Abstract
In this work, we developed a sensitive method to quantify cotinine (COT), norcotinine (NCOT), trans-3′-hydroxycotinine (OHCOT) and cotinine-N-oxide (COTNO) in rat plasma and brain tissue, using solid phase extraction (SPE), hydrophilic interaction liquid chromatography (HILIC) and tandem mass spectrometry (MS/MS). The linear range was 1–100 ng/ml for each analyte in rat plasma and brain homogenate (3–300 ng/g brain tissue). The method was validated with precision within 15% relative standard deviation (RSD) and accuracy within 15% relative error (RE). Stable isotope-labeled internal standards (IS) were used for all the analytes to achieve good reproducibility, minimizing the influence of recovery and matrix effects. This method can be used in future studies to simultaneously determine the concentrations of COT and three major metabolites in rat plasma and brain tissue.
Keywords: cotinine, norcotinine, trans-3′-hydroxycotinine, cotinine-N-oxide, plasma, brain, hydrophilic interaction chromatography, tandem mass spectrometry
1. Introduction
Cotinine (COT), the primary metabolite of nicotine (NIC) in humans and other mammalian species, is currently being evaluated as a prototypical therapeutic agent for Alzheimer’s disease (AD) and related neurodegenerative disorders. Like nicotine, cotinine been observed to have positive effects on attention, working memory, and other domains of cognition in animal models [1–3]. In addition, both in vitro and in vivo studies suggest that COT might have disease-modifying effects (i.e., neuroprotective effects and the ability to delay disease progression) in conditions like AD. For example, COT protects against toxic insults in PC12 cells in culture with potency similar to that of nicotine [1, 3] and it was found (when administered chronically) to prevent memory loss in transgenic (Tg) 6799 AD mice as well as to stimulate the Akt/GSK3β pathway and reduce Aβ aggregation in their brains [4]. As a potential therapeutic agent, COT also appears to have several advantages over nicotine. For example, COT has a longer biological half-life (15–19 hours) and lower toxicity (mouse oral LD50 = 1604 mg/kg) than nicotine (half-life = 2–3 hours, mouse oral LD50 = 50 mg/kg) as well as less addictive potential [3].
COT can be further metabolized into several downstream metabolites, among which norcotinine (NCOT), trans-3′-hydroxycotinine (OHCOT) and cotinine-N-oxide (COTNO) are of interest for similar pharmacological activities and therapeutic potential in AD. In addition, determination of these compounds can also provide distribution and metabolism information for COT.
In order to facilitate further investigations into the effects of COT and its metabolites on the central nervous system (CNS), a sensitive method that can simultaneously quantify these compounds in both plasma and brain tissue is needed. With the determination of the actual concentrations in plasma and brain, blood-brain barrier (BBB) permeabilities, efficacies and toxicities of COT and the metabolites can be assessed in animal studies.
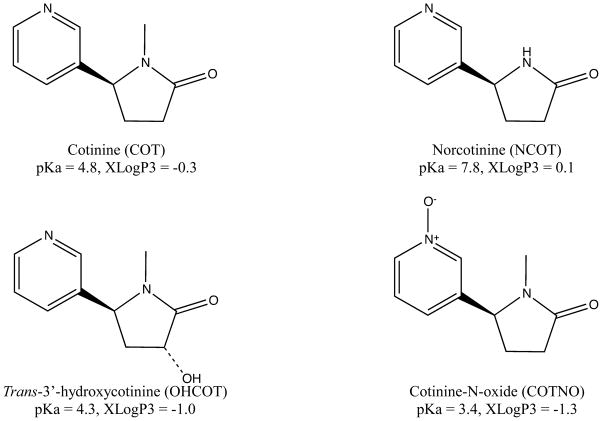
As COT can be used as a biomarker of tobacco exposure, numerous liquid chromatography-tandem mass spectrometry (LC-MS/MS) methods have been reported for the quantification of COT and its metabolites in a variety of biological fluids, i.e. plasma [5–8], serum [9–11], urine [5, 12–14], saliva [15, 16], whole blood [17] and breast milk [18]. Plasma is the most widely used species in animal tests, due to the high drug concentrations and easy accessibility. Because of the great differences in polarity and pKa of COT, NCOT, OHCOT and COTNO (shown in Figure 1), very few current methods have simultaneous determination of all four analytes with good sensitivity [5]. Moreover, some of the LC-MS/MS methods for plasma COT require a large sample volume (1 mL) [5, 7] or complicated sample preparation [5] to achieve high sensitivity.
Figure 1.
Chemical structures, pKa and XLogP values of COT, NCOT, OHCOT and COTNO. Structures were generated by ChemBioDraw Ultra 12.0 software. pKa and XLogP3 values were obtained from PubChem database.
Though plasma concentration can provide information about drug exposure, brain tissue concentrations are also of great importance for such drugs as COT and its metabolites, whose targeting site is the brain. However, there are very limited current quantitation methods for brain tissue. Several gas chromatography-mass spectrometry (GC-MS) methods were reported for NIC and COT quantitation in brain tissue [19–21], the lowest limit of detection (LOD) of COT among which was 10 ng/g [21]. The first LC-MS/MS methods for COT and metabolites quantitation in human brain was reported by Shakleya and Huestis [22], with the linear range 25 – 5,000 ng/g for COT and 50 – 5000 ng/g for OHCOT. Recently Vieira-Brock and coworkers reported an LC-MS/MS method of simultaneous quantification of NIC and all the metabolites, including COT, NCOT, OHCOT and COTNO, in rat brain, with the linearity of 25–7,500 ng/g [23]. However, cotinine metabolites, NCOT, OHCOT and COTNO, in real samples were not detected in these studies, due to their sensitivities.
Hydrophilic interaction chromatography (HILIC) is a type of partition chromatography first introduced by Alpert in 1990 [24]. Its specificity for polar compounds, high organic mobile phase, low buffer concentrations and early elution of hydrophobic impurities make it a good choice for LC-MS/MS quantitation of polar analytes in biological samples [25]. HILIC-MS/MS has been reported for its application for quantitation of NIC, COT and metabolites in biological fluids, due to the high polarities of NIC and COT [9, 26, 27]. HILIC can also be applied with other chromatographic techniques, like capillary LC, to achieve higher sensitivities for the quantitation of COT and metabolites [28]. However, there have not been any HILIC-MS/MS methods for the simultaneous determination of COT and all its major metabolites in plasma or brain.
In this study, we developed and validated a HILIC-MS/MS method for the simultaneous quantitation of COT, NCOT, OHCOT and COTNO in rat plasma and brain tissue. This method was used to quantify COT and its metabolites in preclinical studies on rats, to study the distributions and activities of these compounds for AD therapy.
2. Experimental
2.1 Chemicals and reagents
(−)-Cotinine (COT) was purchase from Sigma-Aldrich (St. Louis, MO). (R,S)-norcotinine (NCOT), trans-3′-hydroxcotinine (OHCOT) and cotinine (S)-cotinine-N-oxide were from Toronto Research Chemicals (Toronto, Canada). Chemical structures of analytes are shown in Figure 1. Stable isotope labeled internal standard (IS) (±)-Cotinine-D3 solution (1mg/mL in methanol) was obtained from Cerilliant (Round Rock, Texas). (R,S)-norcotinine-d4 (NCOT-d4), trans-3′-hydroxycotinine-d3 (OHCOT-d3) and (R,S)-cotinine-N-oxide-d3 (COTNO-d3) were purchased from Toronto Research Chemical (Toronto, Canada). Trichloroacetic acid and ammonium acetate were bought from Baker (Phillipsburg, NJ). Formic acid was from Sigma (St. Louis, MO). Acetonitrile, methanol and water were from Fisher (Pittsburgh, PA) as HPLC/ACS grade.
2.2 Instrumentation
LC-MS/MS analysis was performed by using an Agilent 1100 binary pump HPLC system (Santa Clara, CA) interfaced to a Waters Micromass Quattro Micro triple quadrupole mass spectrometer with an ESI(+) source (Milford, MA). Instrument control was carried out with Masslynx 4.0 software by Waters (Beverly, MA).
2.3 LC-MS/MS conditions
The analytes were separated on a Phenomenex Kinetex™ HILIC column (50 × 2.1 mm ID, 2.6 μm) coupled with a SecurityGuard™ ULTRA HILIC guard column for HILIC UHPLC, sub-2 μm and core-shell columns with 2.1mm internal diameters (ID). Mobile phase A was 10mM ammonium formate aqueous buffer with 0.1% formic acid and mobile phase B was acetonitrile (ACN). After an injection of 10 μL for each sample into the column, analytes were separated with the following gradient (time/minute, % mobile phase B): (0, 95), (8, 50), (8.1, 95), (15, 95). Flow rate was set at 0.3 mL/min and column temperature was 25 °C. The LC system was interfaced by a six-port divert valve to the mass spectrometer, introducing eluents from 1.0 to 6.0 min to the ion source. The autosampler injection needle was washed with methanol after each injection.
The mass spectrometer was operated in positive ion ESI mode. Nitrogen was used as the desolvation gas at a flow rate of 500 L/h and a temperature of 500 °C. The cone gas flow was set to 20 L/h. Argon was the collision gas and the collision cell pressure was 3.5 × 10−3 mbar. The source temperature and capillary voltage were set at 120 °C and 3.5 kV, respectively. Multiple reaction monitoring (MRM) functions were used for the quantification of analytes. The cone voltage was 20 V and collision energy was 20 eV. Ion transitions monitored for analytes were 177-80 for COT, 163-80 for NCOT, 193-80 for OHCOT and 193-96 for COTNO. Ion transitions for IS were 180-80 for COT-d3, 147-84 for NCOT-d4, 196-80 for d3-OHCOT and 196-96 for d3-COTNO.
2.4 Solutions and standards
Individual stock solutions of all the analytes and IS were prepared by dissolving 1.0 mg of compounds in 1.0 mL of methanol to obtain drug concentrations of 1.0 mg/mL, except for COT-d3, which came in as 1.0 mg/mL methanol solution. Combined working solutions were obtained by serial dilution with 90% ACN/water (v/v 9/1). Standard working solutions containing COT, NCOT, OHCOT and COTNO were prepared at concentrations of 10.0, 20.0, 50.0, 100.0, 200.0, 500.0 and 1000.0 ng/mL. Quality control (QC) working solutions were 10.0, 30.0, 300.0 and 750.0 ng/mL. IS working solutions containing COT-d3, NCOT-d4, OHCOT-d3 and COTNO-d3 were prepared at a single concentration of 500.0 ng/mL in the same solvent. Stock solutions were kept at −20 °C when not in use.
2.5 Spiked samples and real samples
Blank rat plasma with sodium EDTA was purchased from Bioreclamation (Westbury, NY). Blank brains were obtained from drug-free control rats and homogenized with two volumes of water to obtain blank brain homogenate. 10 μL of standard or QC working solution was spiked into 100 μL of plasma or brain homogenate to generate corresponding standard or QC samples. The final concentrations of calibration standards were 1.0, 2.0, 5.0, 10.0, 20.0, 50.0 and 100.0 ng/mL in plasma or brain homogenate. The QC samples were 3.0, 30.0 and 70.0 ng/mL.
Real samples were obtained from 1 mg/kg subcutaneously dosed rats after 30 minutes of pretreatment. Plasma was collected via cardiac puncture and transferred to EDTA vacutainers. Brain samples were homogenized in the same manner as blank brain.
All biological samples were stored at −20 °C before use. Fresh standards and QC samples were prepared for each day of validation.
2.6 Sample preparation
Sample preparation was carried out by protein precipitation and solid phase extraction (SPE). Each 100 μL of plasma or brain homogenate was added to 10 μL of IS working solution (500.0 ng/mL), 800 μL of water and 100 μL of 25% (w/v) TCA. The mixture was vortexed for 10 min and then centrifuged at 4500 × g for 10 min to remove the proteins.
An Oasis MCX SPE cartridge from Waters (Milford, MA) was conditioned with 1 mL of methanol and equilibrated with 1 mL of water. The supernatant from protein precipitation was loaded onto the cartridges and allowed to flow by gravity. Then the cartridge was washed twice by 1 mL of 5% methanol, 5% formic acid in water (v/v), followed by vacuum drying for 5 min. Analytes were eluted by 1 mL of fresh 20% methanol, 5% ammonia in water (v/v). The eluent was evaporated to complete dryness in a centrifuge evaporator at 50 °C. The sample was reconstituted by 100 μL of 95% ACN/water (v/v 9/1) with 2% formic acid and ready for injection.
2.7 Method validation
Linearity was tested by spiked standard as well as blank biological samples, since endogenous COT was observed in blank matrices. Calibration curves were made from peak area ratios between analytes and IS, using 1/x weighted linear regression. The intra-day (n = 5) and inter-day (n = 15) precision and accuracy were assessed by QC samples at the lower limit of quantitation (LLOQ), 3.0, 30.0 and 70.0 ng/mL.
Autosampler stability (25 °C, 12 hours), bench-top stability (25 °C, 8 hours) and freeze-thaw stability (3 freeze-thaw cycles, −20 °C, 72 hours) in plasma and brain homogenate were tested for all the analytes at both low (3 ng/mL) and high (75 ng/mL) concentrations (n = 3), by comparing freshly spiked samples and samples subject to stability tests.
Matrix effects, relative recovery and absolute recovery for both plasma and brain homogenate were calculated from peak areas of spiked samples, post-preparation spiked samples and neat standard solutions of concentrations at 3.0, 30.0 and 70.0 ng/mL (n = 3).
Dilution validation was conducted to accommodate real samples with analyte concentrations over the upper limit of quantitation (ULOQ). After diluting spiked samples from 1500 ng/mL into the concentration at ULOQ (100 ng/mL) with corresponding matrices (plasma or brain homogenate), precision and accuracy (n = 5) were tested.
2.8 Animal Study
Male albino Wistar rats (Harlan Sprague-Dawley, Inc., Indianapolis, IN, USA) approximately 2 months old were housed in pairs in a temperature controlled room (25°C), maintained on a 12:12h normal light-dark cycle (lights on at 6AM) with free access to water and food until used for plasma and brain studies (see below). All procedures employed during this study were reviewed and approved by the Georgia Health Sciences University Institutional Animal Care and Use Committee and are consistent with AAALAC guidelines. Measures were taken to minimize pain and discomfort in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996.
Subjects were administered cotinine (dissolved in normal saline) subcutaneously then anesthetized 30 min later with isofluorane. Subsequently, 3.0 ml of blood was collected via cardiac puncture into a Vacutainer® tube containing potassium EDTA. The blood was centrifuged for 15 min at 2500 × g at 4–5°C and the resulting plasma was frozen at −80°C until analyzed. Brains were removed from the same animals, washed with phosphate-buffered saline and frozen at −80°C until analyzed.
3. Results and Discussion
3.1 LC-MS/MS method development
In order to develop a sensitive and selective method for simultaneous quantification of COT and its metabolites, optimizations of different factors and parameters were made in tandem mass spectrometry and liquid chromatography.
To achieve higher sensitivity, the triple quadrupole mass spectrometer was set to unit resolution mode. For instrument tuning, general parameters for desolvation and ionization were obtained by a constant infusion at 10 μL/min of a 1 μg/mL COT solution. The detection of analytes and ISs were conducted using MRM functions, providing high sensitivity and selectivity. A product ion mass spectrum was obtained by collision activated dissociation (CAD) for each analyte and IS, and the most abundant product ions were used in the MRM ion transitions. Collision energy and cone voltages were optimized with injections of 10 μL of 100 ng/mL individual standards for each analyte and IS.
The separation of analytes was carried out by HILIC. During the development of the LC method, both reversed phase liquid chromatography (RPLC) and HILIC were tried for the separation of analytes in both neat samples and spiked samples. The retention of analytes, especially for COTNO with high polarity, on the reversed phase column (Agilent ZORBAX XDB-C18 column) was weak and a high aqueous percentage was required in the eluting mobile phase, which would lower the ionization efficiency when using electrospray. However, all analytes had better retention on the HILIC column (Phenomenex Kinetex™ HILIC column). High organic percentage was used in the mobile phase, which provided better compatibility with the ESI ion source. Moreover, early elution of hydrophobic impurities, especially for brain samples, on the HILIC method contributed to lower possibility of ion source contamination by lipids.
3.2 Sample preparation method development
Before LC-MS/MS analysis, sample preparation was required for biological samples, especially for brain homogenate, which contained more proteins and lipids. In method development, common sample preparation approaches such as, protein precipitation, liquid-liquid extraction (LLE) and solid-phase extraction (SPE), were all tested for plasma and brain homogenate. Samples prepared only by protein precipitation still contained impurities, which became more significant when the samples were evaporated and reconstituted at higher concentrations. Based on this, further sample clean-up, either LLE or SPE, was needed after protein precipitation. LLE was first tried with different extractants, isopropanol, chloroform, ethyl acetate and methylene chloride, among which ethyl acetate provided the highest recovery for COT (73% in brain homogenate, 62% in plasma). Nevertheless, the recovery of the most polar analyte, COTNO, was almost zero. Considering the wide range of polarities among analytes, SPE was used as an alternative for better selectivity. Two types of SPE cartridges, Waters Oasis HLB (hydrophilic-lipophilic balance) and MCX (mixed mode cation exchange) were tested. Since the extraction mechanism of HLB was similar to that of LLE, the recovery for extremely polar analytes was also very low. However, MCX cartridges provided acceptable recoveries, as all analytes were protonated in acidic solution and bound to cartridges via cation exchange interactions. Different levels of matrix effects were observed for the four analytes, which could be reduced by increasing the strength of the washing agent or decreasing the strength of the eluting agent. However, recoveries of the analytes were reduced when the matrix effects were reduced by such approaches. To balance the recovery and matrix effects for all analytes, the strongest washing agent and weakest eluting agent were optimized to provide acceptable recoveries for all of the analytes.
3.3 Linearity and sensitivity
Calibration curves made for COT, NCOT, OHCOT and COTNO in plasma and brain homogenate are shown in Table 1. Good linearity (R2 > 0.99) was observed for all of the analytes over the range from 1 – 100 ng/mL in plasma and brain homogenate (3 – 300 ng/g in brain tissue). A 1/x-weighted linear regression was used to generate all calibration curves. Slopes, intercepts and R2 values are shown in Table 1. A Student t-test was conducted for all the intercept values to determine the statistical significance of the difference from theoretical zero value, which could suggest the endogenous levels of analytes. COT in blank plama is very significantly different from theoretical zero, based on the 0.01 level; while endogenous plasma NCOT, brain COT, brain NCOT and bran OHCOT were significantly different from zero on the 0.05 level. Considering errors caused by signal saturation and linear regression, low endogenous levels of analytes (small intercept values), NCOT and OHCOT, can be negligible even with significant non-zero intercepts. The mean values and statistical differences from theoretical zero suggested COT had significant endogenous levels in blank rat plasma and brain. The sensitivity of the method was defined by the lower limit of quantitation (LLOQ), which was the lowest concentration within 20% precision and accuracy. LLOQs for all the analytes in were 1 ng/mL in plasma or brain homogenate (3 ng/g in brain tissue). Signal-to-noise ratio (S/N), another parameter to assess sensitivity, was greater than 10 at the LLOQ for each analyte in both matrices.
Table 1.
Calibration curves for COT, NCOT, OHCOT and COTNO in plasma and brain homogenate. (n=3)
| Analyte | Plasma | Brain | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Intercept | Slope | R2 | Intercept | Slope | R2 | |
|
| ||||||
| COT | 0.8731±0.0126** | 0.8317±0.0244 | 0.9979 ± 0.0008 | 0.7621±0.1593* | 0.8634±0.0440 | 0.9983 ± 0.0014 |
| NCOT | 0.1312±0.0493* | 0.7453±0.0111 | 0.9977 ± 0.0014 | 0.1099±0.0412* | 0.7122±0.0101 | 0.9989 ± 0.0001 |
| OHCOT | 0.1370±0.0831 | 1.0830±0.0119 | 0.9988 ± 0.0007 | 0.0834±0.0198* | 1.0089±0.0502 | 0.9993 ± 0.0003 |
| COTNO | 0.4582±0.1902 | 1.0717±0.0403 | 0.9973 ± 0.0005 | 0.2483±0.1124 | 1.1244±0.0842 | 0.9947 ± 0.0048 |
P < 0.05, Student t-test.
P < 0.01, Student t-test.
3.4 Precision and accuracy
Precision and accuracy were calculated for LLOQ and QC samples of all four analytes in both matrices, shown in Table 2. Precision, defined as the closeness of measurements of the same concentration, was assessed by the coefficient of variation (CV) or relative standard deviation (RSD) among measured concentrations. Accuracy, defined as the closeness between measured and true values, was assessed by the relative error (RE) between measured concentrations and nominal concentrations. Both intra-day (n = 5) and inter-day (n = 15) precision and accuracy were tested. RSD and RE values for COT, NCOT, OHCOT and COTNO in plasma and brain homogenate are shown in Table 2, which met the FDA requirements of less than 15% for QCs and less than 20% for LLOQs.
Table 2.
The intra-day (n = 5) and inter-day (n = 15) precision (RSD) and accuracy (RE) of the LC–MS/MS method used to quantitate COT, NCOT, OHCOT and COTNO in rat plasma and brain homogenate.
| Intra-day | Inter-day | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Analyte | Nominal Conc. (ng/mL) | Measured Conc. (ng/mL) | RSD (%) | RE (%) | Measured Conc. (ng/mL) | RSD (%) | RE (%) | |
|
|
||||||||
| Plasma | COT | 1.0 | 0.92±0.11 | 12.30 | −8.00 | 0.91±0.12 | 13.44 | −9.27 |
| 3.0 | 3.08±0.31 | 10.03 | 2.67 | 2.87±0.26 | 9.12 | −4.42 | ||
| 30.0 | 30.05±0.89 | 2.97 | 0.15 | 28.63±1.25 | 4.37 | −4.56 | ||
| 75.0 | 74.79±1.73 | 2.31 | −0.28 | 71.86±2.58 | 3.59 | −4.18 | ||
|
| ||||||||
| NCOT | 1.0 | 0.92±0.03 | 3.44 | −8.00 | 0.92±0.06 | 6.13 | −8.33 | |
| 3.0 | 2.99±0.05 | 1.71 | −0.40 | 2.89±0.11 | 3.85 | −3.76 | ||
| 30.0 | 29.49±0.44 | 1.48 | −1.71 | 28.22±1.05 | 3.71 | −5.92 | ||
| 75.0 | 72.05±1.06 | 1.47 | −3.93 | 69.93±2.31 | 3.30 | −6.76 | ||
|
| ||||||||
| OHCOT | 1.0 | 0.87±0.02 | 2.21 | −12.80 | 0.91±0.07 | 7.49 | −8.80 | |
| 3.0 | 2.83±0.08 | 2.93 | −5.53 | 2.81±0.09 | 3.22 | −6.40 | ||
| 30.0 | 28.36±0.64 | 2.26 | −5.47 | 28.38±0.49 | 1.71 | −5.39 | ||
| 75.0 | 70.79±1.12 | 1.59 | −5.61 | 70.70±1.20 | 1.70 | −5.73 | ||
|
| ||||||||
| COTNO | 1.0 | 0.904±0.06 | 6.71 | −9.60 | 0.90±0.17 | 18.85 | −10.20 | |
| 3.0 | 2.886±0.20 | 6.84 | −3.80 | 2.87±0.24 | 8.41 | −4.36 | ||
| 30.0 | 27.234±1.18 | 4.34 | −9.22 | 27.16±0.85 | 3.12 | −9.48 | ||
| 75.0 | 66.53±1.26 | 1.89 | −11.29 | 67.77±3.22 | 4.75 | −9.63 | ||
|
| ||||||||
| Brain | COT | 1.0 | 0.94±0.08 | 9.00 | −6.00 | 0.90±0.10 | 10.72 | −9.53 |
| 30 | 2.82±0.04 | 1.55 | −6.07 | 2.82±0.07 | 2.52 | −6.02 | ||
| 30.0 | 27.12±0.52 | 1.91 | −9.59 | 27.94±0.83 | 2.98 | −6.86 | ||
| 75.0 | 70.14±1.47 | 2.09 | −6.48 | 72.05±2.12 | 2.94 | −3.93 | ||
|
| ||||||||
| NCOT | 1.0 | 0.94±0.03 | 2.81 | −6.00 | 0.94±0.04 | 4.55 | −6.27 | |
| 3.0 | 2.90±0.05 | 1.60 | −3.33 | 2.94±0.08 | 2.59 | −2.00 | ||
| 30.0 | 27.99±0.88 | 3.15 | −6.71 | 28.52±1.11 | 3.89 | −4.94 | ||
| 75.0 | 71.66±3.62 | 5.05 | −4.45 | 72.71±3.23 | 4.45 | −3.05 | ||
|
| ||||||||
| OHCOT | 1.0 | 0.92±0.03 | 3.64 | −7.60 | 0.90±0.04 | 4.35 | −10.13 | |
| 3.0 | 2.92±0.08 | 2.66 | −2.53 | 2.92±0.06 | 2.21 | −2.53 | ||
| 30.0 | 28.63±0.72 | 2.51 | −4.57 | 28.67±0.53 | 1.85 | −4.44 | ||
| 75.0 | 72.64±3.40 | 4.68 | −3.15 | 72.89±2.08 | 2.86 | −2.82 | ||
|
| ||||||||
| COTNO | 1.0 | 1.01±0.05 | 4.73 | 0.80 | 1.03±0.15 | 14.73 | 2.60 | |
| 3.0 | 3.00±0.11 | 3.77 | −0.07 | 2.97±0.14 | 4.57 | −0.84 | ||
| 30.0 | 29.61±0.22 | 0.75 | −1.31 | 27.86±1.44 | 5.17 | −7.14 | ||
| 75.0 | 75.65±4.57 | 6.04 | 0.87 | 72.08±4.01 | 5.56 | −3.89 | ||
3.5 Recovery and matrix effect
Recovery and matrix effect were tested for all the analytes at the three QC concentrations (n = 3) in both matrices, shown in Table 3.
Table 3.
Absolute recovery (%AR), relative recovery (%RR) and matrix effect (%ME) of the method. Mean ± SD values are shown for all the metabolites at 3.0, 30.0 and 75.0 ng/mL concentrations in plasma and brain homogenate. Types of matrix effect are shown in percentage of enhancement or suppression.
| Matrix | Analyte | Conc. (ng/ml) | AR (%) | RR (%) | ME (%) | Type |
|---|---|---|---|---|---|---|
|
| ||||||
| Plasma | COT | 3.0 | 37.26±1.20 | 52.94±1.71 | 70.38 | 29.62% suppression |
| 30.0 | 23.82±8.41 | 40.54±14.31 | 58.75 | 41.25% suppression | ||
| 75.0 | 30.79±7.42 | 41.97±10.12 | 73.36 | 26.64% suppression | ||
|
| ||||||
| NCOT | 3.0 | 62.42±5.66 | 76.80±6.97 | 81.28 | 18.72% suppression | |
| 30.0 | 63.41±9.13 | 83.90±12.09 | 75.57 | 24.43% suppression | ||
| 75.0 | 66.51±8.04 | 73.55±8.89 | 90.43 | 9.57% suppression | ||
|
| ||||||
| OHCOT | 3.0 | 36.04±2.32 | 89.51±5.75 | 40.26 | 59.74% suppression | |
| 30.0 | 37.09±12.42 | 73.61±24.66 | 50.38 | 49.62% suppression | ||
| 75.0 | 41.56±3.49 | 76.77±6.45 | 54.14 | 45.86% suppression | ||
|
| ||||||
| COTNO | 3.0 | 33.54±9.73 | 41.81±12.14 | 80.22 | 19.78% suppression | |
| 30.0 | 45.83±2.21 | 56.99±2.75 | 80.41 | 19.59% suppression | ||
| 75.0 | 34.56±4.00 | 45.06±5.21 | 76.69 | 23.31% suppression | ||
|
| ||||||
| Brain | COT | 3.0 | 41.27±4.39 | 58.75±6.24 | 70.25 | 29.75% suppression |
| 30.0 | 30.88±2.73 | 37.60±3.33 | 82.12 | 17.88% suppression | ||
| 75.0 | 39.15±3.38 | 63.01±5.43 | 62.12 | 37.88% suppression | ||
|
| ||||||
| NCOT | 3.0 | 60.35±1.79 | 75.38±2.23 | 80.07 | 19.93% suppression | |
| 30.0 | 65.68±1.35 | 96.80±2.00 | 67.85 | 32.15% suppression | ||
| 75.0 | 71.84±4.98 | 97.27±6.75 | 73.86 | 26.14% suppression | ||
|
| ||||||
| OHCOT | 3.0 | 27.50±1.82 | 78.61±5.19 | 34.98 | 65.02% suppression | |
| 30.0 | 41.30±2.91 | 82.95±5.85 | 49.79 | 50.21% suppression | ||
| 75.0 | 36.45±1.23 | 100.03±3.36 | 36.44 | 63.56% suppression | ||
|
| ||||||
| COTNO | 3.0 | 19.96±5.08 | 31.88±8.11 | 62.61 | 37.39% suppression | |
| 30.0 | 39.24±6.20 | 68.00±10.74 | 57.71 | 42.29% suppression | ||
| 75.0 | 25.30±7.23 | 39.69±11.35 | 63.74 | 36.26% suppression | ||
For each concentration of analytes in either matrix, three spiked samples and three neat solutions were prepared. Besides, three “post-preparation spiked” samples were made by spiking standard working solutions into blank matrices processed by the same sample preparation. The absolute recoveries were calculated by the peak area ratio between spiked samples and neat standards. Relative recoveries were calculated by the peak area ratio between “post-preparation spiked” samples and spiked samples, quantitating the loss due to sample preparation. Matrix effects were calculated by the peak area ratio between “post-preparation spiked” samples and neat standards, providing the influence of the matrix on the signal response. In addition, types of matrix effects (enhancement or suppression) are shown in Table 3.
As mentioned in the method development section, the sample preparation had been optimized to achieve both acceptable recovery and matrix effects for all the analytes. Since stable isotope-labeled ISs were used in this method, matrix effects became less prominent, because they only slightly affected the sensitivity but not the precision or accuracy. Recovery, which is more directly related to the sensitivity of the method, became more important. Due to the great differences in polarity and pKa among analytes (Figure 1), selectivity of sample preparation had to be compromised to yield satisfactory recoveries for all of the analytes, which would increase the matrix effects at the same time. TCA was used for both protein precipitation and protonating analytes for SPE based cation exchange. In the SPE, the strongest washing agent, which was still very weak, was used for lowest analyte loss; while the weakest eluting agent was used to minimize co-eluting lipid-based impurities as well as providing acceptable recoveries for all the analytes.
All of the matrix effects observed were from ion suppression. Considering the very weak eluting conditions in SPE, lipid-based or protein-based impurities were unlikely to co-elute with the analytes. Therefore, we considered the ion suppression effects to result from salts or positively charged ions introduced by matrices or sample preparation, which could compete with the analytes during ESI and reduce analyte signal response.
3.6 Specificity
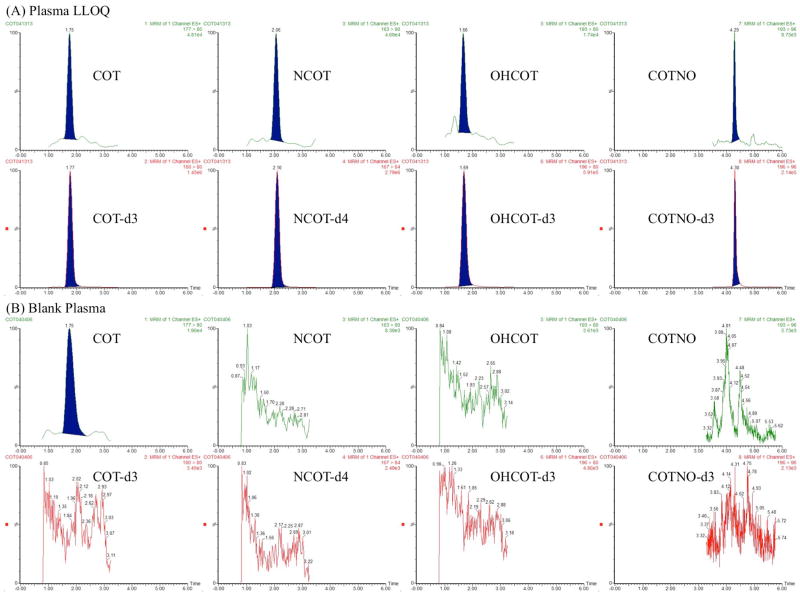
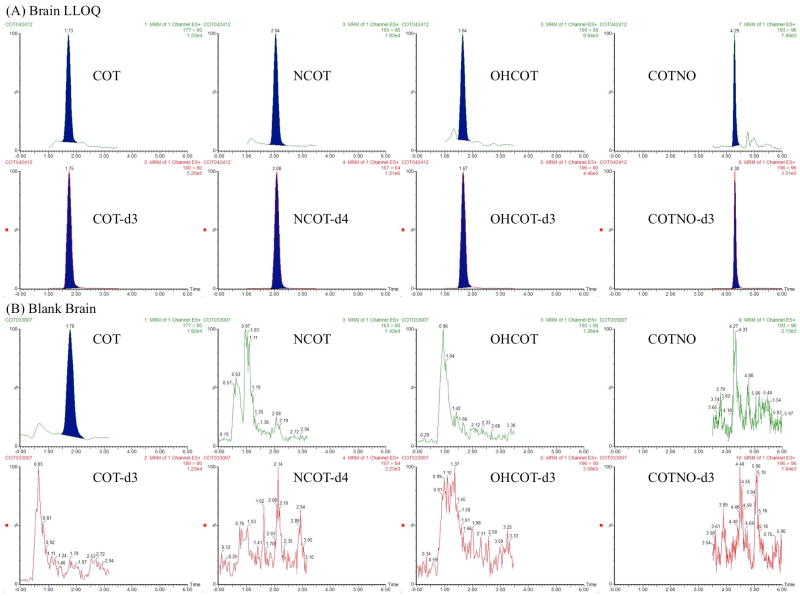
Representative chromatograms obtained from blank biological matrices and spiked with LLOQ standard (1 ng/mL for plasma and brain homogenate) are shown in Figure 2 and Figure 3. No interference from cross-talk was observed among the MRM channels. However, endogenous COT was observed in the blank plasma, as well as, brain homogenate. With matched retention time and ion transitions, the signal in blank matrices was confirmed to result from the same compound. After eliminating the possibility of contamination during sample preparation, the blank matrices were confirmed to contain endogenous COT, the level of which was observed to be stable among individuals. Considering the common contamination of COT in water and air due to smoking, this was thought to be acceptable as long as the endogenous level was consistent and did not affect method robustness. Adjustments were made for calibration curves, including blank matrices as calibration points for all the analytes and matrices.
Figure 2.
Representative chromatograms of plasma samples. For each analyte, chromatograms of the analyte and IS were shown for both a spiked standard at LLOQ (1 ng/mL) (A) and a blank sample (B). The concentrations of IS were all 50 ng/mL.
Figure 3.
Representative chromatograms of brain homogenate samples. For each analyte, chromatograms of the analyte and IS were shown for both a spiked standard at LLOQ (1 ng/mL) (A) and a blank sample (B). The concentrations of IS were all 50 ng/mL.
3.7 Stability
After an intra-day validation, QC samples at 3.0 and 70.0 ng/mL in plasma and brain homogenate (n = 5) were left in the autosampler for 12 hours and reanalyzed for autosampler stability. Spiked plasma and brain homogenate at two concentrations, 3.0 and 70.0 ng/mL were prepared for all the analytes. One set of samples (n = 3) was prepared and analyzed right afterwards, which was used as a time zero control group. At the same time, another two sets of samples (n = 3) spiked together with the first group were subject to bench-top stability and freeze-thaw stability tests. One of the sets was left on the bench-top (25 °C) for 8 hours and then prepared and analyzed. The other set was stored at −20 °C for 24 hours and then completely thawed at 25 °C on the bench-top without assistance. After another two freeze-thaw cycles, the samples were prepared and analyzed. For all the stability tests, response factors (IS concentration times peak ratio between analyte and IS) were obtained for analyzed samples. Stabilities were calculated by the response factor ratio between samples after and before stability tests, shown in Table 4. All the analytes, COT, NCOT, OHCOT and COTNO, at all the concentrations in both plasma and brain homogenate were confirmed to be stable in terms of autosampler, bench-top and freeze-thaw stability, with the deviation from the time zero control of less than 10%.
Table 4.
Autosampler stability (n = 5), bench-top stability (n = 3) and freeze-thaw stability (n = 3) of COT, NCOT, OHCOT and COTNO at 3.0 and 75.0 ng/mL concentrations in plasma and brain homogenate. Stabilities are shown in forms of percentage of relative concentration to time zero control (mean ± SD).
| Matrix | Analyte | Conc. (ng/mL) | Autosampler Stability (%) | Bench-top Stability (%) | Freeze-Thaw Stability (%) |
|---|---|---|---|---|---|
|
| |||||
| Plasma | COT | 3.0 | 92.36±4.57 | 97.43±8.31 | 104.10±4.59 |
| 75.0 | 98.08±3.28 | 100.15±4.88 | 102.29±1.63 | ||
|
| |||||
| NCOT | 3.0 | 98.65±3.63 | 102.65±5.47 | 99.56±2.55 | |
| 75.0 | 96.94±3.03 | 99.76±3.57 | 102.49±5.04 | ||
|
| |||||
| OHCOT | 3.0 | 100.20±2.39 | 100.82±3.47 | 97.26±1.79 | |
| 75.0 | 101.39±1.75 | 99.28±4.76 | 102.46±0.88 | ||
|
| |||||
| COTNO | 3.0 | 99.87±4.88 | 105.72±5.02 | 98.32±1.15 | |
| 75.0 | 100.54±3.19 | 101.17±4.75 | 100.31±1.58 | ||
|
| |||||
| Brain | COT | 3.0 | 101.60±1.80 | 95.26±3.94 | 95.61±10.62 |
| 75.0 | 96.95±2.38 | 102.39±6.13 | 101.88±2.98 | ||
|
| |||||
| NCOT | 3.0 | 101.53±3.28 | 100.49±3.65 | 97.09±9.24 | |
| 75.0 | 98.93±2.69 | 100.64±4.54 | 97.88±0.29 | ||
|
| |||||
| OHCOT | 3.0 | 101.74±3.81 | 100.78±5.88 | 93.70±7.34 | |
| 75.0 | 101.33±3.12 | 101.48±3.08 | 99.14±2.35 | ||
|
| |||||
| COTNO | 3.0 | 99.44±3.73 | 99.68±4.99 | 103.13±6.72 | |
| 75.0 | 98.27±2.89 | 106.59±3.08 | 95.69±2.89 | ||
3.8 Dilution validation
The sensitive method was developed for simultaneous quantification of COT and metabolites at low concentrations. However, these analytes might have different concentrations in the biological samples, especially for COT that usually has much higher concentrations than the others. In order to adjust the method for samples with higher analyte concentrations, the dilution validation was conducted by diluting spiked samples (n = 5) from 1500 ng/mL into the concentration at ULOQ (100 ng/mL) with corresponding blank matrices. Precision and accuracy of these samples were calculated, which are shown in Table 5. The precision and accuracy for all the analytes in both plasma and brain homogenate were within the acceptance of 15%, suggesting sample dilution within 15 fold was validated and applicable to real samples.
Table 5.
Precision (RSD) and accuracy (RE) of spiked samples (n = 5) with 1500.0 ng/mL analyte concentration in plasma and brain homogenate diluted 15 folds into ULOQ (100 ng/mL) concentration.
| Plasma | Brain | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Analyte | Nominal Conc. (ng/mL) | Measured Conc. (ng/mL) | RSD (%) | RE (%) | Measured Conc. (ng/mL) | RSD (%) | RE (%) |
|
| |||||||
| COT | 1500.0 | 1420.92±29.02 | 2.04 | −5.27 | 1530.24±26.03 | 1.70 | 2.02 |
| NCOT | 1362.81±30.01 | 2.20 | −9.15 | 1613.43±15.44 | 0.96 | 7.56 | |
| OHCOT | 1370.49±36.11 | 2.63 | −8.63 | 1656.96±30.67 | 1.85 | 10.46 | |
| COTNO | 1275.69±30.65 | 2.40 | −14.95 | 1583.43±23.89 | 1.51 | 5.56 | |
3.9 Application
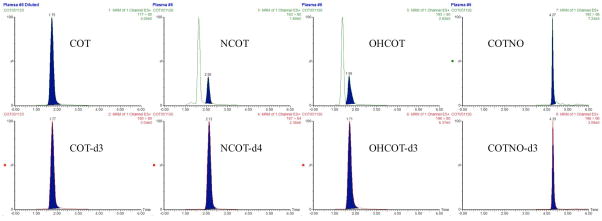
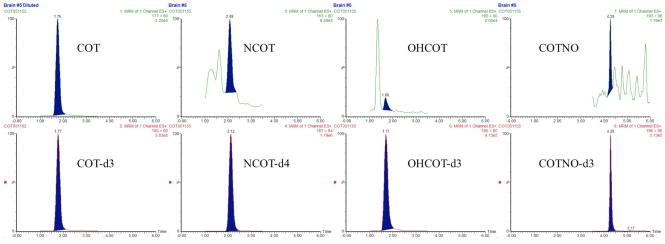
Plasma and brain samples from rats (n = 3) subcutaneously dosed with 1 mg/kg of COT were obtained 30 min after dosing. Paralleled experiments either with or without a 15-fold dilution were conducted for each individual. The same sample preparation and quantitation method were applied to these samples, giving out the result shown in Table 6. The representative chromatograms of these samples are shown in Figure 4 and Figure 5.
Table 6.
Plasma and brain concentrations (mean ± SD) of COT, NCOT, OHCOT and COTNO in biological samples obtained from rats (n = 3) subcutaneously dosed by 1 mg/kg COT.
| Analyte | Plasma Conc. (ng/ml) | Brain Conc. (ng/g) | Brain-to-Plasma Ratio*** |
|---|---|---|---|
|
| |||
| COT | 1364.35±61.58* | 959.4±73.84* | 0.70 |
| NCOT | 0.89±0.09** | 0.48±0.06** | 0.54 |
| OHCOT | 5.31±1.13 | 0.91±0.17** | 0.17 |
| COTNO | 7.13±1.95 | 0.18±0.16** | 0.03 |
Samples over the ULOQ were diluted 15 folds and analyzed with the method.
Concentrations below the LLOQ but still detectable were calculated with extrapolated calibration curves.
Brain-to-plasma ratios were calculated with the assumption that 1 g of brain tissue was equivalent to 1 mL of plasma.
Figure 4.
Representative chromatograms of plasma samples from rats subcutaneously dosed by 1 mg/kg COT: (A) chromatograms of COT and IS in samples that were diluted 15 folds with blank plasma, with the original COT concentration of ng/mL; (B) chromatograms of NCOT and IS, with NCOT concentration below LLOQ; (C) chromatograms of OHCOT and IS, with OHCOT concentration of ng/mL; (D) chromatograms of COTNO and IS, with COTNO concentration of ng/mL.
Figure 5.
Representative chromatograms of brain samples from rats subcutaneously dosed by 1 mg/kg COT: (A) chromatograms of COT and IS in samples that were diluted 15 folds with blank brain homogenate, with the original COT concentration of ng/g; (B) chromatograms of NCOT and IS, with NCOT concentration below LLOQ; (C) chromatograms of OHCOT and IS, with OHCOT concentration below LLOQ; (D) chromatograms of COTNO and IS, with COTNO concentration below LLOQ.
All the analytes could be detected in both plasma and brain. COT concentrations in plasma and brain were largely above the ULOQ, which could still be well quantified after dilution. Concentrations of OHCOT and COTNO were within the linear range in plasma, but below the LLOQ in the brain. NCOT concentrations were below LLOQ in both plasma and brain. All those concentrations below the LLOQ were calculated with extrapolated calibration curves, giving out results with less credibility. Assuming 1 g of brain tissue is equivalent to 1 mL plasma, COT showed great BBB permeability with very high brain-to-plasma concentration ratio 0.7, making COT more promising as an anti-AD drug targeting at the brain. NCOT might also have high BBB permeability, but the credibility of the brain-to-plasma concentration ratio was low. OHCOT and COTNO showed low BBB permeability, due to their high polarity and water solubility. These results provided important information for further investigation of distributions and activities of these drugs in AD therapies.
4. Conclusions
A selective and sensitive LC–MS/MS quantitation method for the simultaneous determination of COT, NCOT, OHCOT and COTNO in rat plasma and brain tissue was developed and validated. This method provided good precision and accuracy for the quantitation of analytes within the linear range of 1 – 100 ng/mL for all the analytes in plasma and brain homogenate (3 – 300 ng/g in brain tissue), with the LLOQ of 1 ng/mL in plasma and 3 ng/g in brain tissue. A low sample volume, 100 μL of rat plasma or brain homogenate, was needed for this method. Protein precipitation and solid-phase extraction was used as sample preparation, yielding acceptable recovery and matrix effect. This method has been successfully applied to preclinical studies of COT, NCOT, OHCOT and COTNO on rats for their anti-AD activity research.
Highlights.
Development of HILIC Tandem MS Method
Optimization of Extraction Process for Multiple Analytes
Validation of Method
Application of Method in Rat Model
Acknowledgments
The experiments described in this manuscript were supported in part by grants from the National Institute on Aging (AG029617), the National Institute on Drug Abuse (DA029127), and the National Institute of Environmental Health Sciences (ES012241)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buccafusco JJ, Terry AV. The potential role of cotinine in the cognitive and neuroprotective actions of nicotine. Life Sciences. 2003;72(26):2931–2942. doi: 10.1016/s0024-3205(03)00226-1. [DOI] [PubMed] [Google Scholar]
- 2.Terry AV, Jr, et al. The nicotine metabolite, cotinine, attenuates glutamate (NMDA) antagonist-related effects on the performance of the five choice serial reaction time task (5C-SRTT) in rats. Biochem Pharmacol. 2012;83(7):941–51. doi: 10.1016/j.bcp.2011.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Terry AV, et al. Cotinine, a neuroactive metabolite of nicotine: Potential for treating disorders of impaired cognition. Cns Drug Reviews. 2005;11(3):229–252. doi: 10.1111/j.1527-3458.2005.tb00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Echeverria V, et al. Cotinine reduces amyloid-beta aggregation and improves memory in Alzheimer’s disease mice. J Alzheimers Dis. 2011;24(4):817–35. doi: 10.3233/JAD-2011-102136. [DOI] [PubMed] [Google Scholar]
- 5.Miller EI, et al. A novel validated procedure for the determination of nicotine, eight nicotine metabolites and two minor tobacco alkaloids in human plasma or urine by solid-phase extraction coupled with liquid chromatography-electrospray ionization-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878(9–10):725–37. doi: 10.1016/j.jchromb.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shakleya DM, Huestis MA. Simultaneous and sensitive measurement of nicotine, cotinine, trans-3′-hydroxycotinine and norcotinine in human plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(29):3537–42. doi: 10.1016/j.jchromb.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim I, Huestis MA. A validated method for the determination of nicotine, cotinine, trans-3′-hydroxycotinine, and norcotinine in human plasma using solid-phase extraction and liquid chromatography-atmospheric pressure chemical ionization-mass spectrometry. J Mass Spectrom. 2006;41(6):815–21. doi: 10.1002/jms.1039. [DOI] [PubMed] [Google Scholar]
- 8.Stolker AL, et al. Determination of nicotine and cotinine in rat plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2003;1020(1):35–43. doi: 10.1016/j.chroma.2003.08.056. [DOI] [PubMed] [Google Scholar]
- 9.Iwasaki Y, et al. Development and validation of a hydrophilic interaction chromatography-tandem mass spectrometry for quantification of nicotine and its metabolites in human maternal and cord sera. Biomedical Chromatography: BMC. 2011;25 (4):503–510. doi: 10.1002/bmc.1475. [DOI] [PubMed] [Google Scholar]
- 10.Bernert JT, Jr, et al. Development and validation of sensitive method for determination of serum cotinine in smokers and nonsmokers by liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Clin Chem. 1997;43(12):2281–91. [PubMed] [Google Scholar]
- 11.Kellogg MD, et al. Rapid and simple tandem mass spectrometry method for determination of serum cotinine concentration. Clin Chem. 2004;50(11):2157–9. doi: 10.1373/clinchem.2004.039594. [DOI] [PubMed] [Google Scholar]
- 12.Tuomi T, Johnsson T, Reijula K. Analysis of nicotine, 3-hydroxycotinine, cotinine, and caffeine in urine of passive smokers by HPLC-tandem mass spectrometry. Clin Chem. 1999;45(12):2164–72. [PubMed] [Google Scholar]
- 13.Heavner DL, et al. Validation and application of a method for the determination of nicotine and five major metabolites in smokers’ urine by solid-phase extraction and liquid chromatography-tandem mass spectrometry. Biomed Chromatogr. 2005;19(4):312–28. doi: 10.1002/bmc.463. [DOI] [PubMed] [Google Scholar]
- 14.Xu X, Iba MM, Weisel CP. Simultaneous and sensitive measurement of anabasine, nicotine, and nicotine metabolites in human urine by liquid chromatography-tandem mass spectrometry. Clin Chem. 2004;50(12):2323–30. doi: 10.1373/clinchem.2004.038489. [DOI] [PubMed] [Google Scholar]
- 15.Shakleya DM, Huestis MA. Optimization and validation of a liquid chromatography-tandem mass spectrometry method for the simultaneous quantification of nicotine, cotinine, trans-3′-hydroxycotinine and norcotinine in human oral fluid. Anal Bioanal Chem. 2009;395(7):2349–57. doi: 10.1007/s00216-009-3157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kataoka H, et al. Determination of nicotine, cotinine, and related alkaloids in human urine and saliva by automated in-tube solid-phase microextraction coupled with liquid chromatography-mass spectrometry. J Pharm Biomed Anal. 2009;49(1):108–14. doi: 10.1016/j.jpba.2008.09.044. [DOI] [PubMed] [Google Scholar]
- 17.Hegstad S, et al. Determination of cotinine in pericardial fluid and whole blood by liquid chromatography-tandem mass spectrometry. J Anal Toxicol. 2009;33(4):218–22. doi: 10.1093/jat/33.4.218. [DOI] [PubMed] [Google Scholar]
- 18.Pellegrini M, et al. Liquid chromatography/electrospray ionization tandem mass spectrometry assay for determination of nicotine and metabolites, caffeine and arecoline in breast milk. Rapid Commun Mass Spectrom. 2007;21(16):2693–703. doi: 10.1002/rcm.3137. [DOI] [PubMed] [Google Scholar]
- 19.Deutsch J, et al. Electron-impact and chemical ionization detection of nicotine and cotinine by gas chromatography-mass spectrometry in rat plasma and brain. J Chromatogr. 1992;579(1):93–8. doi: 10.1016/0378-4347(92)80366-x. [DOI] [PubMed] [Google Scholar]
- 20.Sarasin A, et al. Adrenal-mediated rather than direct effects of nicotine as a basis of altered sex steroid synthesis in fetal and neonatal rat. Reproductive Toxicology. 2003;17 (2):153–162. doi: 10.1016/s0890-6238(02)00119-3. [DOI] [PubMed] [Google Scholar]
- 21.Urakawa N, et al. Simultaneous determination of nicotine and cotinine in various human tissues using capillary gas chromatography/mass spectrometry. International Journal of Legal Medicine. 1994;106(5):232–236. doi: 10.1007/BF01225411. [DOI] [PubMed] [Google Scholar]
- 22.Shakleya DM, Huestis MA. Simultaneous quantification of nicotine, opioids, cocaine, and metabolites in human fetal postmortem brain by liquid chromatography tandem mass spectrometry. Anal Bioanal Chem. 2009;393(8):1957–65. doi: 10.1007/s00216-009-2661-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vieira-Brock PL, et al. Simultaneous quantification of nicotine and metabolites in rat brain by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879(30):3465–74. doi: 10.1016/j.jchromb.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alpert AJ. Hydrophilic-interaction chromatography for the separation of peptides, nucleic acids and other polar compounds. J Chromatogr. 1990;499:177–96. doi: 10.1016/s0021-9673(00)96972-3. [DOI] [PubMed] [Google Scholar]
- 25.Naidong W. Bioanalytical liquid chromatography tandem mass spectrometry methods on underivatized silica columns with aqueous/organic mobile phases. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;796(2):209–24. doi: 10.1016/j.jchromb.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 26.Shou WZ, Naidong W. Simple means to alleviate sensitivity loss by trifluoroacetic acid (TFA) mobile phases in the hydrophilic interaction chromatography-electrospray tandem mass spectrometric (HILIC-ESI/MS/MS) bioanalysis of basic compounds. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;825(2):186–92. doi: 10.1016/j.jchromb.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Naidong W, et al. Novel liquid chromatographic-tandem mass spectrometric methods using silica columns and aqueous-organic mobile phases for quantitative analysis of polar ionic analytes in biological fluids. J Chromatogr B Biomed Sci Appl. 2001;754(2):387–99. doi: 10.1016/s0378-4347(01)00021-4. [DOI] [PubMed] [Google Scholar]
- 28.Murphy SE, et al. Analysis of [3′,3′-d(2)]-nicotine and [3′,3′-d(2)]-cotinine by capillary liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;857(1):1–8. doi: 10.1016/j.jchromb.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]