Abstract
Recently, a unique population of progenitor cells was isolated from human menstrual blood. The human menstrual blood progenitor cells (MBPCs) possess many advantages, such as the noninvasive acquisition procedure, broad multipotency, a higher proliferative rate, and low immunogenicity, and have attracted extensive attention in regenerative medicine. Preclinical studies to test the safety and efficacy of MBPCs have been underway in several animal models. However, relevant studies in type 1 diabetes mellitus (T1DM) have not yet been proceeded. Herein, we studied the therapeutic effect of MBPCs and the mechanism of β-cell regeneration after MBPC transplantation in the T1DM model. Intravenous injection of MBPCs can reverse hyperglycemia and weight loss, prolong lifespan, and increase insulin production in diabetic mice. Histological and immunohistochemistry analyses indicated that T1DM mice with MBPC transplantation recovered islet structures and increased the β-cell number. We further analyzed in vivo distribution of MBPCs and discovered that a majority of MBPCs migrated into damaged pancreas and located at the islet, duct, and exocrine tissue. MBPCs did not differentiate into insulin-producing cells, but enhanced neurogenin3 (ngn3) expression, which represented endocrine progenitors that were activated. Ngn3+ cells were not only in the ductal epithelium, but also in the islet and exocrine tissue. We analyzed a series of genes associated with the embryonic mode of β-cell development by real-time polymerase chain reaction and the results showed that the levels of those gene expressions all increased after cell transplantation. According to the results, we concluded that MBPCs stimulated β-cell regeneration through promoting differentiation of endogenous progenitor cells.
Introduction
Type 1 diabetes mellitus (T1DM), which can lead to hyperglycemia and severe complications [1,2], is an insulin-dependent metabolic disorder characterized by autoimmune destruction of pancreatic islet β cell and inadequate insulin production. Human islet transplantation is an effective therapy by controlling blood glucose and appropriate preventing hyperglycemia without exogenous insulin administration. However, a lack of pancreas donors and the need for long-term immunosuppression limit the widespread use of this treatment [3,4]. Recently, the use of human bone marrow-derived mesenchymal stem cells (BM-MSCs) or human umbilical cord blood (HUCB) cells to treat experimental diabetes had some positive results [5–8]. Transplantation of BM-MSCs or HUCB cells could reduce blood glucose levels [5,6] or improve pancreatic insulitis [7]. Thus, BM-MSCs and HUCB cells may be potential sources for β-cell replacement therapy. However, BM-MSCs and HUCB cells are limited in more widely use for invasiveness of extraction, restricted differentiation potential, or in some cases, a limited proliferative capacity [9–11].
Recently, a novel population of progenitor cells is isolated from human menstrual blood, which can be easily obtained without invasive procedures [12–15]. The human menstrual blood progenitor cells (MBPCs) have showed highly proliferative capabilities and broad multipotency. In absence of induction stimuli, MBPCs are able to expand at least 18 passages without chromosome abnormalities [13]. Meng et al. in vitro induced MBPCs to differentiate into all three germ lineages, including cardiomyocytic, respiratory epithelial, neurocytic, myocytic, endothelial, pancreatic, hepatic, adipocytic, and osteogenic [13]. A clinical trial and an in vitro immunologic test demonstrated that MBPCs possessed low immunogenicity properties and immunomodulatory effects [16,17]. Animal experiments showed that MBPCs had tissue repair effects in some diseases such as Duchenne muscular dystrophy (DMD), myocardial infarction (MI), critical limb ischemia (CLI), and stroke [12,18–21]. Based on the advantages in characteristics and repair effects in diseases, the therapeutic potential and mechanism of MBPCs in diabetes should be notable and investigated.
Thus, in this study, we have two purposes: one is to investigate the therapeutic effect of MBPCs to T1DM mice and the other is to study involved repair mechanism. Using a mouse model of streptozotocin (STZ)-induced type 1 diabetes, we show that transplantation of MBPCs reverses hyperglycemia, recovers islet structures, and stimulates endogenous β-cell regeneration. MBPCs migrate to the pancreatic duct, exocrine tissues, and islet and promote endogenous pancreatic progenitor differentiation.
Materials and Methods
Experimental animals
Six- to eight-week-old male BALB/c mice were purchased from the SLAC Laboratory Animal Corporation (Shanghai, China). Mice were fed ad libitum and housed in a 12-h light and 12-h dark cycle under specified pathogen-free conditions. Eight-week-old male BALB/c mice, weighing 32–36 g, were chosen in animal experiments. All animal experiments were according to the institutional animal welfare guidelines and approved by the Animal Care and Use Committees of Zhejiang University, China.
Isolation and culture of MBPCs
The MBPCs were isolated from female donors according to the protocol previously reported [13] with slight modification. The entire procedures were with consent of the donors and approved by the Ethics Committee of The First Affiliated Hospital, College of Medicine, Zhejiang University, China. The menstrual blood samples were collected with a Divacup (Kitchener, ON) from healthy women (n=9) during a menstrual cycle. The cells were transferred into phosphate-buffered saline (PBS) with mixed antibiotics, including amphotericin B, gentamycin sulfate, kanamycin sulfate, cephalexin, vancomycin hydrochloride, and heparin at 4°C for 24 h. Then, the samples were centrifuged at 1,600 g for 10 min at 4°C, and the supernatants were used for microbiological testing. Mononuclear cells were separated by a density gradient centrifugation with Ficoll-Paque (Fisher Scientific, Portsmouth NH). The interlayer cells were collected and cultured with the Chang Medium [14] in a tissue culture flask (Corning, Corning, NY) to obtain adherent cells. The media were changed every 2–3 days until adherent cells grew to 80%–90% confluency, and then the cells were subcultured using 0.25% trypsin (Invitrogen, Carlsbad, CA) and cultured on flasks or plates seeded at 8,000–10,000 cells/cm2. The cells used in the experiments were at fourth to eighth passage.
Identification of MBPCs by flow cytometry
Isolated MBPCs were utilized for flow cytometry analysis. About 105 cells were resuspended in 100 μL PBS and incubated with primary antibodies (PE-conjugated anti-human CD13, CD29, CD34, CD44, CD45, CD73, CD90, CD105, CD117, CD166, SSEA4, or FITC-conjugated anti-human HLA-DR; Becton Dickinson, Franklin Lakes, NJ) (1:100) at 4°C for 1 h. Then, the cells were washed twice with PBS. The corresponding isotype antibodies (Becton Dickinson) were set as negative controls. Cells were analyzed by a Flow Cytometer (FC500MCL; Beckman Coulter, Pasadena, CA). The results were analyzed by FlowJo software.
Induced differentiation of MBPCs to osteoblasts, adipocytes, and chondroblasts
For osteogenic differentiation, the MBPCs were plated at 3×103 cells/cm2 and treated with the human MSC osteogenic differentiation medium (Cyagen, Guangzhou, China). The osteogenic medium consisted of 100 nM dexamethasone, 10 mM β-glycerophosphate, 0.2 mM ascorbate, 1 mM glutamine, 1% penicillin–streptomycin, and 10% fetal bovine serum (FBS) and was completely changed every 3 days for up to 21 days. Cells were then fixed by 4% formaldehyde and stained with Alizarin red for 5 min.
For adipogenic differentiation, cells were plated at 2×104 cells/cm2 in the human MSC adipogenic differentiation medium A (ADMA; Cyagen); ADMA consisted of 1 mM dexamethasone, 0.5 mM 3-isobutyl-1-methyl-xanthine (IBMX), 10 μg/mL recombinant human insulin, 100 mM rosiglitazone, and 10% FBS. Cells were cultured in the ADMA for 3–5 days, in the adipogenic differentiation medium B (ADMB; 1% penicillin/streptomycin+10% FBS+10 μM insulin+1 mM glutamine) for 24 h, and then interchanged from ADMB to ADMA of three to five cycles of induction/maintenance. The cells were cultured in the ADMB for an additional 7 days by replacing the medium every 3 days. For Oil Red O stain analysis, cells were fixed in 4% formaldehyde and then stained with Oil Red O for 30 min.
For chondrogenic differentiation, cells were plated onto 12-well tissue culture plates at 2.5×105 cells/cm2 in a complete chondrogenic medium. The chondrogenic medium consisted of 1 mM dexamethasone, 0.2 mM ascorbate, 1% ITS, 1 mM sodium pyruvate, 1 mM proline, and 20 ng/mL TGF-β3 (Cyagen). The cells were cultured for 14–28 days by completely replacing the medium every 2 days. Cells were formalin fixed and stained with Alcian blue for 30 min.
Karyotype analysis
The 10th and 20th passages of cultured MBPCs were used to prepare for chromosome samples according to [12]. Karyotype analysis was performed by standard cytogenetic protocol with G staining. For cells, 50 metaphases were prepared for G-banding analysis.
Preparation of mouse T1DM model
The BALB/c mice were injected intraperitoneally (i.p.) with 180 mg/kg STZ (Sigma-Aldrich, St. Louis, MO) at a single injection [22]. STZ was dissolved in 0.1 M of sodium citrate buffer, pH 4.5 [23], and injected within 15 min after preparation. The blood glucose level was assayed with tail vein blood by a standard blood glucose meter (Accu-check, Performa). Hyperglycemia developed within 3–5 days after STZ treatment. Mice with blood glucose levels over 13.9 mM [24] at two random measurements were considered diabetic and used in the subsequent transplantation experiments.
Cell transplantation
Cell transplantation assay was performed to evaluate whether MBPCs had effect to ameliorate diabetic symptoms in the T1DM model. About 3×105 cells were injected through a tail vein into each diabetic mouse, 5–7 days after STZ injection. T1DM mice without cell transplantation were set as control. Untreated normal mice were used as normal control. Body weight and fasting blood glucose were monitored overnight, before and after cell administration. Additionally, the lifespan of cell-treated mice and untreated diabetic mice after cell transplantation was recorded. MBPCs at passage 5 were used in the transplantation model.
Cell labeling and imaging
For cell tracking, MBPCs were labeled by the fluorescent lipophilic tracer 1,1′-dioctadecyl-3,3,-3′,3′-tetramethylindocarbocyanine perchlorate (DiI; Molecular Probes, Eugene, OR). For labeling, the cells were resuspended at 1×106 cells/mL in the αMEM, and added DiI in the cell suspension to obtain a final concentration of 10 μg/mL. After incubation for 20 min at 37°C with 5% humidified CO2, the cells were centrifuged at 400 g for 5 min, washed twice with PBS [25,26], and then resuspended in PBS for 2×106 cells/mL. For transplantation, MBPCs were infused in diabetic mice and normal mice. After 24 h, 3, 7, and 14 days, the mice were sacrificed. The heart, liver, lungs, pancreas, spleen, and kidney were immobilized and prepared for tissue imaging with a Night Owl LB 981 low-light luminograph (Berthold Technologies, Bad Wildbad, Germany) equipped with a cooled CCD camera. Digital images were analyzed with WinLight32 Software. At each time point, the diabetic group and normal group both had three parallel mice. Each mouse received 5×105 cells.
Intraperitoneal glucose tolerance test
To further evaluate the functional recovery effect of islet β cell after MBPC transplantation, an intraperitoneal glucose tolerance (IPGT) test was performed on day 14 after transplantation followed by the protocol from the Animal Models of Diabetic Complications Consortium (AMDCC). Briefly, mice were fasted for 6 h by removal to a clean cage without food at the end of their dark (feeding) cycle and administered i.p. with glucose (1 mg/g body weight; Sigma-Aldrich). Blood samples were obtained from a small tail clip and analyzed by a Roche Accu-check glucose meter. Blood glucose values were obtained at 0, 15, 30, 45, 60, 75, 90, 105, 120, and 135 min.
ELISA
To evaluate the recovering effect of MBPCs, the levels of insulin and C-peptide in serum of T1DM mice, cell-treated mice, and control mice were comparatively analyzed by ELISA kits (Mercodia, Uppsala, Sweden). For this purpose, a human insulin-specific ELISA kit (Ultrasensitive Insulin ELISA), a mouse insulin-specific ELISA kit (Ultrasensitive Mouse Insulin ELISA), and a human C-peptide-specific ELISA kit (Ultrasensitive C-peptide ELISA) were used, respectively. Fasting blood was collected on day 28 after cell administration. Serum samples were obtained from tail vein and allowed to clot for 2 h at room temperature. The clotted material was discarded by centrifugation at 3,000 rpm for 15 min. Serum samples were stored at −80°C for test as above.
Histology, immunohistochemistry, and immunofluorescence
For histological analysis, pancreas samples were fixed in 10% formalin overnight at 4°C and embedded in paraffin. Two micrometer pancreatic sections were cut serially. For histopathology analysis, sections were stained with hematoxylin and eosin (H&E; Sigma-Aldrich). The sliders were visualized using a microscope (Zeiss, Jena, Germany).
For the immunohistochemistry test, sections were peroxidase blocked in 3% H2O2 for 10 min, and then they were pretreated using heat-mediated antigen retrieval with the sodium citrate buffer (pH6.0). Tissue sections were blocked with 10% FBS for 20 min at room temperature and then incubated with a rabbit polyclonal antibody against mouse insulin (1:500 dilution; Abcam, Cambridge, United Kingdom) for 2 h at room temperature. Sliders were washed three times with PBS, 5 min at every time. Then, they were further incubated with a secondary antibody (HRP-conjugated goat anti-rabbit IgG; Chemicon, Billerica, MA). In addition, to detect whether MBPCs migrated into the injured pancreas, we used a mouse monoclonal anti-human nuclei antibody (Millipore, Darmstadt, Germany) to stain MBPCs. The tissues were incubated with this primary antibody (1:50 dilution) for 1 h at room temperature, followed by incubation with a secondary antibody (HRP-conjugated goat anti-mouse IgG; Chemicon) for 1 h at room temperature. 3,3′-diaminobenzidine (DAB) was used as the reaction substrate.
For the immunofluorescence test, sections were costained with a rabbit polyclonal anti-human insulin antibody and a mouse monoclonal anti-human nuclei antibody (1:50 dilution; Millipore) for 90 min at room temperature. Then, they were incubated with secondary antibodies (DyLight 405-labeled goat anti-rabbit IgG, Cy3-labeled goat anti-mouse IgG) for 1 h at room temperature. Additionally, tissues were immunostained with a rabbit polyclonal anti-mouse neurogenin3 (ngn3) antibody (1:500 dilution; Millipore) or costained with a rabbit polyclonal anti-mouse ngn3 antibody and a mouse monoclonal anti-human nuclei antibody for 2 h at room temperature. They were further incubated with secondary antibodies (Alexa Fluor 555-labeled goat anti-rabbit IgG, FITC-labeled goat anti-mouse IgG) for 1 h at room temperature. Immunostaining analysis was performed in a Zeiss fluorescence microscope and images were captured and digitalized with the Axiovision 4.6 software.
Reverse transcriptase–polymerase chain reaction and real-time PCR
Total RNA of pancreas was extracted on day 3, 7, 10, 14, 21, and 28 after cell transplantation by using the TRIzol Reagent (Invitrogen). The samples were reverse transcribed with PrimeScript Reverse Transcriptase (TaKaRa, Shiga, Japan) for 30 min at 42°C in the presence of an oligo-dT primer. Real-time polymerase chain reaction (PCR) analysis was performed by the Eppendorf Mastercycler Realplex detection system using the SYBR Green Premix Ex Taq (Perfect real-time; TaKaRa). The PCR reaction consisted of 5 μL of SYBR Green PCR Ex Taq Mix, 1 μL of 10 mM forward and reverse primers, 3.5 μL of water, and 0.5 μL of template cDNA in a total volume of 10 μL. Cycling was performed using the default conditions of Realplex Software 1.5: 95°C for 2 min, followed by 40 cycles of 95°C for 15 s and 58°C for 20 s. The relative expression of each gene was normalized to endogenous β-actin. The primers of assayed genes, including ngn3, foxa2, pax4, mafa, mafb, and nkx6.1, were designed specifically for mouse. The primers of insulin, glut2, and pdx1 are referenced in [23]. The primer sequences are shown in Table 1. The related gene expression level was calculated using the 2−▵▵CT method. Each sample was run in three parallel reactions.
Table 1.
Primers Used for PCR and Real-Time PCR
| Primer name | Sequence (5′-3′) | Species |
|---|---|---|
| Insulin1 | F: TAGTGACCAGCTATAATCAGAG | Mouse |
| Insulin1 | R: ACGCCAAGGTCTGAAGGTCC | |
| Pdx1 | F: TGTAGGCAGTACGGGTCCTC | Mouse |
| Pdx1 | R: CCACCCCAGTTTACAAGCTC | |
| Glut2 | F: CCACCCAGTTTACAAGCTC | Mouse |
| Glut2 | R: TGTAGGCAGTACGGGTCCTC | |
| Foxa2 | F: GAACTCCATCCGCCACTCT | Mouse |
| Foxa2 | R: GGTCTTCTTGCCTCCGCTA | |
| Pax4 | F: GGATGCGACCCTGTGAC | Mouse |
| Pax4 | R: TCCTGAAGTGCCCGAAG | |
| Ngn3 | F: CGGATGACGCCAAACTTA | Mouse |
| Ngn3 | R: GCCTCCACTACCTCCCACTC | |
| Nkx6.1 | F: GGGGACTTCGGAGAATGAG | Mouse |
| Nkx6.1 | R: GGCGAGCAGCCAGGATA | |
| MafA | F: ATGGCCCGCGGAGCTGGCGATGGGCG | Mouse |
| MafA | R: CGAAGAGGGCACCGAGGAGCAGGGC | |
| MafB | F: CTGCGCCCCTAGCCCTGGACTC | Mouse |
| MafB | R: GGCGGCCCTGGCACTCACAA | |
| β-Actin | F: ATGGATGACGATATCGCTG | Mouse |
| β-Actin | R: ATGAGGTAGTCTGTCAGGT |
PCR, polymerase chain reaction.
Statistical analyses
Statistical data were analyzed by one-way analysis of variance (ANOVA) with SPSS 16.0 software. Results are expressed as mean±standard deviation; a P-value<0.05 or <0.01 was considered significant. Survival cures were plotted using the Kaplan–Meier method.
Results
Characterization of MBPCs
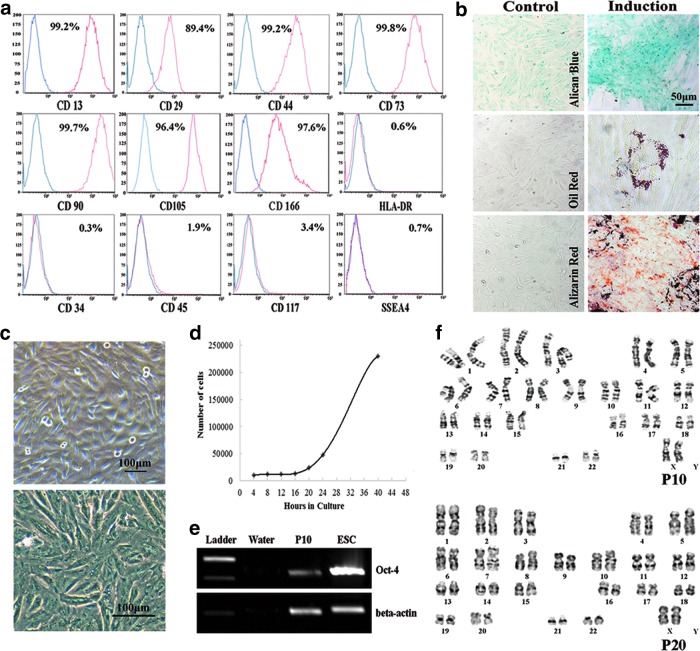
Flow cytometry results revealed that MBPCs exhibited phenotypes of MSCs. They expressed CD13, CD29, CD44, CD73, CD90, CD105, and CD166, but did not express HLA-DR, CD34, CD45, CD117, and SSEA4 (Fig. 1a). MBPCs had the capacity for trilineage mesenchymal differentiation. MBPCs were induced to chondroblasts, adipocytes, and osteoblasts (Fig. 1b) using the commercial differentiating medium. Cell staining showed multipotent characteristics such as sulfated proteoglycans formed in cartilage induction, fat vacuoles detected in adipocyte differentiation, and calcium deposits in bone formation, whereas the controls were negative of trilineage staining (Fig. 1b). Isolated MBPCs were plastic adherent, fibroblast like, and spindle shaped (Fig. 1c). One doubling time of MBPCs at passage 5 was near 20 h (Fig. 1d). MBPCs expressed the multipotent marker Oct-4 at passage 10 (Fig. 1e). MBPCs maintained diploid karyotype without chromosomal aberrations at passage 10 and 20 (Fig. 1f).
FIG. 1.
Identification of human menstrual blood progenitor cells (MBPCs). (a) Representative mesenchymal stem cell (MSC) phenotypes of MBPCs (four to six passages) by flow cytometry. MBPCs are positive for MSC markers such as CD13, CD29, CD44, CD73, CD90, CD105, and CD166 and negative for HLA-DR, CD34, CD45, CD117, and SSEA4. (b) Representative MBPCs having the potential to differentiate into chondroblasts, adipocytes, and osteoblasts of mesoderm tissues under conditioned culture. (c) Representative photomicrographs showing adherent MBPCs with spindle shapes on a plastic tissue culture flask. (d) Growth curve of MBPCs at passage 5 by MTT assay. (e) MBPCs expressing the multipotent marker Oct-4 at the RNA level at passage 10 by reverse transcriptase–polymerase chain reaction (RT-PCR). (f) Representative normal chromosome morphology of MBPCs by karyotype analysis at passage 10 and 20.
Therapeutic effect of MBPCs in diabetic mice
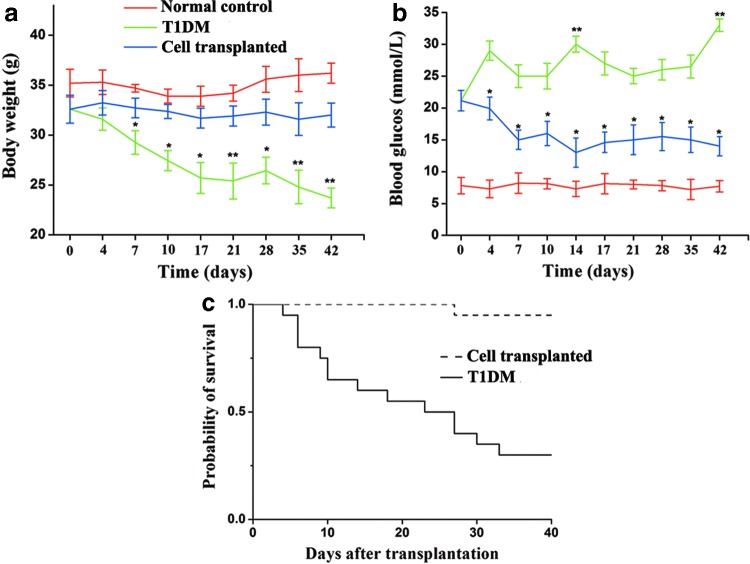
To analyze the potential therapeutic effect of MBPCs to T1DM, we transplanted MBPCs into T1DM mice at the onset of hyperglycemia by tail vein infusion and recorded body mass, blood glucose levels, and survival rates for 42 days. T1DM mice experienced a significant mean weight loss from 32.6±1.3 to 23.7±1 g, whereas mice transplanted with MBPCs had a relative stable weight at 32.3±1.6 g (Fig. 2a). The mean body mass of cell-transplanted mice was significantly greater compared with T1DM mice. Glycemic monitoring showed that blood glucose increased in T1DM mice at all time points (from 21±1.6 to >33 mM) (Fig. 2b), but it dramatically reduced in cell-transplanted mice on day 7 post-transplantation (from 21.2±1.6 to 15±1.5 mM). The mean blood glucose level from day 10 to 42 was 14.7±1.5 mM in cell-transplanted mice and was 7.7±1 mM in normal mice (Fig. 2b). Cell-transplanted mice had a survival rate of 95%, in contrast to T1DM mice with a rate of 30% (100% morbidity) (Fig. 2c). Furthermore, polyuria was significantly improved compared with untreated diabetic mice (data not shown).
FIG. 2.
Transplantation of MBPCs ameliorates diabetic symptoms in streptozotocin (STZ)-induced mice. Each STZ-treated (1 injection at 180 mg/kg) hyperglycemic mouse received 3×105 MBPCs by tail vein transplantation. STZ, streptozocin. (a) Compared with type 1 diabetes mellitus (T1DM) mice (green line, n=8), cell-transplanted mice (blue line, n=10) maintained their body weight and had no significant difference from normal controls (red line, n=6). (b) After transplantation, the mean level of blood glucose from day 10 to 42 reduced to 14.7±1.5 mM in MBPC-treated mice (blue line, n=10). The mean level of blood glucose in T1DM mice (green line, n=8) was at 27.4±2.8 mM. (c) Compared with T1DM mice (full line, n=20), mice transplanted with MBPCs (dotted line, n=18) showed a higher survival rate (95% vs. 30%). Data are presented as mean±standard deviation (SD). *P<0.05, **P<0.01.
Islet function and structure repair by MBPC engraftment
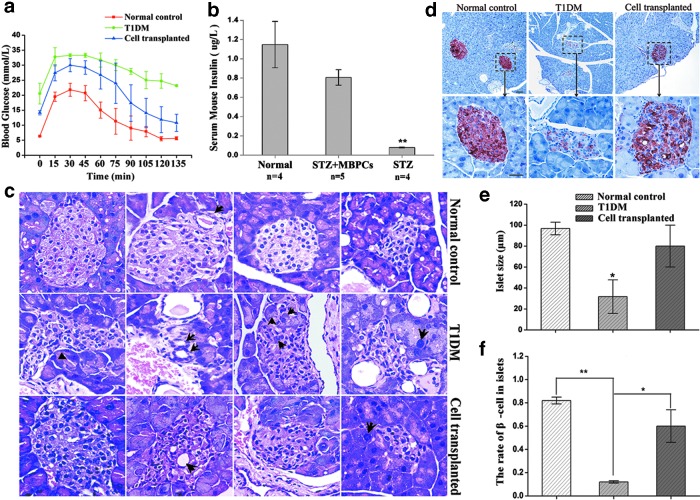
To test the repair effect of MBPCs to impaired islet, we estimated glucose control in cell-transplanted mice using an IPGT test and found a similar rate of hypoglycemic activity as normal control, but T1DM mice showed an impaired glucose control (Fig. 3a). The serum insulin levels in cell-transplanted mice (0.81±0.08 μg/L) were ∼10-fold higher than in T1DM mice (0.08±0.005 μg/L), but were lower than in normal control (1.12±0.24 μg/L) (Fig. 3b).
FIG. 3.
Transplantation of MBPCs improves the function and structure of damaged islets in STZ-induced mice. (a) An intraperitoneal glucose tolerance (IPGT) test was performed on day 14 after transplantation. Compared with normal control (red line, n=5), cell-transplanted mice (blue line, n=6) showed a similar rate of lowering blood glucose, whereas T1DM mice had uncontrolled blood glucose levels during 135 min (green line, n=5). (b) The serum samples of mice were collected on day 28 after cell administration for ELISA. Compared with T1DM mice (right bar, n=4), mice transplanted with MBPCs (middle bar, n=5) showed a significantly higher level of serum insulin (P<0.01), but lower than normal control (left bar, n=4) with no significant difference. (c) Representative pathological variances in the pancreas on day 21 after cell infusion. Islet in T1DM mice was shrinking and deformed (arrow on the left), and exocrine acinar cells swelled (arrow on the right), and exocrine cells inserted in islet (arrows on third line), and pseudoductal structures appeared in the islet (arrows on the second line). However, islets in cell-transplanted mice had no obvious pathological changes compared with normal control. The four to six sliders of each group were used to analyze. Original magnification, 400×. (d) Representative immunohistochemistry results showed that insulin production in islet tissue increased in the cell-transplanted mice relative to T1DM mice. Mice were sacrificed on day 28 after cell transplantation. The three to five sliders of each group were used to analyze. Scale bar=10 μm. (e, f) These two figures are the statistical analysis of figure 3d. The islet size of T1DM mice decreased significantly compared with cell-transplanted mice. The islet size of cell-transplanted mice had no significant difference from normal control. The number of β cells in cell-transplanted mice significantly increased compared with T1DM mice and had no significant difference from normal control. The average 20 islets of each group were used to analyze. Data are mean±SD (n=3). *P<0.05, **P<0.01.
Pathological and histological results (Fig. 3c) indicated that the islet structure and morphology were restored by MBPC administration. Following transplantation of MBPCs, mice exhibited normal islet structure and size in the pancreas, whereas in T1DM mice, the islet changed morphologically and appeared atrophied. In the inner part of the islet, some endocrine cells of T1DM mice formed pseudoducts, while in the normal mice and cell-treated mice, there were just normal vessels in the islet. In addition, in T1DM mice, some acinar cells of the exocrine gland were swollen and trapped in the islet, whereas in the cell-transplanted mice, there were very few acinar cells around islet displaying intumescence. From the results of H&E staining, it displayed that cell-transplanted mice did not experience apparent pathological changes compared with normal control (Fig. 3c).
Immunohistochemical results (Fig. 3d) showed that the islet size and β-cell number in cell-transplanted mice were higher than in T1DM mice. We carried out a statistical analysis for islet size and β-cell number in normal control mice, T1DM mice, and cell-transplanted mice (Fig. 3e, f). According to the statistical result, compared with T1DM mice, islet size (Fig. 3e) and the quantity of β cell in visible islet (Fig. 3f) were significantly increased (P<0.05) in cell-transplanted mice, but were not significantly different from normal control (P>0.05). Even though there was a slight reduction in the β-cell number in cell-transplanted mice, islet size and morphology still remained normal relative to normal control mice (Fig. 3d–f). Compared with normal control, the total number of islets in T1DM mice also had a significant decrease (data not shown).
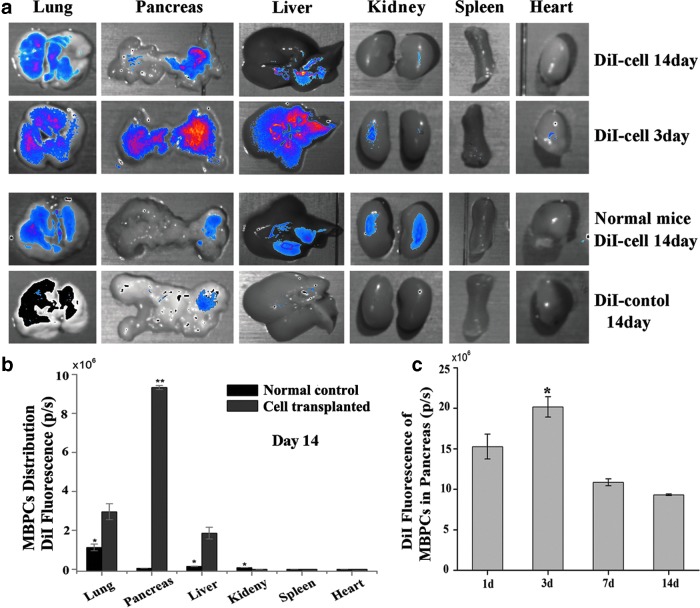
MBPC distribution in vivo
Tissues were dissected under the CCD camera for tracking distribution of DiI-marked MBPCs in the diabetic mice and normal mice. In the diabetic mice, MBPCs mainly located in the lungs, pancreas, and liver on day 3 after cell implantation, and relatively more cells resided in diabetic pancreas on day 3 and 14 (Fig. 4a). In the normal mice, on day 14 after transplantation, MBPCs mainly distributed in the lungs, liver, and kidney and most of them located in the lungs (Fig. 4a,b); the quantity of MBPCs significantly reduced compared with cell-transplanted diabetic mice, and MBPCs were almost undetectable in normal pancreas (Fig. 4b). However, on day 14 after transplantation, many MBPCs still survived in diabetic mice, and most of them existed in the injured pancreas (Fig. 4a,b). During the first 24 h after cell infusion, part of the MBPCs migrated into diabetic pancreas and the number of MBPCs increased on day 3, then had a significant reduction on day 7 and 14 (Fig. 4c). The DiI signal increased during the first 3 days (Fig. 4c), indicating the acceptance of the grafted cells and proliferation of the cells. However, after 3 days, the DiI signal dropped step by step (Fig. 4c), indicating cell death in long-term implantation. Additionally, we also evaluated MBPC distribution on day 28 and 42. The quantity of MBPCs in pancreas gradually decreased on day 28 and 42, but there were still a few MBPCs in pancreas. Although the infused cell number had an obviously decrease in course of time, the number of MBPCs in diabetic pancreas was still higher than in diabetic other tissues (data not shown).
FIG. 4.
The distribution of MBPCs in vivo. (a) 1,1′-dioctadecyl-3,3,-3′,3′-tetramethylindocarbocyanine perchlorate (DiI) marked MBPC distribution in vivo of diabetic mice and normal mice on day 3 and/or 14 after transplantation. Each mouse had an intravenous infusion with 5×105 MBPCs. In diabetic mice, MBPCs mainly distributed in the pancreas, lungs, and liver (upper two rows). And diabetic pancreatic tissue acquired more cells. In the normal mice, MBPCs disseminated in the lungs, liver, and kidney, and were almost undetectable in the pancreas on day 14. (b) Representative quantity of MBPCs migrated in different tissues after infusion in T1DM mice and normal mice. The majority of MBPCs resided in injured pancreas in T1DM mice, while most of cells resided in the lungs in normal mice on day 14. (c) Representative quantity of MBPCs in diabetic pancreas at different time points. Data are mean±SD (n=3). *P<0.05, **P<0.01.
Recruitment and differentiation of MBPCs in damaged islet
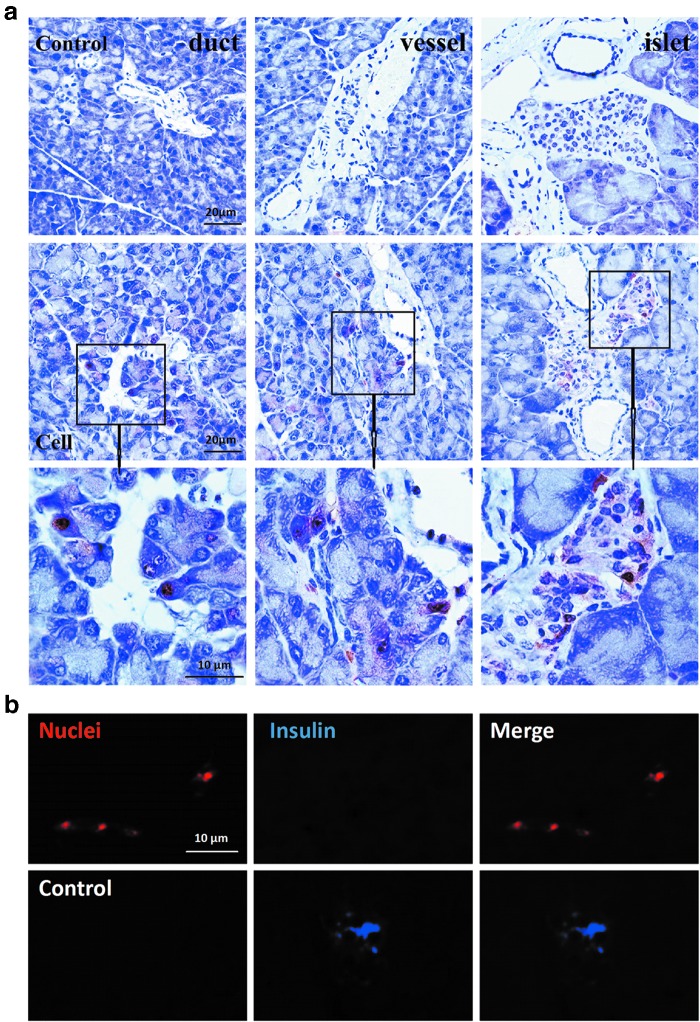
To confirm whether MBPCs migrated to the diabetic pancreas, we immunohistochemically stained human specialized nuclei antigen (Fig. 5a). The results showed that infused MBPCs migrated to the injured pancreatic tissue and located at islet and exocrine tissue near the pancreatic duct and pancreatic vessel (Fig. 5a).
FIG. 5.
Immunostaining analysis of location and differentiation of MBPCs in pancreatic tissue. To detect MBPC migration and differentiation in pancreas, mice were sacrificed on day 28 after cell transplantation. (a) Immunohistochemical results showed that MBPCs migrated to the damaged pancreas and located at the exocrine regions near the pancreatic duct and vessel, and islet. T1DM mice without cell transplantation were as control. (b) Fluorography indicated that MBPCs in pancreas did not differentiate into insulin-producing cells. Red fluorescent staining was representative of specific human nuclei, whereas blue fluorescence was representative of human insulin.
To test whether MBPCs promoted β-cell regeneration via self-differentiation into insulin-producing cells (IPCs) under the injured microenvironment, ELISA for human insulin and human C-peptide were performed by using serum samples from cell-transplanted mice. The results showed that no human insulin or human C-peptide was detected (data not shown). To further confirm the in vivo differentiation of MBPCs, we costained the human-specific nuclei antibody and the human insulin antibody on day 28 after cell transplantation by immunofluorescence. The results displayed a lack of insulin expression in MBPCs (Fig. 5b). Based on the specificity of the human ELISA kit and antibodies, we concluded that MBPCs did not differentiate into IPCs in injured pancreas.
MBPC-promoted endogenous progenitor cell differentiation
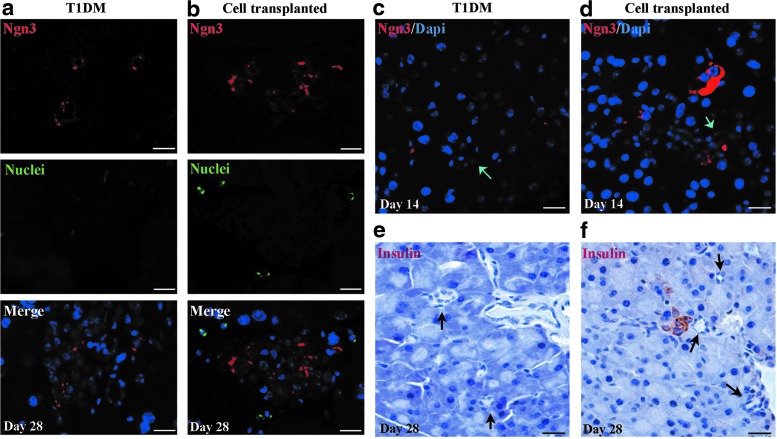
To confirm whether ngn3+ cells differentiated from MBPCs, we costained ngn3 and the human nuclei antibody (Fig. 6a, b) on day 28 after transplantation. As a result, in cell-transplanted mice, ngn3+ cells were detected in islet, but they were negative for the human nuclei antigen (Fig. 6b). Compared with cell-transplanted mice (Fig. 6b), fewer ngn3+ cells were observed in islet in T1DM mice (Fig. 6a). To verify whether MBPCs stimulate the differentiation of pancreatic progenitor cells associated with the duct epithelium, we analyzed ngn3 expression in pancreatic structures, including the duct and exocrine tissue (Fig. 6c, d). We observed that in cell-transplanted mice, on day 14 after transplantation, a few of the ngn3+ cells were detected not only in the ductal epithelium, but also in exocrine tissue near the duct (Fig. 6d), while in T1DM mice, no ngn3+ cells were detected in the exocrine tissue and duct epithelium (Fig. 6c). On day 28 after cell transplantation, a small cluster of insulin-positive cells were detected in exocrine tissue near the duct (Fig. 6f), where ngn3+ cells also appeared on day 14 in cell-transplanted mice (Fig. 6d). The results demonstrated that MBPCs could activate more endocrine progenitor cells, which resided not only in the duct but also in islet and exocrine tissue.
FIG. 6.
MBPCs promote endogenous pancreatic progenitor differentiation to compensate for β-cell loss. (a, b) Fluorescence photomicrographs showed that neurogenin3 (ngn3)-positive cells, which were representative of endocrine progenitor cells, appeared in islet of cell-transplanted mice on day 28 after MBPC transplantation, and there were a few of MBPCs around islet. Less of ngn3+ cells were observed in islet of T1DM mice. (c) Ngn3 did not express in the exocrine tissue close to the ductal epithelium in T1DM mice. However, (d) ngn3+ cells were detected in exocrine tissue and ductal epithelium in MBPC-transplanted mice. (c, d) Arrows are representative of pancreatic ducts. (e) No insulin-expressing cells around ducts were detected in T1DM mice. However, (f) in cell-transplanted mice, a small cluster of insulin-positive cells was detected in exocrine tissue near the duct on day 28 after transplantation. (e, f) Arrows are representative of pancreatic ducts.
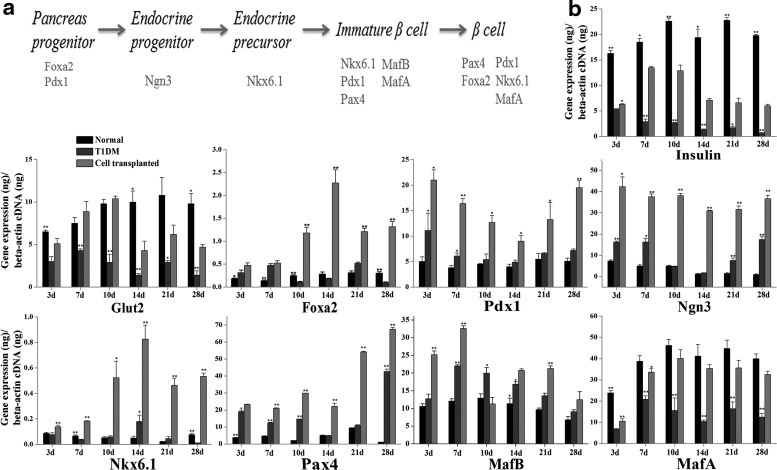
To further analyze whether endogenous progenitor cell differentiation was involved in β-cell regeneration, we assayed a series of genes associated with embryonic β-cell development (Fig. 7a) by real-time PCR. In cell-transplanted mice, the average expression levels of foxa2 and pdx1 genes (associated with early stages of pancreatic differentiation) were much higher than that in the other two groups. Compared with T1DM mice and normal control, the expression of ngn3 significantly increased throughout 28 days after MBPC treatment. The expression of nkx6.1 (associated with midstage of β-cell development and maturation) was significantly upregulated in cell-transplanted mice at all time points. The expression of pax4 (associated with immature and mature stages of β cell) also significantly increased in cell-transplanted mice compared with T1DM mice and normal mice at all time points. In cell-transplanted mice, the gene mafb only expressed in immature β cells had significantly increased on day 3 and 7, and was lower on day 10, 14, 21, and 28. It indicated a transition from immature β cells to mature β cells. The gene mafa only expressed in mature β cells had a higher expression in the cell-transplanted mice than in the T1DM mice, and there was no difference in the levels of mafa between the cell-transplanted mice and the normal control on day 10, 14, 21, and 28. Moreover, insulin and glut2, which were expressed in functional β cells, were significantly increased in cell-transplanted mice compared with T1DM mice.
FIG. 7.
MBPCs activated the expression of genes associated with embryonic β-cell development and promoted differentiation of pancreatic progenitor to β cell. (a) Representative of important genes in the development of islet β cell, from pancreatic progenitor to mature β cell. (b) Representative of real-time PCR analysis of genes relating to islet β-cell development at time point of day 3, 7, 10, 14, 21, and 28 after cell transplantation. In functional β cells, the insulin and glut2 gene levels significantly increased in cell-transplanted mice compared with T1DM control. Genes associated with embryonic β-cell development (foxa2, pdx1, ngn3, nkx6.1, and pax4) were time dependent and significantly increased in cell-transplanted mice compared with normal mice and T1DM mice. Compared with T1DM mice and normal control, gene mafb representative of immature β cell was significantly upregulated in cell-transplanted mice on day 3, 7, 14, and 21. Mafa associated with β-cell maturation was significantly upregulated in cell-transplanted mice compared with T1DM mice at all time points. Values are expressed as mean±SD (n=3).
In T1DM mice, those genes are of irregular expression. On day 3 and 7, gene foxa2 and pdx1 significantly increased compared with normal control and ngn3 increased on day 3, 7, 21, and 28. On day 3, 7, 10, and 28, pax4 comparatively increased relative to normal control and nkx6.1 was only upregulated on day 14. Levels of mafa, insulin, and glut2 were much lower than cell-transplanted mice and normal control at all time points. In the normal mice, the above genes, except mafa, insulin, and glut2, were lower than in the other two groups (Fig. 7b), which indicated their homeostasis in the β-cell mass.
Discussion
The characteristics of MBPCs
In this study, we isolated and identified a population of progenitor cells from human menstrual blood (MBPCs). Although MBPCs exhibit some characteristics of MSCs, they also have some distinct properties. First, MBPCs do not express SSEA4 and CD117 (Fig. 1a), which are common markers of BM-MSCs [27–31], whereas they express an embryonic stem cell maker Oct-4 (Fig. 1e). Second, MBPCs are much more proliferative. Under in vitro culture conditions, they can stably expand more than 20 passages without karyotype abnormalities (Fig. 1f), and their one doubling time is near 20 h (Fig. 1d). MSCs isolated from BM or UCB have a longer doubling time [27,32] and their proliferation capacity will slow down in long-term culture. Additionally, compared with BM-MSCs, MBPCs seem to possess broader multipotent plasticity. MBPCs are able to generate cells of all three germ lines [13]. Thus, these characteristics make MBPCs a unique population different from MSCs obtained from other tissues such as BM and UCB.
The therapeutic effect of MBPCs to T1DM mice
Because of the unique characteristics of MBPCs, their therapeutic potential in diseases deserves to be studied. In our study, we first investigated the therapeutic effect of MBPCs in STZ-induced mice. The results of animal experiments demonstrated that transplantation of MBPCs was an effective treatment for T1DM mice by ameliorating diabetic symptoms, such as reversing hyperglycemia, maintaining the body weight, improving the survival rate, insulin levels, and glucose tolerance. The levels of blood glucose could be reduced and stabilized at about 15 mM after an infusion of 3×105 MBPCs for each mouse. However, to obtain a similar hypoglycemic effect with BM-MSC treatment, the cells need to be purified and sorted with high-aldehyde dehydrogenase activity (ALDHhi), and the dose of cells must achieve to at least 2×105 for each mouse [33]. A 10-fold or more of transplanted UCB-MSCs still could not improve hyperglycemia in NOD mice [34]. So, MBPCs reduced blood glucose more efficiently than BM-MSCs and UCB-MSCs.
Recovery effect of MBPCs to diabetic islet
Pathological and histological analyses showed that diabetic mice without MBPCs had severe histological changes in islet and exocrine tissue. However, in cell-transplanted mice, islet structures and morphology remained normal. This showed that MBPCs had a recovery effect in islet. It has reported that STZ selectively destroyed pancreatic β cells through the generation of reactive oxygen species (ROS) and alkylation of DNA [35,36]. In addition, the oxidative stress (OS) caused by ROS contributed to β-cell death or dysfunction in type 1 diabetes [37–39]. Some studies showed that MSCs are able to resist OS under in vitro culture and ionizing radiation circumstances [40,41]. Valle-Prieto et al. found that human MSCs had a high resistance to OS-induced death by expression of enzymes required to manage OS and basal glutathione (GSx) [42]. The study of Cho et al. proved that MSCs could restore liver injury by promoting an antioxidant response [43]. In view of these results together, the fact that MBPCs have a recovery effect on the pathologic structure of diabetic pancreas possibly has a link with OS.
Endogenous β-cell regeneration stimulated by MBPCs
β-cell regeneration stimulated by MBPCs was another important observation in our study. According to the immunohistochemistry analyses, the islet size and β-cell number were recovered to normal levels. Previous studies have indicated that exogenous human progenitor cells migrated into impaired pancreas and then differentiated into IPCs [34,44,45]. To investigate whether MBPCs differentiate into IPCs, we detected the in vivo distribution and differentiation of MBPCs. The results demonstrated two points: (i) the injury signal can recruit a majority of MBPCs to the diabetic pancreas and make them locate at the ductal, exocrine, and islet structures. In normal mice, although part of cells still survive in the lungs and liver tissues on day 14 after transplantation, their survival time is much shorter than cells in diabetic pancreas. The different survival time of MBPCs between injured pancreas and other normal tissues indicates a sealed dependence relationship between injured tissues and progenitor cells; and (ii) MBPCs do not differentiate into IPCs under this diabetic environment. That MBPCs do not differentiate into IPCs implies an endogenous β-cell regeneration mechanism stimulated by MBPCs.
Endogenous pancreatic progenitors have been identified in adult mice pancreas, which revealed that the adult pancreas retained the potential to reactivate β-cell development. During embryonic islet development, ngn3 is considered as an essential master switch for differentiation of endocrine progenitors [46–48]. To investigate whether the differentiation of pancreatic progenitor cells contributed to endogenous β-cell regeneration, we detected ngn3 and insulin expression by immunostaining in our models. In T1DM mice, ngn3 was activated, but insulin was reduced (Figs. 3d and 6a). This indicated that injury signals could stimulate endogenous progenitor cell differentiation, but have no effect on functional β-cell formation [49]. This result could be confirmed by quantitative analysis. In T1DM mice, although those genes associated with β-cell development (pdx1, foxa2, ngn3, nkx6.1, pax4, and mafb) are not all upregulated at all time points, the expression levels of them were higher than in normal mice in some time points, but almost much lower than in cell-transplanted mice. Gene mafa, insulin, and glut2 (associated with mature β cell) are much less than in cell-transplanted mice and normal mice. Thus, a damage signal of STZ induction can stimulate pancreatic progenitor differentiation, but cannot repair β-cell damage.
In cell-transplanted mice, ngn3 was also activated and was higher than in T1DM mice. Insulin was increased as well. Both higher expression of ngn3 and insulin in cell-transplanted mice indicate that MBPCs promote pancreatic progenitor cell differentiating into β cell. In addition, the results of quantitative analysis further confirm this conclusion. Compared with T1DM mice and normal mice, average levels of the key genes associated with embryonic β-cell development are all the more significantly upregulated in cell-transplanted mice during 28 days after transplantation. Also, the gene levels of insulin, glut2, and mafa (associated with mature β cells) in cell-transplanted mice also significantly increased compared with T1DM mice. Thus, MBPC-promoted pancreatic progenitor differentiation is a possible mechanism of β-cell regeneration in our models. Interestingly, Bell et al. [33] did not detect any ngn3+ cells in diabetic mice and cell-transplanted mice in their study. They demonstrated that another putative neogenic mechanism may contribute to β-cell regeneration. It is noteworthy that we use different cell sources and mouse models. The unique characteristics of MBPCs may stimulate different pathways of β-cell regeneration.
Additionally, we also discovered that in cell-transplanted mice, ngn3+ cells not only resided in the ductal epithelium but also appeared in exocrine tissue and islet, which was in contrast to the viewpoint that ngn3+ progenitor cells associated with the ductal epithelium [49–51]. The result indicates that MBPCs activated both duct progenitors and other population of pancreatic progenitor cells, which are distinct from ductal progenitor cells [52].
Conclusions
Our study reveals a novel promising cell source for cell replacement and regeneration therapy for diabetes mellitus. MBPCs can be easily isolated under a noninvasive manner. After being transplanted in vivo, MBPCs are efficient to reverse hyperglycemia and restore islet structures. We have also discussed the in vivo repair mechanism about MBPC-stimulated β-cell regeneration. Our data demonstrate that MBPCs promote endogenous progenitor cells differentiating into β cells. The β-cell regeneration may be due to the paracrine mechanism of MBPCs [22,53]. Clarifying which factors are involved in MBPC-induced β-cell regeneration will aid the future use of MBPCs to treat diabetes.
Acknowledgments
This work was supported by grants from the National High-tech R&D Program (863 program, no. 2011AA020102 and no. 2012AA020905), the Key Technologies R&D Program of Zhejiang Province (no. 2012C03SA170003), and the Hangzhou Key Technologies R&D Program (no. 20122513A49). The authors also wish to thank Lvyun Zhu and Weiping Chen for their technical support. We also want to thank Yingying Huang at Biochemical platform, Zhejiang University School of Medicine for flow cytometry analysis.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B, Rabelink TJ. and van Zonneveld AJ. (2004). Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes 53:196–199 [DOI] [PubMed] [Google Scholar]
- 2.Laing SP, Swerdlow AJ, Slater SD, Burden AC, Morris A, Waugh NR, Gatling W, Bingley PJ. and Patterson CC. (2003). Mortality from heat diseases in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia 46:760–765 [DOI] [PubMed] [Google Scholar]
- 3.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR. and Shapiro AM. (2005). Five-year follow-up after clinical islet transplantation. Diabetes 54:2060–2069 [DOI] [PubMed] [Google Scholar]
- 4.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM. and Rajotte RV. (2000). Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 343:230–238 [DOI] [PubMed] [Google Scholar]
- 5.Ezquer FE, Ezquer ME, Parrau DB, Carpio D, Yañez AJ. and Conget PA. (2008). Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transplant 14:631–640 [DOI] [PubMed] [Google Scholar]
- 6.Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD. and Prockop DJ. (2006). Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci U S A 103:17438–17443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ende N, Chen R. and Reddi AS. (2004). Effect of human umbilical cord blood cells on glycemia and insulitis in type 1 diabetic mice. Biochem Biophys Res Commun 325:665–669 [DOI] [PubMed] [Google Scholar]
- 8.Ende N, Chen R. and Reddi AS. (2004). Transplantation of human umbilical cord blood cells improves glycemia and glomerular hypertrophy in type 2 diabetic mice. Biochem Biophys Res Commun 321:168–171 [DOI] [PubMed] [Google Scholar]
- 9.Baksh D, Yao R. and Tuan RS. (2007). Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells 25:1384–1392 [DOI] [PubMed] [Google Scholar]
- 10.Kern S, Eichler H, Stoeve J, Klüter H. and Bieback K. (2006). Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24:1294–1301 [DOI] [PubMed] [Google Scholar]
- 11.Stenderup K, Justesen J, Clausen C. and Kassem M. (2003). Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone 33:919–926 [DOI] [PubMed] [Google Scholar]
- 12.Cui CH, Uyama T, Miyado K, Terai M, Kyo S, Kiyono T. and Umezawa A. (2007). Menstrual blood-derived cells confer human dystrophin expression in the murine model of Duchenne muscular dystrophy via cell fusion and myogenic transdifferentiation. Mol Biol Cell 18:1586–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meng X, Ichim TE, Zhong J, Rogers A, Yin Z, Jackson J, Wang H, Ge W, Bogin V, et al. (2007). Endometrial regenerative cells: a novel stem cell population. J Transl Med 5:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel AN, Park E, Kuzman M, Benetti F, Silva FJ. and Allickson JG. (2008). Multipotent menstrual blood stromal stem cells: isolation, characterization, and differentiation. Cell Transplant 17:303–311 [DOI] [PubMed] [Google Scholar]
- 15.Rossignoli F, Caselli A, Grisendi G, Piccinno S, Burns JS, Murgia A, Veronesi E, Loschi P, Masini C, et al. (2013). Isolation, characterization, and transduction of endometrial decidual tissue multipotent mesenchymal stromal/stem cells from menstrual blood. Biomed Res Int 2013:901821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhong Z, Patel AN, Ichim TE, Riordan NH, Wang H, Min WP, Woods EJ, Reid M, Mansilla E, et al. (2009). Feasibility investigation of allogeneic endometrial regenerative cells. J Transl Med 7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nikoo S, Ebtekar M, Jeddi-Tehrani M, Shervin A, Bozorgmehr M, Kazemnejad S. and Zarnani AH. (2012). Effect of menstrual blood-derived stromal stem cells on proliferative capacity of peripheral blood mononuclear cells in allogeneic mixed lymphocyte reaction. J Obstet Gynaecol Res 38:804–809 [DOI] [PubMed] [Google Scholar]
- 18.Murphy MP, Wang H, Patel AN, Kambhampati S, Angle N, Chan K, Marleau AM, Pyszniak A, Carrier E, Ichim TE. and Riordan NH. (2008). Allogeneic endometrial regenerative cells: An “Off the shelf solution”. for critical limb ischemia? J Transl Med 6:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hida N, Nishiyama N, Miyoshi S, Kira S, Segawa K, Uyama T, Mori T, Miyado K, Ikegami Y, et al. (2008). Novel cardiac precursor-like cells from human menstrual blood-derived mesenchymal cells. Stem Cells 26:1695–1704 [DOI] [PubMed] [Google Scholar]
- 20.Borlongan CV, Kaneko Y, Maki M, Yu SJ, Ali M, Allickson JG, Sanberg CD, Kuzmin-Nichols N. and Sanberg PR. (2010). Menstrual blood cells display stem cell–like phenotypic markers and exert neuroprotection following transplantation in experimental stroke. Stem Cells Dev 19:439–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang Z, Hu X, Yu H, Xu Y, Wang L, Chen H, Chen H, Wu R, Zhang Z, et al. (2013). Human endometrial stem cells confer enhanced myocardial salvage and regeneration by paracrine mechanisms. J Cell Mol Med 17:1247–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xavier S, Efi EM, Feng YZ, Erin W. and Hugh ST. (2011). Derivation of insulin producing cells from human endometrial stromal stem cells and use in the treatment of murine diabetes. Mol Ther 19:2065–2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R. and McKay R. (2001). Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science 292:1389–1394 [DOI] [PubMed] [Google Scholar]
- 24.Jiang W, Shi Y, Zhao DZ, Chen S, Yong J, Zhang J, Qing TT, Sun X, Zhang P, et al. (2007). In vitro derivation of functional insulin-producing cells from human embryonic stem cells. Cell Res 17:333–334 [DOI] [PubMed] [Google Scholar]
- 25.Koga H, Muneta T, Ju YJ, Nagase T, Nimura A, Mochizuki T, Ichinose S, von der Mark K. and Sekiya I. (2007). Synovial stem cells are regionally specified according to local microenvironments after implantation for cartilage regeneration. Stem Cells 25:689–696 [DOI] [PubMed] [Google Scholar]
- 26.Wiebke S, Friedrich JH, Marc B, Matthias H, Alexander B, Claudia R, Markus S, Thomas L, Andreas N, et al. (2008). Tracking of human cells in mice. Histochem Cell Bio 130:329–338 [DOI] [PubMed] [Google Scholar]
- 27.Gang EJ, Bosnakovski D, Figueiredo CA, Visser JW. and Perlingeiro RC. (2007). SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood 109:1743–1751 [DOI] [PubMed] [Google Scholar]
- 28.Rosu-Myles M, McCully J, Fair J, Mehic J, Menendez P, Rodriguez R. and Westwood C. (2013). The globoseries glycosphingolipid SSEA-4 is a marker of bone marrow-derived clonal multipotent stromal cells in vitro and in vivo. Stem Cells Dev 22:1387–1397 [DOI] [PubMed] [Google Scholar]
- 29.Huss R. and Moosmann S. (2002). The co-expression of CD117 (c-kit) and osteocalcin in activated bone marrow stem cells in different diseases. Br J Haematol 118:305–312 [DOI] [PubMed] [Google Scholar]
- 30.Romanov YA, Darevskaya AN, Meralikina NV. and Buravkova LB. (2005). Mesenchymal stem cells from human bone marrow and adipose tissue: isolation, characterization, and differentiation potentialities. Bull Exp Biol Med 140:138–143 [DOI] [PubMed] [Google Scholar]
- 31.Grajales L, García J, Banach K. and Geenen DL. (2010). Delayed enrichment of mesenchymal cells promotes cardiac lineage and calcium transient development. J Mol Cell Cardiol 48:735–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu LL, Liu YJ, Yang SG, Zhao QJ, Wang X, Gong W, Han ZB, Xu ZS, Lu YX, et al. (2006). Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica 91:1017–1026 [PubMed] [Google Scholar]
- 33.Bell GI, Broughton HC, Levac KD, Allan DA, Xenocostas A. and Hess DA. (2012). Transplanted human bone marrow progenitor subtypes stimulate endogenous islet regeneration and revascularization. Stem cells Dev 21:97–109 [DOI] [PubMed] [Google Scholar]
- 34.Koblas T, Zacharovová K, Berková Z, Leontovic I, Dovolilová E, Zámecník L. and Saudek F. (2009). In vivo differentiation of human umbilical cord blood-derived cells into insulin-producing β cells. Folia Biol 55:224–232 [PubMed] [Google Scholar]
- 35.Lenzen S. (2008). The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 51:216–226 [DOI] [PubMed] [Google Scholar]
- 36.Szkudelski T. (2001). The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res 50:537–546 [PubMed] [Google Scholar]
- 37.Lenzen S. (2008). Oxidative stress: the vulnerable beta-cell. Biochem Soc Trans 36(Pt 3):343–347 [DOI] [PubMed] [Google Scholar]
- 38.Cnop M, Welsh N, Jonas JC, Jörns A, Lenzen S. and Eizirik DL. (2005). Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 54(Suppl 2):S97–S107 [DOI] [PubMed] [Google Scholar]
- 39.Lenzen S, Drinkgern J. and Tiedge M. (1996). Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med 20:463–466 [DOI] [PubMed] [Google Scholar]
- 40.Halliwell B. and Whiteman M. (2004). Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol 142:231–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen MF, Lin CT, Chen WC, Yang CT, Chen CC, Liao SK, Liu JM, Lu CH. and Lee KD. (2006). The sensitivity of human mesenchymal stem cells to ionizing radiation. Int J Radiat Oncol Biol Phys 66:244–253 [DOI] [PubMed] [Google Scholar]
- 42.Valle-Prieto A. and Conget PA. (2010). Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells Dev 19:1885–1893 [DOI] [PubMed] [Google Scholar]
- 43.Cho KA, Woo SY, Seoh JY, Han HS. and Ryu KH. (2012). Mesenchymal stem cells restore CCl4-induced liver injury byan antioxidative process. Cell Biol Int 36:1267–1274 [DOI] [PubMed] [Google Scholar]
- 44.Hess D, Li L, Martin M, Sakano S, Hill D, Strutt B, Thyssen S, Gray DA. and Bhatia M. (2003). Bone marrow-derived stem cells initiate pancreatic regeneration. Nat Biotechnol 21:763–770 [DOI] [PubMed] [Google Scholar]
- 45.Lin P, Chen L, Yang N, Sun Y. and Xu YX. (2009). Evaluation of stem cell differentiation in diabetic rats transplanted with bone marrow mesenchymal stem cells. J Transplant Proc 41:1891–1893 [DOI] [PubMed] [Google Scholar]
- 46.Kubo A, Stull R, Takeuchi M, Bonham K, Gouon-Evans V, Sho M, Iwano M, Saito Y, Keller G. and Snodgrass R. (2011). Pdx1 and Ngn3 overexpression enhances pancreatic differentiation of mouse ES cell-derived endoderm population. PLoS One 6:e24058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gradwohl G, Dierich A, LeMeur M. and Guillemot F. (2000). Neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci U S A 97:1607–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwitzgebel VM, Scheel DW, Conners JR, Kalamaras J, Lee JE, Anderson DJ, Sussel L, Johnson JD. and German MS. (2000). Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development 127:3533–3542 [DOI] [PubMed] [Google Scholar]
- 49.Xu X, D'Hoker J, Stangé G, Bonné S, De Leu N, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, et al. (2008). β cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 132:197–207 [DOI] [PubMed] [Google Scholar]
- 50.Li WC, Rukstalis JM, Nishimura W, Tchipashvili V, Habener JF, Sharma A. and Bonner-Weir S. (2010). Activation of pancreatic-duct-derived progenitor cells during pancreas regeneration in adult rats. J Cell Sci 123:2793–2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inada A, Nienaber C, Katsuta H, Fujitani Y, Levine J, Morita R, Sharma A. and Bonner-Weir S. (2008). Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci U S A 105:19915–19919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gu G, Dubauskaite J. and Melton DA. (2002). Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 129:2447–2457 [DOI] [PubMed] [Google Scholar]
- 53.Xu YX, Chen L, Wang R, Hou WK, Lin P, Sun L, Sun Y. and Dong QY. (2008). Mesenchymal stem cell therapy for diabetes through paracrine mechanisms. Med Hypotheses 71:390–393 [DOI] [PubMed] [Google Scholar]