Abstract
Small animal models are indispensable for research on nerve injury and reconstruction, but their superlative regenerative potential may confound experimental interpretation. This study investigated time-dependent neuroregenerative phenomena in rodents. Forty-six Lewis rats were randomized to three nerve allograft groups treated with 2 mg/(kg day) tacrolimus; 5 mg/(kg day) Cyclosporine A; or placebo injection. Nerves were subjected to histomorphometric and walking track analysis at serial time points. Tacrolimus increased fiber density, percent neural tissue, and nerve fiber count and accelerated functional recovery at 40 days, but these differences were undetectable by 70 days. Serial walking track analysis showed a similar pattern of recovery. A ‘blow-through’ effect is observed in rodents whereby an advancing nerve front overcomes an experimental defect given sufficient time, rendering experimental groups indistinguishable at late time points. Selection of validated time points and corroboration in higher animal models are essential prerequisites for the clinical application of basic research on nerve regeneration.
Much of our understanding of nerve regeneration is derived from experiments in small animal models. This research is premised upon the notion that observations in mice or rats have direct implications for related biological phenomena in human patients. While rodent models offer advantages of small size and suitability for performing experimental neurorrhaphy, data from these models must be evaluated critically. In the rodent, exceptional regenerative capacity, short limb length, and biological variability among animals all conspire to obscure key differences between experimental groups. As a result, type II errors—in which a difference exists between groups but fails to be detected— may be more common than generally recognized.
It was hypothesized that, due to superior regenerative potential observed in rodents, there exists only a finite window of opportunity for making accurate assessments of nerve regeneration in this model. The present study employed an established nerve allograft model of neuroenhancement using the agent tacrolimus (FK506), which possesses immunosuppressive properties and is the most well studied agent for neuroenhancement in small animals.1 Allografted animals that were treated with cyclosporine A (CsA) or that were treated with no agent served as negative controls. In this model, the nerve allograft is a scaffold for nerve regeneration, and allogeneic Schwann cells are the antigenic target for rejection. Under the influence of immu nosuppression, host Schwann cells migrate into allograft nerve and support regenerating nerve fibers as they extend towards target end-organs. Previously validated histological2 and functional assessments3–5 of nerve regeneration were made at serial time points.
MATERIALS AND METHODS
Experimental Design
Adult male American Cancer Institute (ACI RT1a) rats served as nerve donors to adult male Lewis rats (RT1(1)) (Charles Rivers Laboratories, Wilmington, MA). Major histocompatibility disparity exists in these two inbred strains at both class I and II loci and this model reliably demonstrates rejection of untreated allografts. Animals were housed in flat bottomed cages and allowed standard rat chow and water ad libitum. All experimental procedures and interventions as well as housing, diet, and animal care regimens were performed in strict accordance with guidelines from the National Institutes of Health. The experimental protocol was approved by the Institution Animal Studies Committee.
Methods
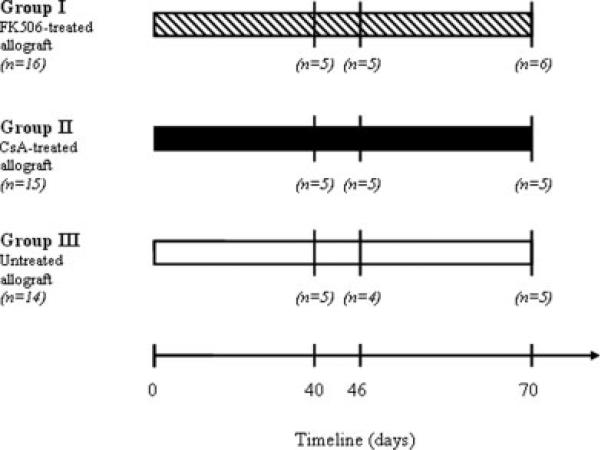
Animals were randomly assigned to one of the three groups (see Fig. 1). Group I animals (n = 16) received 2 mg/(kg day) of tacrolimus, group II animals (n = 15) received 5 mg/(kg day) of cyclosporine A, and group III animals (n = 14), functioned as a negative control and received a vehicle injection of anhydrous alcohol (Quantum Chemical, Tuscola, IL). Animals from all three groups were then sacrificed at either 40, 46, or 70 days postoperatively (see Fig. 1). In group I, animals were sacrificed at 40 (n = 5), 46 (n = 5), and 70 (n = 6) days postoperatively. In group II, five animals were sacrificed at each of the three time points. In group III, animals were sacrificed at 40 (n = 5), 46 (n = 4), and 70 (n = 5) 70 days postoperatively. The postoperative day 40 and 46 endpoints were selected based on the stabilization of print length factor (marker for tibial nerve functional recovery), in the tracrolimus and CsA groups, respectively.
Figure 1.
Schematic representation of the experimental design. Group sizes are indicated in italics. Animals were sacrificed 40, 46, and 70 days postoperatively.
Immunosuppressive Regimen
Tacrolimus (Fujisawa USA, Deerfield, IL) was diluted to a concentration of 5 mg/ml in anhydrous alcohol. Cyclosporine A (Sandoz Pharmaceuticals, Sandoz Canada, Dorval, Quebec) was diluted to a concentration of 15 mg/ml with 20% Tween 80 (polyxyethylene sorbitan monolaurate; Sigma Chemical, St. Louis, Missouri) and 65% anhydrous alcohol. All animals were injected via the intraperitoneal route, and received their first dose 2 days prior to surgery. Subsequently, animals were dosed daily beginning 1 day after surgery. Dosage selections were based on prior work demonstrating the immunosuppressive dose of tacrolimus [2 mg/(kg day)]6 and CsA [5 mg/(kg day)]7,8 in the rodent peripheral nerve allograft model. Rats were weighed weekly and the dosages adjusted accordingly.
Surgical Procedure
An intramuscular injection of ketamine (Fort Dodge Animal Health, Fort Dodge, IA) at a dose of 50 mg/kg and medetomidine HCl (Pfizer Animal Health, Exton, PA) at a dose of 0.2 mg/kg was used to induce anesthesia. All surgical procedures were performed aseptically using standard microsurgical techniques and an operating microscope. The posterior tibial nerves of the ACI donors were identified bilaterally with sharp muscle-splitting incisions and blunt dissection. Under magnification, a 2-cm segment of the posterior tibial nerve was isolated and carefully neurolysed. This 2-cm segment would serve as the nerve graft. Simultaneously, an identical incision on the Lewis rat exposed the right posterior tibial nerve, which was transected 0.5-cm distal to the sciatic nerve trifurcation. The orientation of the donor nerve graft was reversed, and the nerve graft was then interposed into the defect and coapted to the recipient nerve ends with four interrupted 10-0 nylon microepineurial sutures.
Regimen Toxicity Assessment
Nephrotoxicity was determined from the blood urea nitrogen (BUN) and creatinine (Cr) levels obtained from 2 ml of blood drawn from each animal at the time of sacrifice.
Functional Assessment
A standardized walking track analysis (WTA) technique allowed for assessment of hindlimb recovery by examination of footprint patterns. After the hind feet of the animals were dipped in D76 developer (Eastman-Kodak, Rochester, NY), they were allowed to ambulate down a 14 × 56 cm corridor in which the bottom was lined with exposed, underdeveloped X-ray film. The footprint lengths of both the normal (NPL, left) and experimental (EPL, right) feet were measured by means of a computer-linked digital pen and morphometry software developed in our laboratory. The print length factor (PLF) was then calculated by the formula:
Since this technique relates the unoperated left limb to the experimental right limb, prior to grafting, the mean PLF approximates zero, demonstrating no difference between the two hindlimb footprints. In the rat, posterior tibial nerve injury is associated with an increased EPL, and therefore an increased PLF. As hindlimb function recovers over time, the PLF progressively decreases providing a marker for functional recovery of the tibial nerve.3–5 WTA began 2 weeks after surgery and continued every 3 days until postoperative day 50 after which, walking tracks were performed every 10 days until the 70-day endpoint.
Histomorphometric Evaluation
At the determined time points, the nerve graft was reexposed under general anesthesia, and excised en bloc to include proximal and distal host segments. Immediately following nerve harvest, animals were sacrificed with a lethal intravenous dose of Pentobarbital (Delmarva Laboratories, Midlothian, VA). The sample was then fixed in a cold, buffered 3% glutaraldehyde solution for 24 h, post-fixed with osmium tetroxide, and embedded in Araldite 502 (Polysciences, Warrington, PA). About 1 μm-thick cross-sections were cut with an LKB III Ultra-microtome (LKB-Produkter A.B., Bromma, Sweden) and stained with 1% toluidine blue. Under light microscopy, these stained cross-sections were evaluated for overall nerve architecture, quality and quantity of regenerated nerve fibers, extent of myelination, and presence of Wallerian degeneration.
Using an automated digital image-analysis system linked to morphometry software (Leco Instruments, St. Joseph, Michigan), the microscope image was digitized and displayed on a video monitor with a calibration of 0.125 μm/pixel. Computer analysis of the digitized information based on gray and white scales allowed measurements of total fascicular area and total fiber number in the recipient nerve 5 mm distal to the graft repair. At 1,000× magnification, six randomly selected fields per nerve, or a minimum of 500 myelinated fibers, were evaluated for myelin width, axon width, and fiber width. From these, calculations of nerve fiber density (fibers/mm2), total number of myelinated fibers and percentage of neural tissue (100 × neural area/intrafascicular area) were made. An observer blinded to the experimental groups performed all measurements using the previously validated technique described above.2
Statistical Analysis
The computer program Statistica (StatSoft, Tulsa, OK) was used to calculate statistics, and the performance of appropriate statistical procedures was based on the distribution frequency of all of the data. Statistical analysis was performed on the mean values for PLFs obtained from the walking tracks. A one-way repeated measures analysis of variance was performed on the same groups of rats over time. If the analysis demonstrated significance, then the means of variables from specific groups were compared using the Student-Newman-Keuls test. For the histomorphometry, an overall analysis of the differences between group means was calculated by a one-way analysis of variance (ANOVA). Differences between groups with respect to body weight, BUN, and Cr were identified with an independent t test. In all cases, statistical significance was set at P < 0.05.
RESULTS
General Observations
Animals weighed 272 ± 31 g (199–338 g) preoperatively and all lost weight during the first 2 weeks of the postoperative period. Subsequently, body weight recovered in all animals, and no statistically significant differences regarding weight gain were demonstrated between treatment groups. At the time of sacrifice, control animals weighed 288.9 ± 38 g, Cyclosporine A-treated animals weighed 285 ± 47 g, and tacrolimus-treated animals weighed 281 ± 35 g. Nephrotoxicity did not develop in any of the experimental animals based upon BUN and Cr levels in the immunosuppressed groups within the normal range (BUN < 30, Cr < 1.2), and did not differ significantly from the immunocompetent control group.
Functional Assessment
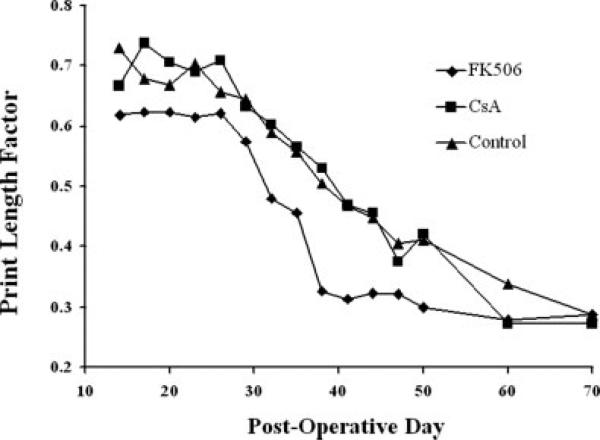
Based upon stabilization of functional recovery (Fig. 2), nerve harvest time points were determined as 40 days for tacrolimus-treated animals and 46 days for Cyclosporine A-treated animals. Onset of recovery, as determined by significant functional improvement relative to the first postoperative walking track, was 35 days (P < 0.05) for the tacrolimus-treated animals while the Cyclosporine A-treated animals showed signs of significant recovery on day 38 post-engraftment (P < 0.05). At 70 days, there were no statistically significant differences between PLFs between groups. The stabilization of functional recovery in animals treated with tacrolimus occurred at 38 days while recovery in Cyclosporine A-treated or untreated animals reached a plateau at 60 and 70 days, respectively.
Figure 2.
Mean print length factors of surviving animals in Groups I-III plotted over 70 days. Animals treated with tacrolimus appear to normalize and stabilize more quickly than those left untreated or treated with cyclosporine-A. Differences were not statistically significant.
Histomorphometric Evaluation
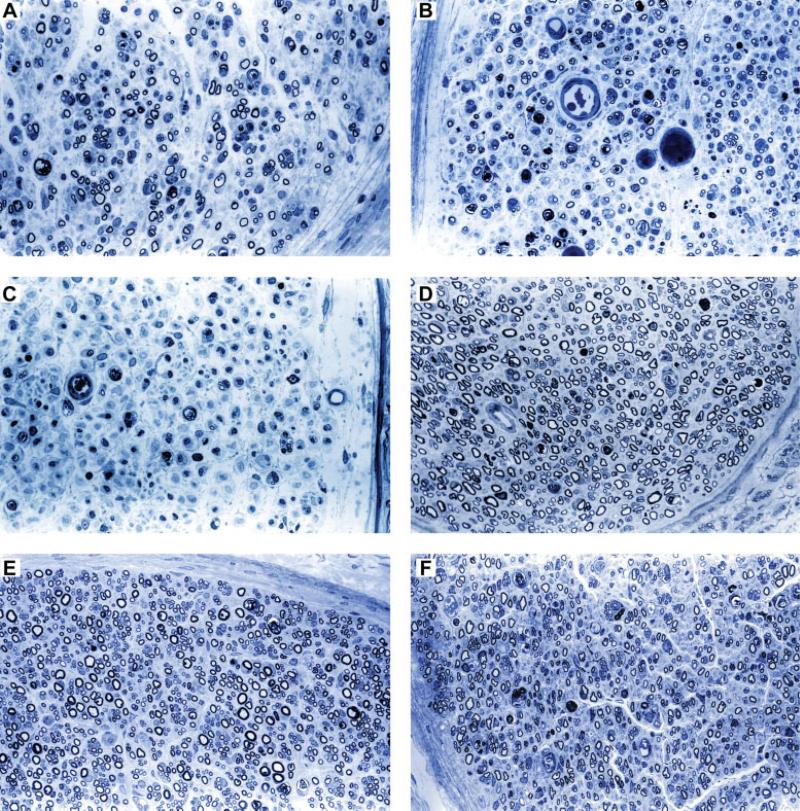
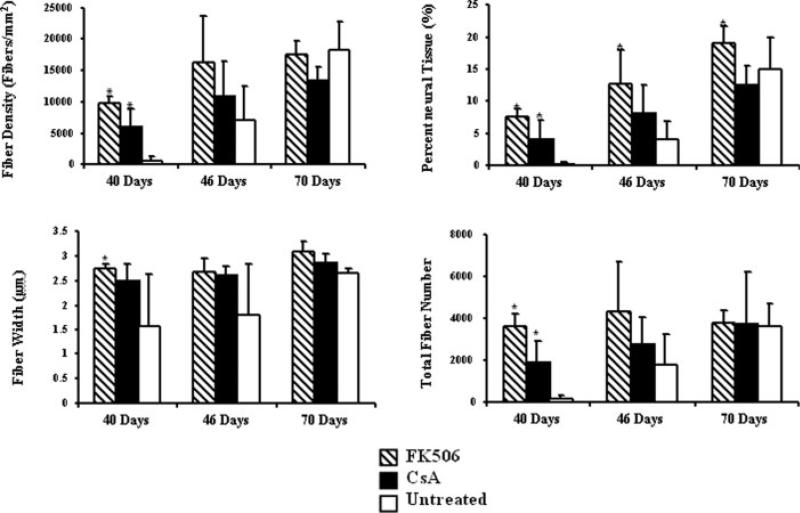
Representative photomicrographs of the toluidine blue-stained sections from nerves harvested at 40 and 70 days are illustrated in Figure 3. Analyses of the histomorphometric data comparing animal groups at 40, 46, and 70 days are shown in Figure 4. At 40 days, the fiber density, percent neural tissue, and total number of fibers in the tacrolimus group (n = 5), were significantly greater than both the Cyclosporine A (P < 0.05) group (n = 5), and the untreated control (P < 0.01) group (n = 5). At the same time point, the fiber width of the tacrolimus-treated animals was significantly greater than untreated controls (P < 0.05). In addition, the Cyclosporine A-treated animals had a significantly greater fiber density, percent neural tissue, and total number of fibers when compared with the controls at 40 days (P < 0.05). There were no significant differences between groups regarding fiber density, fiber width, or total number of fibers at 70 days.
Figure 3.
Representative photomicrographs of the toluidine blue-stained sections 5-mm distal to nerve allografts harvested at 40 and 70 days at ×400 magnification. By 40 days postoperatively, treatment with (A) Tacrolimus increased nerve regeneration compared with (B) Cyclosporine-A and (C) untreated allografts, while at 70 days, differences between (D) tacrolimus, (E) Cyclosporine-A, and (F) untreated groups could not be distinguished from one another. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Figure 4.
Mean histomorphometric parameters for groups I–III harvested at time points of 40, 46, and 70 days postoperatively. Tacrolimus-treated animals demonstrated significantly greater nerve regeneration than Cyclosporine-A treated or untreated animals at 40 days (* denotes a significant difference at P < 0.05 in comparisons against untreated group, error bars indicate standard deviation).
DISCUSSION
Timing is a critical consideration in the study of nerve regeneration. Accelerating nerve regeneration has the potential to optimize functional recovery through early reinnervation and salvaging of functional motor end-plates.9,10 Conversely, impaired nerve regeneration not only delays functional recovery but may adversely impact upon the degree of recovery that is ultimately achieved. A large body of experimental work investigates approaches to enhance nerve regeneration with the goal of improving outcomes after nerve injury.11 To place this research in a meaningful context, it is essential to understand the limitations of the rodent model. The present study provides evidence in support of a “blow-through” effect whereby superior regenerative potential inherent in the rodent nervous system allows small animals to overcome challenges unlikely to be surmounted in humans and higher animals.
In the present study, treatment of rodents with the neuroregenerative agent tacrolimus was correlated with a significantly greater fiber density, percent neural tissue, and total number of nerve fibers compared with both untreated and Cyclosporine A-treated animals at 40 days postallotransplantation. Cyclosporine A therapy was effective in preventing any histologic evidence of rejection and supported axonal regeneration through nerve allografts. Numerous studies of the nerve allograft response in rodents have shown that CsA can be used as monotherapy to prevent rejection of nerve using mixed lymphocyte reactions (MLRs),8,12 enzyme-linked immunosorbent spot assays (ELISPOT),13 and skin graft evaluation.6
In comparison, tacrolimus actively enhanced neuroregeneration as indicated by the histomorphometric data distal to the nerve graft repair. Additionally, animals treated with tacrolimus demonstrated an increased rate of normalization of the PLF and earlier stabilization of hindlimb recovery, as indicated by a plateau in the PLF value. This trend towards more rapid recovery is indicative of earlier motor end plate reinnervation, consistent with prior studies showing that tacrolimus not only accelerates regeneration but also accelerates functional recovery in various nerve injury models.11
At later time points, the rodent model becomes insensitive for detection of differences between experimental groups. At 46 days, some differences persist between experimental groups, but most comparisons were no longer significant. By 70 days, the rodent nervous system had entirely surmounted nerve allograft rejection, as reflected by robust nerve regeneration, restoration of perineurial architecture, and functional recovery. This finding appears unique to small animals in that nerve allograft rejection in large animal models and human subjects have been associated with irreversible scarring and regeneration failure.14,15 Thus, given enough time for regeneration, rodents exhibit a pattern of regeneration in negative control groups that is not observed in large animal models and humans.
These data suggest that a relatively narrow window of opportunity exists for reliably evaluating nerve regeneration in rodents. The inherent capacity for nerve regeneration and small anatomy in the rat helps to explain this “blow-through” effect.16,17 Selecting a time point to accurately evaluate the efficacy of various treatment modalities poses a challenge to short nerve allograft experimentation. Specifically, evaluation must be conducted before the short nerve graft is repopulated with neural tissue from the host18–20 but should allow enough time for some nerve regeneration to occur. Untreated nerve allografts in excess of 3 cm are prone to undergo rejection and support very limited neural regeneration.21 These grafts are characterized by the persistence of antigenic donor Schwann cells22 and incomplete repopulation of the graft with host neural tissue. Therefore, to demonstrate this “blow-through” effect we chose a 2-cm gap which is not a critical gap length, allowing for the negative control to approach the tacrolimus group at a late timepoint. Graft lengths of 2 cm or less are commonly used in the rodent literature on nerve regeneration.23,24 In large animal models, which better simulate large gap injuries seen in patients, a 5- or 7-cm defect can be studied.14,25 A review of prior peripheral nerve research in rodents can help identify the appropriate time of evaluation for various graft lengths based on the desired variable of evaluation. A standardized endpoint by graph length would allow for more accurate interpretation of results from research in rodent nerve regeneration models.
The findings of this study might prompt one to question whether tacrolimus actually does have the potential to confer a lasting benefit in nerve regeneration. Several pieces of evidence substantiate the efficacy of tacrolimus as a neuroenhancing agent. While multiple pharmacological agents have been investigated for their putative neuroregenerative properties,26–28 only tacrolimus has been shown to enhance nerve regeneration and accelerate functional recovery in crush,10,29,30 transection,31,32 isografts,23 conduits,33 and allograft34,35 rodent nerve injury models, in addition to promoting neurite extension with in vitro models.11 Furthermore, the neuroregenerative effects of tacrolimus have been documented in a large animal model36 and FK506 has been used in human nerve allotransplantation,37 laryngeal transplantation,38 and hand transplantation39; all examples of nerve regeneration influencing functional outcomes. The clear demonstration of a lack of neuroenhancement at late time points in this small animal model therefore establishes that the effect is a true limitation of the small animal model.
Prior experimental data do support the notion that differences in nerve regeneration detected transiently in small animals15,40–42 may prove to be long-lasting and significant phenomena in larger animals.36,43 In a rodent study by Calvert et al. comparing long tibial nerve isografts to allografts, significant differences in nerve regeneration were evident after 10 weeks postoperatively but failed to be reproduced at 14 weeks.42 This relatively late finding suggests that the timing of the “blow-through” effect is partially a function of graft length. In one study, the sciatic nerve was transected at the sciatic notch and 4.5-cm length excised. Peripheral nerves were found to spontaneously regenerate a mean distance of ~2.5 cm.17
Additional data in support of a small animal phenomenon derives from prior experiments on tolerance induction conducted in a murine model.41 Anti-CD40 ligand monoclonal antibody is capable of preventing nerve allograft rejection through an immunomodulatory mechanism involving costimulatory blockade of T-cell activation. At a 3-week endpoint, untreated mice will exhibit unequivocal evidence of nerve allograft rejection, and treated animals demonstrate no evidence of rejection by histological and MLR immunoassay. However, at 6 weeks, treated and untreated animals have similar nerve histology. This latter observation is of particular interest when one observes that anti-CD40 ligand therapy prevents rejection and improves nerve regeneration over placebo in primates several months after transplantation.44
Another relevant example is recent work describing the use of longitudinally oriented sutures to reconstruct short nerve defects. Scaffolds comprised of polyglactin or polyamide suture will provide a linear support for extension of nerve fibers but lack Schwann cells and the associated capacity for synthesis of structural and neurotrophic components. This suture scaffold model has been reported as equivalent to autogenous nerve grafting based on the similar appearance of experimental groups at 12 weeks.45,46 Subsequent work demonstrated nerve regeneration with the suture scaffold at 4 weeks was poor when compared with the autograft gold standard.40 Thus, the finding of equivalency between 2 groups at 12 weeks may represent an artifact of the rodent experimental model (“blow-through” effect). This critique may also pertain to literature on a variety of conduit-based approaches to nerve reconstruction in rodents.
While small animals are vital to research on nerve regeneration; work using these models must be undertaken with an informed perspective on the unique biology of small animals and how it differs from that of higher animals. When rodents are evaluated at a late time point, beneficial treatments may be overlooked as conferring no advantage. A more pervasive problem is the tendency to overestimate the efficacy of a new therapy that appears equivalent to a gold standard at late time points. These observations underscore the importance of rigorous controls and appropriate timing in functional and morphometric assessments of nerve regeneration.
In certain circumstances the increased sprouting observed early in a study may not translate into an improved functional outcome at later timepoints. For example, studies have now shown that, while GDNF will enhance sprouting in a midgraft, this GDNF-induced sprouting will not be manifested as increased regeneration in the distal graft or improve functional outcome measures.47 At times this may even result in functionally inappropriate target reinnervation as evident by spinal cord retrograde label staining demonstrating that increased sprouting results in more misdirected fibers resulting in improper function.48 In terms of tacrolimus, accelerated functional recovery has been demonstrated and this correlates with the nerve histomorphometry. Therefore, some questions are best answered with early timepoints of evaluation to identify histologic differences and later timepoints to evaluate for functional differences. An alternative approach would be to confirm early timepoint findings in a larger animal model before application of results to humans.
CONCLUSION
Selection of appropriate time points for evaluation of nerve regeneration and functional recovery is a crucial consideration in small animal models. The superlative regenerative potential inherent in the rodent nervous system may mask key differences in experimental comparisons at late time points. This “blow-through” effect can skew results and must therefore be taken into account when interpreting data on nerve regeneration and functional recovery in small animal models.
ACKNOWLEDGMENTS
We are particularly grateful to Fujisawa USA Inc. (Deerfield, IL) for their generous donation of the tacrolimus, and to Sandoz Canada Inc. (Dorval, Quebec) for their generous donation of the Cyclosporine-A used for this study.
Grant sponsor: National Institute of Health; Grant numbers: 2R01 NS33406-12; T32 DC 0022-15
REFERENCES
- 1.Gold BG. FK506 and the role of the immunophilin FKBP-52 in nerve regeneration. Drug Metab Rev. 1999;31:649–663. doi: 10.1081/dmr-100101940. [DOI] [PubMed] [Google Scholar]
- 2.Hunter DA, Moradzadeh A, Whitlock EL, Brenner MJ, Myckatyn TM, Wei CH, Tung THH, Mackinnon SE. Binary Imaging analysis for comprehensive quantitative histomorphometry of peripheral nerve. J Neurosci Methods. 2007;166:116–124. doi: 10.1016/j.jneumeth.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hare GM, Evans PJ, Mackinnon SE, Best TJ, Bain JR, Szalai JP, Hunter DA. Walking track analysis: A long-term assessment of peripheral nerve recovery. Plast Reconstruc Surg. 1992;89:251–258. [PubMed] [Google Scholar]
- 4.Hare GM, Evans PJ, Mackinnon SE, Best TJ, Midha R, Szalai JP, Hunter DA. Walking track analysis: Utilization of individual footprint parameters. Ann Plast Surg. 1993;30:147–153. doi: 10.1097/00000637-199302000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Nichols CM, Myckatyn TM, Rickman SR, Fox IK, Hadlock T, Mackinnon SE. Choosing the correct functional assay: A comprehensive assessment of functional tests in the rat. Behav Brain Res. 2005;163:143–158. doi: 10.1016/j.bbr.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Yang RK, Lowe JB, Sobol JB, Sen SK, Hunter DA, Mackinnon SE. Dose-dependent effects of FK506 on neuroregeneration in a rat model. Plast Reconstr Surg. 2003;112:1832–1840. doi: 10.1097/01.PRS.0000091167.27303.18. [DOI] [PubMed] [Google Scholar]
- 7.Bain J, Mackinnon SE, Hudson AR, Falk RE, Falk JA, Hunter DA. The peripheral nerve allograft: an assessment of regeneration across nerve allografts in rats immunosuppressed with cyclosporine A. Plast Reconstr Surg. 1988;82:1052–1064. [PubMed] [Google Scholar]
- 8.Bain J, Mackinnon SE, Hudson AR, Falk RE, Falk JA, Hunter DA. The peripheral nerve allograft: A dose-response curve in the rat immunosuppressed with cyclosporin A. Plast Reconstr Surg. 1988;82:447–455. doi: 10.1097/00006534-198809000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi J, MacKinnon SE, Watanabe O, Ball DJ, Gu XM, Hunter DA, Kuzon WM., Jr The effect of duration of muscle denervation on functional recovery in the rat model. Muscle Nerve. 1997;20:858–866. doi: 10.1002/(sici)1097-4598(199707)20:7<858::aid-mus10>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 10.Lee M, Doolabh VB, Mackinnon SE, Jost S. FK506 promotes functional recovery in crushed rat sciatic nerve. Muscle Nerve. 2000;23:633–640. doi: 10.1002/(sici)1097-4598(200004)23:4<633::aid-mus24>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 11.Myckatyn TM, Mackinnon SE. A review of research endeavors to optimize peripheral nerve reconstruction. Neurol Res. 2004;26:124–138. doi: 10.1179/016164104225013743. [DOI] [PubMed] [Google Scholar]
- 12.Ramsay AEBM, Mackinnon SE, Myckatyn TM, Hunter DA. Use of mixed lymphocyte reaction to identify subimmunosuppresive FK-506 levels in mice. Microsurgery. 2003;23:276–282. doi: 10.1002/micr.10117. [DOI] [PubMed] [Google Scholar]
- 13.Brenner MJ, Mackinnon SE, Rickman SR, Jaramillo A, Tung TH, Hunter DA, Mohanakumar T. FK506 and anti-CD40 ligand in peripheral nerve allotransplantation. Restor Neurol Neurosci. 2005;23:237–249. [PubMed] [Google Scholar]
- 14.Brenner MJ, Lowe JB, 3rd, Fox IK, Mackinnon SE, Hunter DA, Darcy MD, Duncan JR, Wood P, Mohanakumar T. Effects of Schwann cells and donor antigen on long nerve allograft regeneration. Microsurgery. 2005;25:61–70. doi: 10.1002/micr.20083. [DOI] [PubMed] [Google Scholar]
- 15.Mackinnon S, Doolab VB, Novak CB, Trulock EP. Clinical outcome following nerve allograft transplantation. Plast Reconstr Surg. 2001;107:1419–1429. doi: 10.1097/00006534-200105000-00016. [DOI] [PubMed] [Google Scholar]
- 16.Mackinnon S, Midha R, Bain J, Hunter DA, Wade J. An assessment of regeneration across peripheral nerve allograft in rats receiving shot courses of clyclosporin A immunosuppression. Neuroscience. 1992;46:585–593. doi: 10.1016/0306-4522(92)90146-s. [DOI] [PubMed] [Google Scholar]
- 17.Mackinnon S, Hudson AR, Hunter DA. Histologic assessment of nerve regeneration in the rat. Plast Reconstr Surg. 1985;75:384–388. doi: 10.1097/00006534-198503000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Levi AD, Guenard V, Aebischer P, Bunge RP, Levi AD, Guenard V, Aebischer P, Bunge RP. The functional characteristics of Schwann cells cultured from human peripheral nerve after transplantation into a gap within the rat sciatic nerve. J Neurosci. 1994;14(3, Part 1):1309–1319. doi: 10.1523/JNEUROSCI.14-03-01309.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levi AD, Sonntag VK, Dickman C, Mather J, Li RH, Cordoba SC, Bichard B, Berens M. The role of cultured Schwann cell grafts in the repair of gaps within the peripheral nervous system of primates. Exp Neurol. 1997;143:25–36. doi: 10.1006/exnr.1996.6344. [DOI] [PubMed] [Google Scholar]
- 20.Midha R, Mackinnon SE, Becker LE. The fate of Schwann cells in peripheral nerve allografts. J Neuropathol Exp Neurol. 1994;53:316–322. doi: 10.1097/00005072-199405000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Zalewski AA, Gulati AK, Silvers WK. Loss of host axons in nerve allografts after abolishing immunological tolerance in rats. Exp Neurol. 1981;72:502–506. doi: 10.1016/0014-4886(81)90239-9. [DOI] [PubMed] [Google Scholar]
- 22.Zalewski AA, Silvers WK, Gulati AK. Failure of host axons to regenerate through a once successful but later rejected long nerve allograft. J Comp Neurol. 1982;209:347–351. doi: 10.1002/cne.902090404. [DOI] [PubMed] [Google Scholar]
- 23.Doolabh VB, Mackinnon SE. FK506 accelerates functional recovery following nerve grafting in a rat model. Plast Reconstr Surg. 1999;103:1928–1936. doi: 10.1097/00006534-199906000-00018. [DOI] [PubMed] [Google Scholar]
- 24.Strasberg SR, Hertl MC, Mackinnon SE, Lee CK, Watanabe O, Tarasidis G, Hunter DA, Wong PY. Peripheral nerve allograft preservation improves regeneration and decreases systemic cyclosporin A requirements. Exp Neurol. 1996;139:306–316. doi: 10.1006/exnr.1996.0104. [DOI] [PubMed] [Google Scholar]
- 25.Hess BM JR, Fox IK, Nichols CM, Myckatyn TM, Hunter DA, Rickman SR, Mackinnon SE. Use of cold-preserved allografts seeded with autologous Schwann cells in the treatment of a long-gap peripheral nerve injury. Plast Reconstr Surg. 2007;119:246–259. doi: 10.1097/01.prs.0000245341.71666.97. [DOI] [PubMed] [Google Scholar]
- 26.Angelov DN, Neiss WF, Streppel M, Andermahr J, Mader K, Stennert E. Nimodipine accelerates axonal sprouting after surgical repair of rat facial nerve. J Neurosci. 1996;16:1041–1048. doi: 10.1523/JNEUROSCI.16-03-01041.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Birge RB, Wadsworth S, Akakura R, Abeysinghe H, Kanojia R, MacIelag M, Desbarats J, Escalante M, Singh K, Sundarababu S, Parris K, Childs G, August A, Siekierka J, Weinstein DE. A role for Schwann cells in the neuroregenerative effects of a non-immunosuppressive fk506 derivative, jnj460. Neuroscience. 2004;124:351–366. doi: 10.1016/j.neuroscience.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Gold BG, Villafranca JE. Neuroimmunophilin ligands: The development of novel neuroregenerative/neuroprotective compounds. Curr Top Med Chem. 2003;3:1368–1375. doi: 10.2174/1568026033451880. [DOI] [PubMed] [Google Scholar]
- 29.Gold BG, Storm-Dickerson T, Austin DR. The immunosuppressant FK506 increases functional recovery and nerve regeneration following peripheral nerve injury. Restor Neurol Neurosci. 1994;6:287–296. doi: 10.3233/RNN-1994-6404. [DOI] [PubMed] [Google Scholar]
- 30.Gold BG, Katoh K, Storm-Dickerson T. The immunosuppressant FK506 increases the rate of axonal regeneration in rat sciatic nerve. J Neurosci. 1995;15:7509–7516. doi: 10.1523/JNEUROSCI.15-11-07509.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aydin MA, Urbanchek MG, Kuzon WM. Improved early muscle recovery using FK506 in a rat nerve-repair model. J Reconstr Micro-surg. 2004;20:183–192. doi: 10.1055/s-2004-820776. [DOI] [PubMed] [Google Scholar]
- 32.Jost SC, Doolabh VB, Mackinnon SE, Lee M, Hunter D. Acceleration of peripheral nerve regeneration following FK506 administration. Restor Neurol Neurosci. 2000;17:39–44. [PubMed] [Google Scholar]
- 33.Udina E, Verdu E, Navarro X. Effects of the immunophilin ligand FK506 on nerve regeneration in collagen guides seeded with Schwann cells in rats. Neurosci Lett. 2004;357:99–102. doi: 10.1016/j.neulet.2003.11.070. [DOI] [PubMed] [Google Scholar]
- 34.Buttemeyer R, Rao U, Jones NF. Peripheral nerve allograft transplantation with FK506: functional, histological, and immunological results before and after discontinuation of immunosuppression. Ann Plast Surg. 1995;35:396–401. [PubMed] [Google Scholar]
- 35.Weinzweig N, Grindel S, Gonzalez M, Kuy D, Fang J, Shahani B. Peripheral-nerve allotransplantation in rats immunosuppressed with transient or long-term FK-506. J Reconstr Microsurg. 1996;12:451–459. doi: 10.1055/s-2007-1006618. [DOI] [PubMed] [Google Scholar]
- 36.Jensen JN, Brenner MJ, Tung TH, Hunter DA, Mackinnon SE. Effect of FK506 on peripheral nerve regeneration through long grafts in inbred swine. Ann Plast Surg. 2006;54:420–427. doi: 10.1097/01.sap.0000151461.60911.c0. [DOI] [PubMed] [Google Scholar]
- 37.Mackinnon SE. Nerve allotransplantation following severe tibial nerve injury. Case report. J Neurosurg. 1996;84:671–676. doi: 10.3171/jns.1996.84.4.0671. [DOI] [PubMed] [Google Scholar]
- 38.Strome M, Stein J, Esclamado R, Hicks D, Lorenz RR, Braun W, Yetman R, Eliachar I, Mayes J. Laryngeal transplantation and 40-month follow-up. N Engl J Med. 2001;344:1676–1679. doi: 10.1056/NEJM200105313442204. [DOI] [PubMed] [Google Scholar]
- 39.Petruzzo P, Revillard JP, Kanitakis J, Lanzetta M, Hakim NS, Lefrancois N, Owen E, Dubernard JM. First human double hand transplantation: Efficacy of a conventional immunosuppressive protocol. Clin Transplant. 2003;17:455–460. doi: 10.1034/j.1399-0012.2003.00005.x. [DOI] [PubMed] [Google Scholar]
- 40.Keune JD, Brenner MJ, Schwetye KE, Yu JW, Fox IK, Hunter DA, Mackinnon SE. Temporal factors in peripheral nerve reconstruction with suture scaffolds: An experimental study in rodents. Restor Neurol Neurosci. 2006;24:181–190. [PubMed] [Google Scholar]
- 41.Jensen JN, Tung TH, Mackinnon SE, Brenner MJ, Hunter DA. Use of anti-CD40 ligand monoclonal antibody as antirejection therapy in a murine peripheral nerve allograft model. Microsurgery. 2004;24:309–315. doi: 10.1002/micr.20028. [DOI] [PubMed] [Google Scholar]
- 42.Calvert GT, Doolabh VB, Grand AG, Hunter DA, Mackinnon SE. Rat-strain differences in recovery following peripheral-nerve allotransplantation. J Reconstr Microsurg. 2001;17:185–191. doi: 10.1055/s-2001-14350. [DOI] [PubMed] [Google Scholar]
- 43.Brenner MJ, Jensen JN, Lowe JB, Myckatyn TM, Fox IK, Hunter DA, Mohanakumar T, Mackinnon SE. Anti-CD40 ligand antibody permits regeneration through peripheral nerve allografts in a nonhuman primate model. Plast Reconstr Surg. 2004;114:1802–1814. doi: 10.1097/01.prs.0000143575.88064.d0. [DOI] [PubMed] [Google Scholar]
- 44.Brenner MJ, Tung TH, Mackinnon SE, Myckatyn TM, Hunter DA, Mohanakumar T. Anti-CD40 ligand monoclonal antibody induces a permissive state, but not tolerance, for murine peripheral nerve allo-grafts. Exp Neurol. 2004;186:59–69. doi: 10.1016/j.expneurol.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 45.Scherman P, Lundborg G, Kanje M, Dahlin LB. Sutures alone are sufficient to support regeneration across a gap in the continuity of the sciatic nerve in rats. Scand J Plast Reconstr Surg Hand Surg. 2000;34:1–8. doi: 10.1080/02844310050160105. [DOI] [PubMed] [Google Scholar]
- 46.Scherman P, Kanje M, Dahlin LB. Sutures as longitudinal guides for the repair of nerve defects—influence of suture numbers and reconstruction of nerve bifurcations. Rest Neurol Neurosci. 2005;23:79–85. [PubMed] [Google Scholar]
- 47.Piquilloud GCT, Pfister LA, Gander B, Papaloizos MY. Variations in glial cell-line derived neurotrophic factor release from biodegradable conduits modify the rate of functional motor recovery after rat primary nerve repairs. Eur J Neurosci. 2007;26:1109–1117. doi: 10.1111/j.1460-9568.2007.05748.x. [DOI] [PubMed] [Google Scholar]
- 48.English A. Enhancing Axonal regeneration in peripheral nerves also increases functionally inappropriate reinnervation of targets. J Compar Neurol. 2005;490:427–441. doi: 10.1002/cne.20678. [DOI] [PubMed] [Google Scholar]