)90018-x. [DOI] [PubMed] [Google Scholar]
- 32.Sloan JW, Todd GD, Martin WR. Nature of nicotine binding to rat brain P2 fraction. Pharmacol Biochem Behav. 1984 Jun;20(6):899–909. doi: 10.1016/0091-3057(84)90015-7. [DOI] [PubMed] [Google Scholar]
- 33.Anderson DJ, Arneric SP. Nicotinic receptor binding of [3H]cytisine, [3H]nicotine and [3H]methylcarbamylcholine in rat brain. Eur J Pharmacol. 1994 Mar 3;253(3):261–267. doi: 10.1016/0014-2999(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 34.Vainio PJ, Tuominen RK. Cotinine binding to nicotinic acetylcholine receptors in bovine chromaffin cell and rat brain membranes. Nicotine Tob Res. 2001 May;3(2):177–182. doi: 10.1080/14622200110043095. [DOI] [PubMed] [Google Scholar]
- 35.Buccafusco JJ, Beach JW, Terry AV., Jr Desensitization of nicotinic acetylcholine receptors as a strategy for drug development. J Pharmacol Exp Ther. 2009 Feb;328(2):364–370. doi: 10.1124/jpet.108.145292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Serres F, Carney SL. Nicotine regulates SH-SY5Y neuroblastoma cell proliferation through the release of brain-derived neurotrophic factor. Brain Res. 2006 Jul 26;1101(1):36–42. doi: 10.1016/j.brainres.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 37.Formaggio E, Fazzini F, Dalfini AC, Di Chio M, Cantù C, Decimo I, Fiorini Z, Fumagalli G, Chiamulera C. Nicotine increases the expression of neurotrophin receptor tyrosine kinase receptor A in basal forebrain cholinergic neurons. Neuroscience. 2010 Mar 17;166(2):580–589. doi: 10.1016/j.neuroscience.2009.12.073. [DOI] [PubMed] [Google Scholar]