Abstract
Skeletal muscle (SkM) comprise ∼40% of human body weight. Injury or damage to this important tissue can result in physical disability, and in severe cases is difficult for its endogenous stem cell—the satellite cell—to reverse effectively. Mesenchymal stem cells (MSC) are postnatal progenitor/stem cells that possess multilineage mesodermal differentiation capacity, including toward SkM. Adult bone marrow (BM) is the best-studied source of MSCs; however, aging also decreases BMMSC numbers and can adversely affect differentiation capacity. Therefore, we asked whether human sources of developmentally early stage mesenchymal stem cells (hDE-MSCs) isolated from embryonic stem cells, fetal bone, and term placenta could be cellular sources for SkM repair. Under standard muscle-inducing conditions, hDE-MPCs differentiate toward a SkM lineage rather than cardiomyocytic or smooth muscle lineages, as evidenced by increased expression of SkM-associated markers and in vitro myotube formation. In vivo transplantation revealed that SkM-differentiated hDE-MSCs can efficiently incorporate into host SkM tissue in a mouse model of SkM injury. In contrast, adult BMMSCs do not express SkM-associated genes after in vitro SkM differentiation nor engraft in vivo. Further investigation of possible factors responsible for this difference in SkM differentiation potential revealed that, compared with adult BMMSCs, hDE-MSCs expressed higher levels of serum response factor (SRF), a transcription factor critical for SkM lineage commitment. Moreover, knockdown of SRF in hDE-MSCs resulted in decreased expression of SkM-related genes after in vitro differentiation and decreased in vivo engraftment. Our results implicate SRF as a key factor in age-related SkM differentiation capacity of MSCs, and demonstrate that hDE-MSCs are possible candidates for SkM repair.
Introduction
Skeletal muscle (SkM) is a major component of the body, comprising ∼40% of adult human weight and responsible for the wide array of conscious motor functions. Injury and damage to this important tissue can lead to disability and poor quality of life. While it has long been known that a resident progenitor/stem cell population—the satellite cell—exists in this tissue, these are very rare cells, and mild to severe SkM injury is still mostly treated conservatively [1]. Thus, there has been interest in using other types of stem cells for SkM repair and regeneration [2], including multilineage mesenchymal stem cells (MSCs). MSCs are post-natal progenitor/stem cells that can differentiate into multiple mesodermal lineages including bone, cartilage, adipose tissue, and some muscle lineages [3,4]. First isolated from the adult bone marrow (BM), MSCs have now been isolated from many other adult organs [5,6]. However, the number of BMMSCs decline with increasing donor age [7] and procurement of these cells from adult organs require invasive procedures. These issues have led researchers to search for developmentally naïve and more accessible sources of MSCs for therapeutic application.
Increasing number of reports show that fetal tissue and fetal extraembryonic tissues such as umbilical cord and placenta are good sources for MSCs [6,8–11]. These developmentally early stage mesenchymal stem cells (DE-MSCs) can be isolated without performing additional invasive procedures, and these are more proliferative than adult BMMSCs [12,13]. Moreover, many DE-MSCs express markers associated with embryonic stem cells (ESCs), likely due to the closer developmental origin of these MSCs to ESCs [11,14]. While it is known that increasing donor age of BMMSCs can limit the therapeutic applicability of these adult-source MSCs by enhancing adipogenic differentiation capacity at the expense of decreasing osteogenic differentiation capacity [15–17], little is known whether muscle differentiation capacity may be affected as well. We therefore studied the in vitro differentiation capacity of diverse sources of human DE-MSCs (hDE-MSCs)—including MSCs derived from human ESCs, human term placenta, and human fetal bone—and adult BMMSCs toward myogenesis. We found that hDE-MSCs most readily differentiated into a SkM lineage rather than cardiomyocytes. To test the in vivo relevance of our in vitro findings, we used a mouse model of SkM injury and applied hDE-MSCs and adult BMMSCs. We found that in both in vitro and in vivo studies, hDE-MSCs but not adult BMMSCs can undergo efficient differentiation into a SkM lineage. To elucidate the reason behind these findings, we examined for relevant molecules and found that serum response factor (SRF)—a key transcription factor in SkM lineage commitment—was involved, with higher baseline expression in hDE-MSCs compared with adult BMMSCs. SRF is known to be a crucial transcription factor for muscle-specific gene expression, involved in the control of MyoD expression in skeletal myoblasts and in satellite cell activation during muscle regeneration [18,19]. Our results demonstrate that hDE-MSCs can be good candidates for SkM regeneration, and the role of SRF in contributing to the efficient SkM differentiation of hDE-MSCs.
Materials and Methods
Cell culture
hDE-MSCs were derived from human ESCs, term placenta, and fetal bone. MSCs were derived from HSF-6 human ESCs (NIH code UC06, obtained from the University of California, San Francisco under a Materials Transfer Agreement) as previously reported [20,21]. Placenta-derived MSCs were isolated from term placenta obtained from healthy mothers with informed consent obtained as approved by the institutional review board [11]. For hDE-MSCs from human fetal bone, the conditionally immortalized human fetal osteoblastic cell line hFOB 1.19 (hFOB; obtained from American Type Culture Collection; Manassas, VA) was utilized. All cells were cultured and characterized for tri-lineage mesodermal differentiation capacity and expression of BMMSC surface markers as previously reported [11,20,22]. Human embryonal rhabdomyosarcoma cell line RD (obtained from Bioresource Collection and Research Center, BCRC, Hsinchu City, Taiwan) was utilized as SkM positive control.
Myogenic differentiation
Cells were plated on 10 cm tissue dishes (4×105 cells/dish) and incubated overnight in expansion medium to allow for adherence. Myogenic differentiation was induced with myogenic-differentiation medium (MM), which consisted of DMEM-low glucose and 2% horse serum (Gibco-Invitrogen, Carlsbad, CA) supplemented with (MM2) or without 5 μM 5-azacytidine (MM1) (Sigma-Aldrich, St. Louis, MO) [23,24].
Reverse transcription–polymerase chain reaction
Reverse transcription–polymerase chain reaction (RT-PCR) was performed as previously reported [22]. Briefly, total RNA was extracted with TRIzol (Gibco-Invitrogen) from cells, and cDNA was synthesized in a 20-μL reaction volume containing 5 μg of total RNA and SuperScript II (Promega Corporation, Madison, WI) reverse transcriptase. The thermal profile for PCR was 95°C for 5 min, followed by 30 cycles at 95°C for 1 min, 55°C for 30 s, 72°C for 40 s, and finally, 72°C for 5 min. The primers used in this study were as follows: β-actin, sense 5′-TGG CAC CAC ACC TTC TAC AAT GAG C-3′, and antisense 5′-GCA CAG CTT CTC CTT AAT GTC ACG C-3′ (PCR product 400 bp); MyoD, sense 5′-AAG CGC CAT CTC TTG AGG TA-3′, and antisense 5′-GCG CCT TTA TT TGA CC-3′ (PCR product 500 bp) [25]; TNT, sense 5′-GGC AGC GGA AGA GG TGC TGAA-3′, antisense 5′-GAG GCA CCA AGT TGG GCA TGA ACG A-3′ (PCR product 150 bp); SRF, sense 5′-TGA GTG CCA CTG GCT TTG AAG AGA-3′, antisense 5′-AGA GGT GCT AGG TGC TGT TTG GAT-3′ (PCR product 146 bp). Quantification of RT-PCR results was performed by using Image J [26] and normalized with the internal control β-actin.
Immunofluorescence staining
Cells were plated onto chamber slides and fixed for 15 min in phosphate-buffered saline (PBS) containing 4% paraformaldehyde and 0.1% Triton×100. After washing thrice with PBS, cells were incubated at 4°C overnight with the primary antibodies directed against α-actinin (1:100; Abcam, Cambridge, United Kingdom), with subsequent incubation with secondary antibodies (1:200) at 37°C for 2 h. Cell nuclei were counterstained with 4′-6-diamidino-2-phenylindole (DAPI) for 5 min and visualized by immunofluorescence (IF) microscopy (Olympus, Tokyo, Japan). Image J Software (Version 1.47; National Institutes of Health, Bethesda, MD) was used to quantify IF results using total cell count using DAPI nuclear staining within the visualized field for normalization.
Western blotting
Western blotting was performed as previously reported [27]. Briefly, cells were washed by PBS and lysed by RIPA buffer (RIPA buffer: 50 mM Tris pH 7.5, 150 mM NaCl, 10 mM EDTA, 1% NP-40, 0.1% sodium dodecyl sulfate (SDS), 1 mM PMSF, and 10 μg/mL aprotinin). Protein samples were applied to 10% SDS–polyacrylamide gel electrophoresis and transferred to nitrocellulose paper. Blots were incubated with primary antibodies against α-actinin (1:1,000) or tubulin (1:3,000; Santa Cruz Biotechnology, Santa Cruz, CA), and further incubated with horseradish peroxidase-conjugated secondary antibodies. Proteins were detected by using electrogenerated chemiluminescence. Quantification of western blotting results was performed by using Image J and normalized with the internal control tubulin.
SkM injury animal model and histology
Nude mice (Nu/Nu; 8-week-old) were purchased from the National Laboratory Animal Center (NLAC, Taipei, Taiwan), and all animal work was performed in accordance with protocols approved by the Institutional Animal Care and Use Committee. SkM injury was induced as previously reported [28]. Briefly, SkM injury was induced by injection of 4% bupivacaine hydrochloride (Sigma-Aldrich) in normal saline (50 μL/mouse) into the midpoint of the tibialis anterior muscle of the mice, with the needle inserted at an angle and advanced proximally following the longitudinal axis of the muscle. Sham injury was induced with injection of normal saline only. After 48 h of muscle injury, 5×105 cells (differentiated or undifferentiated hDE-MSCs or BMMSCS) were injected into midpoint of the injured muscle. Prior to injection, cells were labeled with the fluorescent dye chloromethylbenzamido 1,1′-dioctadecyl-3,3,3′3′- tetramethylindocarbocyanine perchlorate (CM-DiI; Gibco-Invitrogen). Mice were sacrificed for tissue processing 72 h after cell injection. Hematoxylin and eosin (H&E) and Masson Trichrome staining was performed on paraffin-embedded sections. IF staining was performed on OCT (Tissue Tek, Torrance, CA)-embedded frozen sections (6 μm thick), and fixation was performed for 15 min in 4% paraformaldehyde and 0.1% Triton×100. After washing thrice with PBS, tissue sections were incubated at 4°C overnight with the primary antibodies directed against α-actinin, with subsequent incubation with secondary antibodies (1:200) at 37°C for 2 h. Cell nuclei were counterstained with DAPI for 5 min and visualized by IF microscopy. Image J Software was used for quantification, with counts of human cells in mouse SkM tissue frozen sections performed by counting DAPI-stained nuclei within DiI-positive cell outlines; and counts of actinin-positive human cells were performed by counting actinin-positive DAPI-stained nuclei within DiI-positive cell outlines.
Statistics
All data are expressed as mean±standard error of mean from at least three experiments. Statistical analysis was performed using two-tailed Student's paired t-test. In all cases, differences between groups were considered significant when P<0.05.
Results
hDE-MSCs poorly differentiate toward a cardiomyocytic phenotype
We and others have previously shown the surface marker profile and mesodermal trilineage differentiation potential of hDE-MSCs to adipocytes, chondrocytes, and osteoblasts (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/scd) [9,11,21,22,29,30]. The ability of hDE-MSCs to differentiate into various myogenic lineages, however, has not been investigated. We therefore explored the myogenic differentiation capacity of these progenitor cells. It has been reported that low levels of horse serum or 5-azacytidine has the ability to promote BMMSC differentiation into myogenic lineages including cardiomyocytes and SkM [24,31]. To examine whether the hDE-MSCs could differentiate into a cardiomyocytic lineage, we cultured these cells in MM without or with 5-azacytodine supplementation (MM1 and MM2, respectively), assessing first for expression of the cardiac-specific genes cardiac troponin T (TNT) and connexin-43. While slight increases in TNT expression could be found in some of the cells after up to 2 weeks of differentiation, no expression of connexin-43 could be found (Supplementary Fig. S2). Moreover, hDE-MSCs cultured in MM did not undergo spontaneous beating, a common phenomenon seen during in vitro differentiation of stem cells into cardiomyocytes. Thus, we concluded that hDE-MSCs could not differentiate into a mature cardiomyocytic phenotype under MM.
hDE-MSCs express SkM-specific genes after MM differentiating conditions
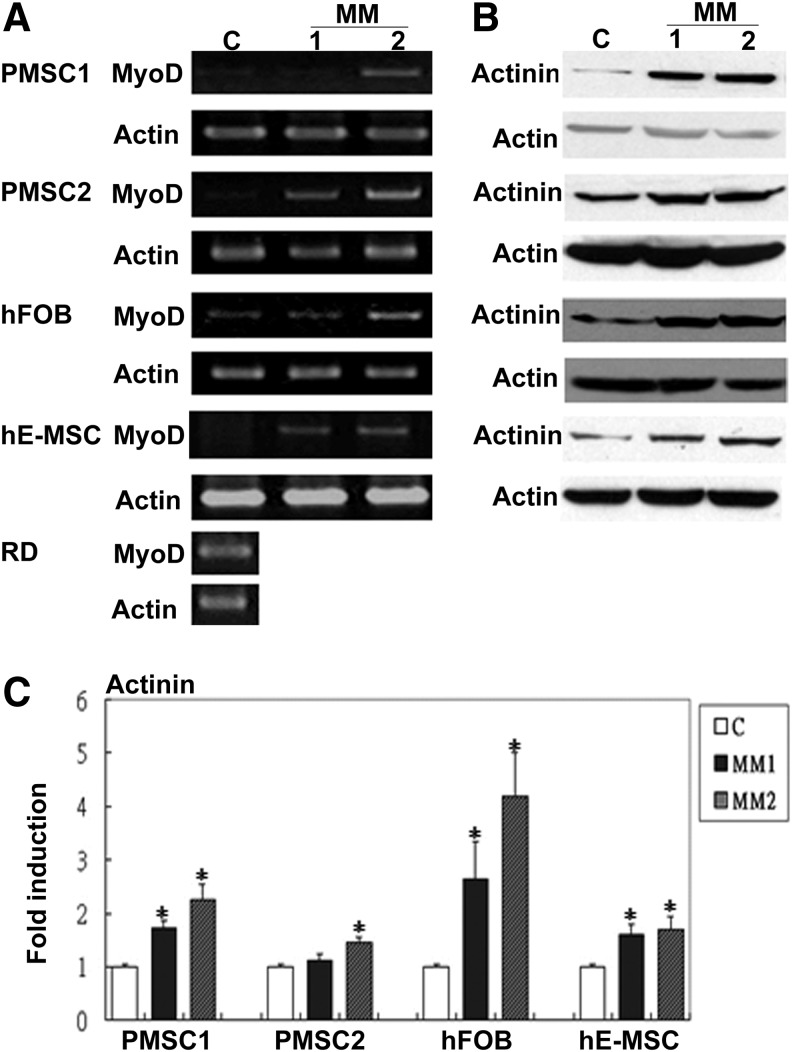
Since MM has been used to also induce other non-cardiomyocytic muscle lineages including SkM [32–34], we then assayed for SkM differentiation of hDE-MSCs under MM conditions. As quickly as 3 days after differentiation, RT-PCR results showed that MyoD, a transcription factor required for the determination of the SkM lineage, was increased by both MM1 and MM2 in hDE-MSCs, with a higher expression level with MM2 (Fig. 1A); other SkM-related genes including Pitx2, Tbx1, Myf5, Mrf4, and Myogenin were also similarly increased in hDE-MSCs after culturing in MM1 and/or MM2 conditions (Supplementary Fig. S3). Western blot results also indicated that expression of the SkM-structural protein, α-actinin [35], in hDE-MSCs was enhanced by MM (Fig. 1B; quantification in Fig. 1C). Thus, hDE-MSCs differentiate toward a SkM lineage under MM conditions.
FIG. 1.
Human sources of developmentally early stage mesenchymal stem cells (hDE-MSCs) express skeletal muscle (SkM)-specific genes after standard myogenic-differentiation medium (MM). (A) Gene expression of SkM transcription factor MyoD in various sources of hDE-MSCs after 3–7 days of induction in MM as assayed by reverse transcription–polymerase chain reaction (RT-PCR); C, control (hDE-MSCs maintain in growth medium); MM1, 2% horse serum; MM2, MM1 with 5-azacytadine (5 μM); placental MSCs (PMSC) (donor 1 and 2); hFOB, hFOB 1.19 cell line; hE-MSCs, human embryonic stem cell-derived MSCs. RD, human rhabdomyosarcoma cells as positive control. (B) Protein expression of SkM-structural protein α-actinin in various sources of hDE-MSCs after 7–14 days of induction in MM1 and MM2 as assayed by western blotting. (C) Quantification of western blot analysis of α-actinin. *P<0.05 compared to control.
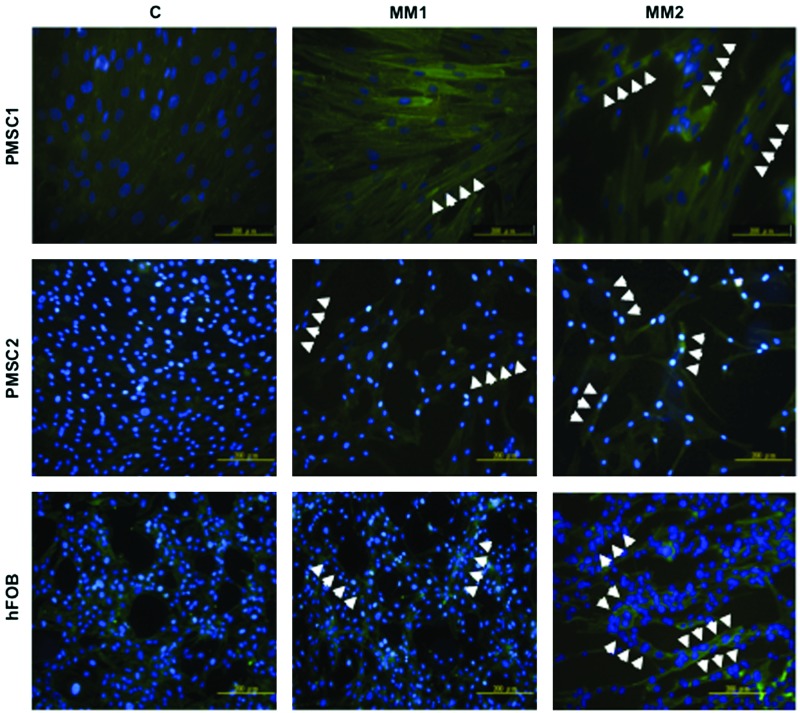
MM conditions induce hDE-MSCs to form myotubes in vitro
To further confirm that hDE-MSCs can differentiate into functional SkM when cultured in MM, we assessed myotube formation, which is a structure comprised of multinucleated cells derived from the fusion of individual SkM cells [36–38]. As showed in Figure 2, hDE-MSCs formed multinucleate myotubes in vitro after culturing for 14 days in MM (white arrowheads), with MM2 being more efficient than MM1. IF staining show the formation of fused cells with multiple nuclei, and increased expression for α-actinin, in cells cultured in MM compared with control medium. Based on the increased expression of various SkM-related proteins and the formation of myotubes in vitro, MM can induce hDE-MSCs to undergo SkM differentiation.
FIG. 2.
MM conditions induce hDE-MSCs to form myotubes in vitro. Immunoflourescence staining for SkM-structural protein α-actinin (FITC) in myotubes. Myotubes were generated from hDE-MSCs (two donors of PMSCs and hFOB) by culturing in MM1 and MM2 for 14 days. Nuclei of cells were stained with 4′-6-diamidino-2-phenylindole (DAPI). White arrow-heads: multi-nucleated cells. Color images available online at www.liebertpub.com/scd
SkM differentiation of hDE-MSCs was associated with suppression of the non-muscle lineage master transcription factors Runx2 and PPAR-γ
MSCs are capable of multilineage differentiation into mesodermal lineages other than muscle cells, including osteoblasts and adipocytes, and as differentiation into one lineage occurs, suppression of differentiation into other lineages occur through the downregulation of master-lineage transcription factors [39]. hDE-MSCs, as a type of MSCs, are capable of multilineage differentiation [9,11,21,22,29,30], and we sought to ascertain the specificity of MM-induced SkM differentiation in these MSCs by assaying for changes in the levels of Runx2 and PPAR-γ, the master lineage transcription factors for osteogenesis and adipogenesis, respectively [40,41]. We found that in all types of hDE-MSCs undergoing SkM differentiation, the expression levels of Runx2 and PPAR-γ are decreased compared with undifferentiated hDE-MSCs (Supplementary Fig. S4). These findings further confirmed that the SkM differentiation induced by MM in hDE-MSCs is specific, and results in lineage commitment away from other non-muscle mesodermal lineages.
SkM-differentiated hDE-MSCs reduce fiber damage in an in vivo model of SkM injury
To investigate whether hDE-MSCs can engraft in SkM in vivo, we established a SkM-injury animal model to test this question [28] (timeline and brief protocol shown in Fig. 3A). We first ascertained whether injury was successfully induced; H&E staining and Masson Trichrome staining—to stain for collagen deposition after injury [42,43]—show fiber disruption and cellular infiltration at 2 days after injury with a large area of fiber necrotic damage seen in mice injected with bupivacaine hydrochloride, an agent that induces SkM necrosis [44], compared with no fiber necrotic damage in sham-injured, normal saline-injected mice. We intramuscularly injected undifferentiated or SkM-differentiated hDE-MSCs at 48 h after injury and found that compared with no injection of cells, injection of hDE-MSCs reduced SkM necrotic damage (Fig. 3B, C; higher magnification shown in Supplementary Fig. S5). Moreover, MM2-differentiated hDE-MSCs appear to reduce the area of SkM fiber necrotic damage more strongly than undifferentiated hDE-MSCs. Thus, it appears that injection of hDE-MSCs can decrease necrotic damage of injured SkM, and results may be enhanced with prior SkM differentiation of these progenitor cells.
FIG. 3.
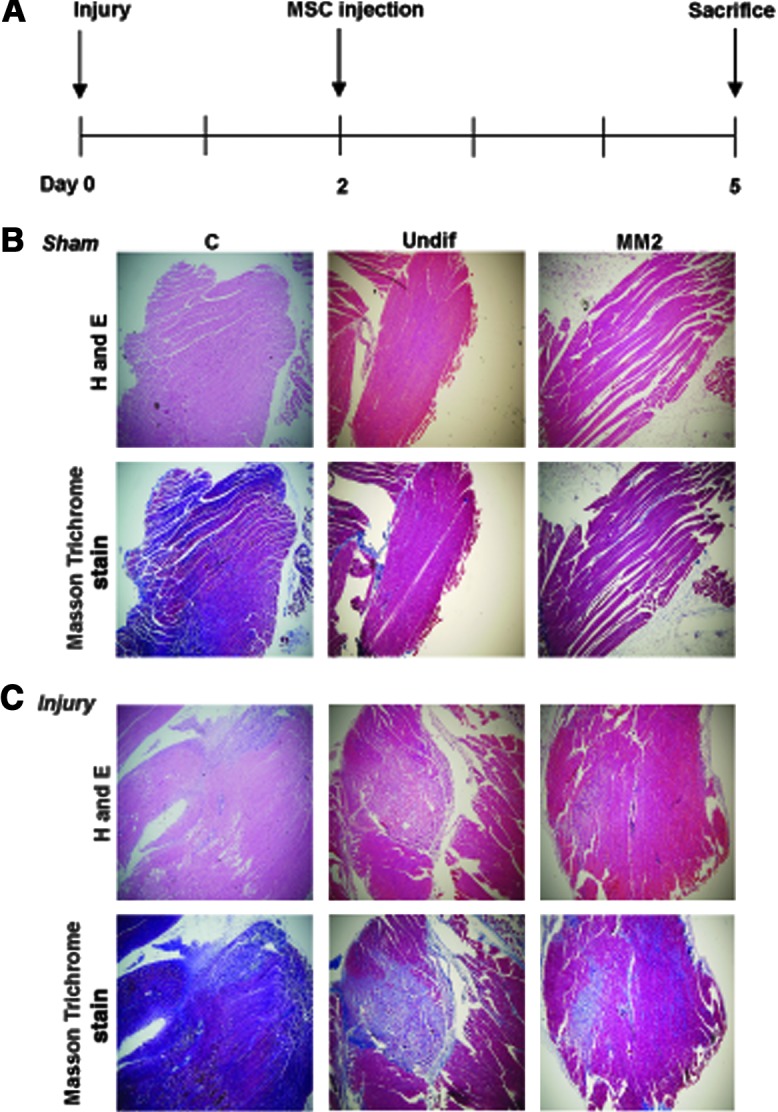
SkM-differentiated hDE-MSCs can reduce fiber necrotic damage an in vivo model of SkM injury. (A) Experimental design of mouse SkM injury model (please see Materials and Methods section for further details). Hematoxylin and eosin and Masson Trichrome staining of SkM tissue paraffin sections from (B) sham (normal saline)-injured (sham) and (C) bupivicaine hydrochloride-injured (injury) mice after local injection of phosphate-buffered saline only (C, control), undifferentiated PMSCs (Undif), or MM2-cultured PMSCs (MM2). Three mice per group; magnification, 50×. Color images available online at www.liebertpub.com/scd
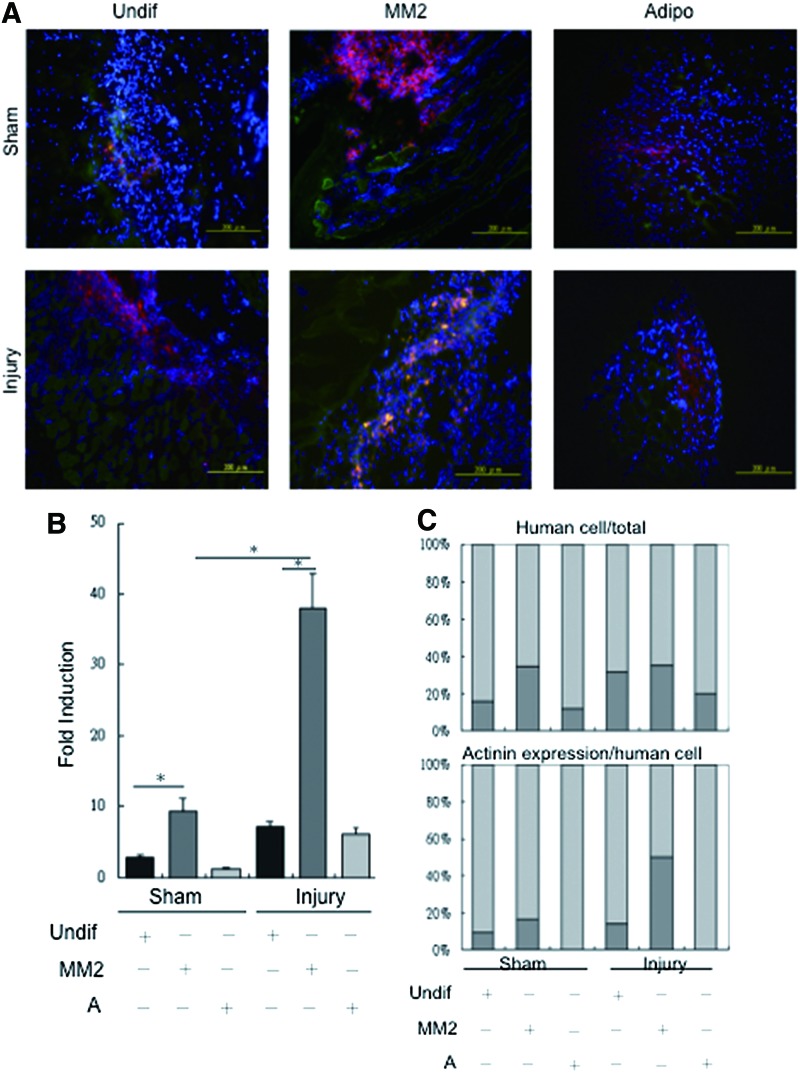
SkM-differentiated hDE-MSCs can engraft in an in vivo model of SkM injury
We next sought to answer whether hDE-MSCs are capable of in vivo engraftment into SkM. A large body of data suggests that in a number of disease models, the paracrine factors secreted by MSCs are actually responsible for the therapeutic effects, rather than the actual cells themselves [6]. To answer this question, hDE-MSCs were stained with the cell tracker DiI dye prior to injection. We intramuscularly injected hDE-MSCs cultured in different conditions—undifferentiated, SkM-differentiated, or adipocyte-differentiated hDE-MSCs—at 48 h after injury to ascertain the specificity of engraftment, with regard to lineage commitment status of the injected stem cells. IF microscopic visualization of tissue sections taken 72 h after cell injection showed the presence of DiI(+) cells (red fluorescence) in both the injured and sham-injured mice, with the tissue section of injured mice showing more DiI(+) cells. Further, cells with co-localization of the SkM-structural protein α-actinin (stained in green fluorescence) are dramatically increased in the injured mice injected with SkM-differentiated hDE-MSCs (Fig. 4A). Quantification for double-positive DiI/α-actinin cells in the tissue sections showed that engraftment of hDE-MSCs in injured mice was generally higher than that for sham-injured mice; specifically, SkM-differentiated hDE-MSCs engrafted at a significantly higher rate compared with undifferentiated hDE-MSCs or adipocyte-differentiated hDE-MSCs (Fig. 4B). While the percentage of engrafted DiI(+) hDE-MSCs regardless of differentiation status in injured mice was generally higher than sham-injured mice (Fig. 4C, upper panel), the proportion of α-actinin(+) cells was highest when SkM-differentiated hDE-MSCs was injected (Fig. 4C, lower panel). In contrast, when adult BMMSCs are injected in this model, we saw only minimal engraftment of human cells (data not shown). Thus, our data suggest that SkM differentiation is efficient prior to using hDE-MSCs as cellular therapeutic agents for SkM injury.
FIG. 4.
SkM-differentiated hDE-MSCs can engraft in an in vivo model of SkM injury. (A) Visualization of tissue sections of sham or injured mice after local injection of hDE-MSCs (labeled by DiI; red fluorescence) and α-actinin (FITC; green fluorescence) by immunofluorescent microscopy with cell nuclei stained with DAPI (blue fluorescence). hDE-MSCs were injected in an undifferentiated state (Undif), after MM2 culture (MM2), or adipogenic differentiation medium (Adipo). (B) Quantification/fold-induction of DiI/α-actinin double-positive cells in tissue sections, as compared to levels in sham mice injected with undifferentiated hDE-MSCs (left-most bar). Quantification was performed by analysis of 12 sections per mouse, with 600–1,000 nuclei counted per section. *P<0.05 compared to undifferentiated hDE-MSCs; n=3 per group. (C) Percentage of DiI(+) human cells in all cells as represented by DAPI staining (upper panel), and percentage of α-actinin(+) cells within DiI(+) human cells (lower panel) in tissue sections. Color images available online at www.liebertpub.com/scd
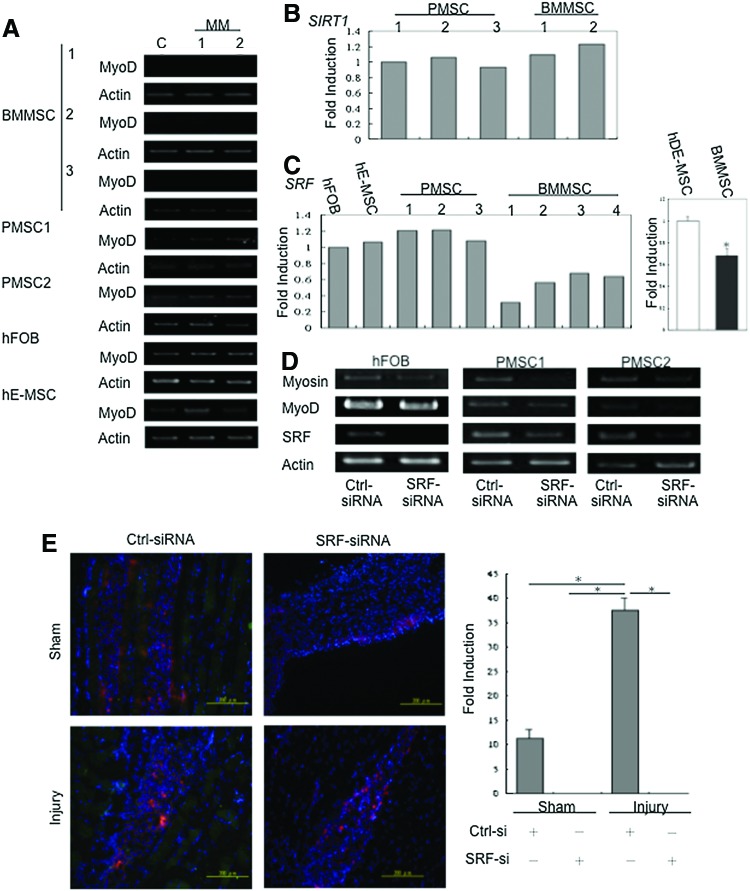
Expression levels of SRF affect the ability of differentiation into SkM in adult BMMSCs compared to hDE-MSCs
Our in vivo data showed that hDE-MSCs can engraft in injured SkM of mice, and that differentiation into a SkM lineage prior to injection also enhanced engraftment and in vivo expression of the SkM-structural protein α-actinin. In contrast, adult BMMSCs, poorly engraft in injured SkM even after in vitro SkM differentiation prior to injection into mice. To elucidate the reason behind these discrepant findings for MSCs from different developmental ages, we went back to test the in vitro SkM differentiation ability of human adult BMMSCs. To our surprise, using the same MM that rapidly induced SkM differentiation in hDE-MSCs, lower levels of MyoD expression was seen with adult BMMSCs than hDE-MSCs (Fig. 5A) and no myotube formation could be elicited (data not shown). We then searched for a molecular reason to explain this discrepant finding. Since adult BMMSCs are clearly obtained from donors who are older than donors for hDE-MSCs, we first assayed for age-related molecules that have been reported to affect SkM. SIRT1, a histone deactylase, which has increasingly been shown to link metabolism with longevity, has been found to be increased in many types of senescent cells [45], including SkM stem cells/satellite cells [46,47]. However, we did not find SIRT1 levels to differ between hDE-MSCs and adult BMMSCs (Fig. 5B). A key marker for SkM is MyoD, and SRF, which belongs to the MCM1-agamous-ARG80-deficiens-SRF (MADS)-box family of transcription factors, is known to directly regulate MyoD expression [48,49]. Very recent studies have suggested that the expression of this important SkM transcription factor is decreased in aged SkM [50,51]. We therefore assessed the role of SRF in the discrepant SkM differentiation capacity of hDE-MSCs versus adult BMMSCs. We found that adult BMMSCs express SRF at approximately only 40% of the level found in hDE-MSCs (Fig. 5C). To further confirm the role of SRF in SkM differentiation of hDE-MSCs, we used specific siRNA to knockdown SRF expression in these cells. We found that after knockdown of SRF, lower levels of SkM-associated genes were expressed by hDE-MSCs undergoing in vitro SkM differentiation (Fig. 5D). These findings were corroborated in the in vivo injury model; injection of SkM-differentiated hDE-MSCs with SRF knockdown showed a lower engraftment rate as seen by the presences of fewer DiI(+) cells, and no expression of α-actinin (Fig. 5E). Thus, our results demonstrate that SRF is involved in the enhanced in vitro and in vivo SkM differentiation capacity of hDE-MSCs.
FIG. 5.
Expression levels of serum response factor (SRF) affect the ability of in vitro and in vivo differentiation into SkM in adult bone marrow mesenchymal stem cells (BMMSCs) compared to hDE-MSCs. (A) Gene expression analysis by RT-PCR for expression of MyoD three donors of adult BMMSCs and various hDE-MSCs. (B) Quantification of SIRT1 gene expression levels in three donors of PMSCs and two donors of adult BMMSCs. (C) Quantification of SRF gene expression levels in various hDE-MSCs (one sample for hFOB; one sample for hE-MSCs; and three donors for PMSCs) and adult BMMSCs (four donors). (D) Gene expression level of SkM-associated genes myosin and MyoD in hDE-MSCs silenced for SRF expression after MM2 induction. Ctrl-siRNA, control (non-target) small interfering RNA; SRF-siRNA, SRF-specific siRNA. (E) Tissue section of sham or injured mice after injection of DiI-labeled hDE-MSCs (red fluorescence) after non-target (Ctrl-siRNA) or SRF-specific siRNA (SRF-siRNA) knockdown. Chart (right side) is quantification of cell engraftment and α-actinin expression (FITC; green fluorescence). *p<0.05, for (C) as compared to control or for (E) between groups as indicated by bars. Color images available online at www.liebertpub.com/scd
Discussion
SkM comprise a major proportion of the body, and injury/damage of this important organ results in reduced mobility, independence, and quality of life. There exist a resident stem cell population, the satellite cell but regeneration from these SkM stem cells may be insufficient if the injury or damage is severe [1]. Thus, there is increasing interest in strategies using other sources of stem cells, and adult BMMSCs are a popular choice due to their accessibility, multilineage differentiation capacity, and close mesodermal tissue origin [52,53]. While it has been long known that these progenitors possess the capacity for SkM differentiation, the data with regard to the use of adult BMMSCs for SkM repair is surprisingly scarce. Our data suggest that the relatively older age of adult BMMSCs decreased the capacity for efficient SkM differentiation, and that SRF play a role in this process. Using many sources of human MSCs from either embryonic, fetal, or postnatal sources, we demonstrate that these hDE-MSCs rapidly differentiated into a SkM lineage under standard general myogenic differentiation in vitro conditions, and engraft in vivo in a mouse model of SkM injury. Lineage commitment of hDE-MSCs into SkM involved SRF, an important SkM-related transcription factor, which is highly expressed in these cells compared with adult BMMSCs. Collectively, our data indicate that hDE-MSCs may be a possible source for SkM repair, and highlight the detrimental effect of aging on SkM lineage commitment as mediated by SRF. The multipotency and accessibility of MSCs have made these cells popular sources for testing in clinical trials [54]. Given that these are primary-isolated cells, the question of whether donor age can affect the differentiation capacity of these somatic stem cells has come into scrutiny [15–17]. A number of molecules and factors have been put forth as important to SkM stem cell capacity, including the Notch ligand Delta-1, Wnt3a of the β-catenin pathway, and Spry1 [55,56]. One molecule recently proposed to play a role in aging of various processes including SkM satellite cells is SIRT1 [46,47]. Known to be important in linking organism aging and metabolism, SIRT1 is a type of histone deacetylase that also can interact and modulate the actions of transcription factors through its deacetylase activity [57]. We have very recently found that replicative senescence of fetal MSCs is associated with decreased expression of SIRT1 [13] and SIRT1 has also been reported to affect lineage commitment in MSCs [58,59]. In this study, however, we did not find SIRT1 levels differ between adult BM and hDE-MSCs (Fig. 5B); rather, we found that levels of SRF, an important transcription factor in the lineage determination of all types of muscle, was higher in hDE-MSCs than its adult counterpart. Indeed, one recent report has shown that in aging mice, the levels of SRF are reduced in SkM; moreover, tissue-specific knockout of SRF in postnatal mice results in an accelerated SkM aging phenotype [50]. Our findings suggest that SRF exerts strong SkM lineage determination effects at the level of the MSC, and support that SRF may be an important factor in the age-related decline of SkM. Further research is clearly indicated to elucidate the molecular mechanisms linking aging to SRF in SkM. One key issue to keep in mind in stem cell therapy for SkM is the impact of the donor, endogenous SkM niche on such strategies, since it is known that an aged SkM adversely affects the regenerative capacity of its endogenous stem cell [60–62]. The use of exogenous sources of stem cells from donors of young age in such an aged SkM environment has not been evaluated for efficacy; more research is needed to test the possible applicability of such a strategy.
We found that standard in vitro conditions using 5-azacytidine for general myogenic differentiation lead to SkM differentiation rather than cardiomyogenesis in hDE-MSCs. 5-azacytidine, a demethylating agent, has been reported to induce BMMSCs to differentiate into various myogenic cell types including cardiomyocytes and SkM [24]. However, the ability of 5-azacytadine to induce different myogenic lineages in MSCs may vary depending on the source of these progenitors, since in another report, only BMMSCs but not umbilical cord- or cord blood-derived MSCs can be induced by this agent into cardiomyocytes [63]. Thus, our data may partially reflect the different tissue-of-origin of the MSCs used in this study. Oxytoxin, a hypothalamic peptide hormone, has also been reported to induce cardiomyocyte lineage differentiation in ESCs [64,65], but we found that oxytocin did not induce hDE-MSCs toward cardiomyogenesis, but rather toward SkM (data not shown). Moreover, the in vitro timeline of SkM differentiation of hDE-MSCs is rather fast, with SkM-related genes being expressed as early as 3 days and proteins seen by 7–12 days, compared with up to 30 days for adult-sources of MSCs [66,67]. The use of horse serum and 5-azacytidine together (as MM2) in our study—both often used alone to induce various myogenic lineage in MSCs—may have also contributed to the increased efficiency. Thus, it may be that these developmentally early sources of MSCs more preferentially differentiate toward SkM. Our in vivo data also helps to support our in vitro findings; however, the transplanted human cells were not seen 15 days after transplantation (data not shown). It is known that despite the use of an immunocompromised mouse strain, engraftment of human cells is still difficult in xenogeneic studies [68–70]. For future applications, therefore, investigation using same-species transplantation models should be carried out in which xenogeneic immune incompatibility is not an issue. Interestingly, of the many reports on cardiomyocyte differentiation from MSCs, there are only a few studies in which both in vitro and in vivo evidence of differentiation are demonstrated, leading to continued controversy in this particular area of MSC research [71,72].
In conclusion, in standard myogenic in vitro differentiation conditions, hDE-MSCs can differentiate rapidly toward a SkM lineage, and engraft in injured SkM in an in vivo mouse model, in contrast to adult BMMSCs. Higher expression of SRF, a transcription factor important in SkM-lineage commitment, in hDE-MSCs may be responsible, since knockdown of SRF in hDE-MSCs abrogated both in vitro SkM differentiation capacity and in vivo SkM marker expression. Our data demonstrate the important role of SRF in SkM differentiation, and the use of hDE-MSCs as possible sources for cell therapy of SkM injury and damage.
Supplementary Material
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Jarvinen TA, Jarvinen TL, Kaanainen M, Kalimo H. and Jarvinen M. (2005). Muscle injuries: biology and treatment. Am J Sports Med 33:745–764 [DOI] [PubMed] [Google Scholar]
- 2.Paylor B, Natarajan A, Zhang RH. and Rossi F. (2011). Nonmyogenic cells in skeletal muscle regeneration. Curr Top Dev Biol 96:139–165 [DOI] [PubMed] [Google Scholar]
- 3.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S. and Marshak DR. (1999). Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147 [DOI] [PubMed] [Google Scholar]
- 4.Minguell JJ, Erices A. and Conget P. (2001). Mesenchymal stem cells. Exp Biol Med 226:507–520 [DOI] [PubMed] [Google Scholar]
- 5.da Silva Meirelles L, Chagastelles PC. and Nardi NB. (2006). Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci 119:2204–2213 [DOI] [PubMed] [Google Scholar]
- 6.Brooke G, Cook M, Blair C, Han R, Heazlewood C, Jones B, Kambouris M, Kollar K, McTaggart S, et al. (2007). Therapeutic applications of mesenchymal stromal cells. Semin Cell Dev Biol 18:846–858 [DOI] [PubMed] [Google Scholar]
- 7.Rao MS. and Mattson MP. (2001). Stem cells and aging: expanding the possibilities. Mech Ageing Dev 122:713–734 [DOI] [PubMed] [Google Scholar]
- 8.Romanov YA, Svintsitskaya VA. and Smirnov VN. (2003). Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells 21:105–110 [DOI] [PubMed] [Google Scholar]
- 9.Fukuchi Y, Nakajima H, Sugiyama D, Hirose I, Kitamura T. and Tsuji K. (2004). Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells 22:649–658 [DOI] [PubMed] [Google Scholar]
- 10.In‘t Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE. and Kanhai HH. (2004). Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 22:1338–1345 [DOI] [PubMed] [Google Scholar]
- 11.Yen BL, Huang HI, Chien CC, Jui HY, Ko BS, Yao M, Shun CT, Yen ML, Lee MC. and Chen YC. (2005). Isolation of multipotent cells from human term placenta. Stem Cells 23:3–9 [DOI] [PubMed] [Google Scholar]
- 12.Guillot PV, Gotherstrom C, Chan J, Kurata H. and Fisk NM. (2007). Human first-trimester fetal MSC express pluripotency markers and grow faster and have longer telomeres than adult MSC. Stem Cells 25:646–654 [DOI] [PubMed] [Google Scholar]
- 13.Ho PJ, Yen ML, Tang BC, Chen CT. and Yen BL. (2013). H2O2 accumulation mediates differentiation capacity alteration, but not proliferative decline, in senescent human fetal mesenchymal stem cells. Antioxid Redox Signal 18:1895–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai MS, Lee JL, Chang YJ. and Hwang SM. (2004). Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum Reprod 19:1450–1456 [DOI] [PubMed] [Google Scholar]
- 15.D'Ippolito G, Schiller PC, Ricordi C, Roos BA. and Howard GA. (1999). Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res 14:1115–1122 [DOI] [PubMed] [Google Scholar]
- 16.Stenderup K, Justesen J, Clausen C. and Kassem M. (2003). Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone 33:919–926 [DOI] [PubMed] [Google Scholar]
- 17.Zhou S, Greenberger JS, Epperly MW, Goff JP, Adler C, Leboff MS. and Glowacki J. (2008). Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell 7:335–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li S, Czubryt MP, McAnally J, Bassel-Duby R, Richardson JA, Wiebel FF, Nordheim A. and Olson EN. (2005). Requirement for serum response factor for skeletal muscle growth and maturation revealed by tissue-specific gene deletion in mice. Proc Natl Acad Sci U S A 102:1082–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guerci A, Lahoute C, Hebrard S, Collard L, Graindorge D, Favier M, Cagnard N, Batonnet-Pichon S, Precigout G, et al. (2012). Srf-dependent paracrine signals produced by myofibers control satellite cell-mediated skeletal muscle hypertrophy. Cell Metab 15:25–37 [DOI] [PubMed] [Google Scholar]
- 20.Yen BL, Chang CJ, Liu KJ, Chen YC, Hu HI, Bai CH. and Yen ML. (2009). Brief report—human embryonic stem cell-derived mesenchymal progenitors possess strong immunosuppressive effects toward natural killer cells as well as T lymphocytes. Stem Cells 27:451–456 [DOI] [PubMed] [Google Scholar]
- 21.Yen ML, Hou CH, Peng KY, Tseng PC, Jiang SS, Shun CT, Chen YC. and Kuo ML. (2011). Efficient derivation and concise gene expression profiling of human embryonic stem cell-derived mesenchymal progenitors (EMPs). Cell Transplant 20:1529–1545 [DOI] [PubMed] [Google Scholar]
- 22.Yen ML, Chien CC, Chiu IM, Huang HI, Chen YC, Hu HI. and Yen BL. (2007). Multilineage differentiation and characterization of the human fetal osteoblastic 1.19 cell line: a possible in vitro model of human mesenchymal progenitors. Stem Cells 25:125–131 [DOI] [PubMed] [Google Scholar]
- 23.Lawson MA. and Purslow PP. (2000). Differentiation of myoblasts in serum-free media: effects of modified media are cell line-specific. Cells Tissues Organs 167:130–137 [DOI] [PubMed] [Google Scholar]
- 24.Burlacu A, Rosca AM, Maniu H, Titorencu I, Dragan E, Jinga V. and Simionescu M. (2008). Promoting effect of 5-azacytidine on the myogenic differentiation of bone marrow stromal cells. Eur J Cell Biol 87:173–184 [DOI] [PubMed] [Google Scholar]
- 25.Mizuno H, Zuk PA, Zhu M, Lorenz HP, Benhaim P. and Hedrick MH. (2002). Myogenic differentiation by human processed lipoaspirate cells. Plast Reconstr Surg 109:199–209; discussion 210–191. [DOI] [PubMed] [Google Scholar]
- 26.Schneider CA, Rasband WS. and Eliceiri KW. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu CC, Chao YC, Chen CN, Chien S, Chen YC, Chien CC, Chiu JJ. and Linju Yen B. (2008). Synergism of biochemical and mechanical stimuli in the differentiation of human placenta-derived multipotent cells into endothelial cells. J Biomech 41:813–821 [DOI] [PubMed] [Google Scholar]
- 28.Hill M. and Goldspink G. (2003). Expression and splicing of the insulin-like growth factor gene in rodent muscle is associated with muscle satellite (stem) cell activation following local tissue damage. J Physiol 549:409–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lian Q, Lye E, Yeo Suan K, Khia Way Tan E, Salto-Tellez M, Liu TM, Palanisamy N, El Oakley RM, Lee EH, Lim B. and Lim SK. (2007). Derivation of clinically compliant MSCs from CD105+, CD24− differentiated human ESCs. Stem Cells 25:425–436 [DOI] [PubMed] [Google Scholar]
- 30.Liu KJ, Wang CJ, Chang CJ, Hu HI, Hsu PJ, Wu YC, Bai CH, Sytwu HK. and Yen BL. (2011). Surface expression of HLA-G is involved in mediating immunomodulatory effects of placenta-derived multipotent cells (PDMCs) towards natural killer lymphocytes. Cell Transplant 20:1721–1730 [DOI] [PubMed] [Google Scholar]
- 31.Wakitani S, Saito T. and Caplan AI. (1995). Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve 18:1417–1426 [DOI] [PubMed] [Google Scholar]
- 32.Secco M, Zucconi E, Vieira NM, Fogaca LL, Cerqueira A, Carvalho MD, Jazedje T, Okamoto OK, Muotri AR. and Zatz M. (2008). Multipotent stem cells from umbilical cord: cord is richer than blood!. Stem Cells 26:146–150 [DOI] [PubMed] [Google Scholar]
- 33.Vieira NM, Brandalise V, Zucconi E, Jazedje T, Secco M, Nunes VA, Strauss BE, Vainzof M. and Zatz M. (2008). Human multipotent adipose-derived stem cells restore dystrophin expression of Duchenne skeletal-muscle cells in vitro. Biol Cell 100:231–241 [DOI] [PubMed] [Google Scholar]
- 34.Pozzobon M, Piccoli M, Ditadi A, Bollini S, Destro R, Andre-Schmutz I, Masiero L, Lenzini E, Zanesco L, et al. (2009). Mesenchymal stromal cells can be derived from bone marrow CD133+ cells: implications for therapy. Stem Cells Dev 18:497–510 [DOI] [PubMed] [Google Scholar]
- 35.Lazarides E. and Burridge K. (1975). Alpha-actinin: immunofluorescent localization of a muscle structural protein in nonmuscle cells. Cell 6:289–298 [DOI] [PubMed] [Google Scholar]
- 36.Biressi S, Molinaro M. and Cossu G. (2007). Cellular heterogeneity during vertebrate skeletal muscle development. Dev Biol 308:281–293 [DOI] [PubMed] [Google Scholar]
- 37.Choi J, Costa ML, Mermelstein CS, Chagas C, Holtzer S. and Holtzer H. (1990). MyoD converts primary dermal fibroblasts, chondroblasts, smooth muscle and retinal pigmented epithelial cells into striated mononucleated myoblasts and multinucleated myotubes. Proc Natl Acad Sci U S A 87:7988–7992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mancini A, Sirabella D, Zhang W, Yamazaki H, Shirao T. and Krauss RS. (2011). Regulation of myotube formation by the actin-binding factor drebrin. Skelet Muscle 1:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee KS, Kim HJ, Li QL, Chi XZ, Ueta C, Komori T, Wozney JM, Kim EG, Choi JY, Ryoo HM. and Bae SC. (2000). Runx2 is a common target of transforming growth factor beta1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol Cell Biol 20:8783–8792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tontonoz P, Hu E. and Spiegelman BM. (1994). Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 79:1147–1156 [DOI] [PubMed] [Google Scholar]
- 41.Ducy P, Schinke T. and Karsenty G. (2000). The osteoblast: a sophisticated fibroblast under central surveillance. Science 289:1501–1504 [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Fu FH. and Huard J. (2005). Cutting-edge muscle recovery: using antifibrosis agents to improve healing. Phys Sportsmed 33:44–50 [DOI] [PubMed] [Google Scholar]
- 43.Luna L. (1968). Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. McGraw-Hill Press, New York, NY [Google Scholar]
- 44.Nonaka I, Takagi A, Ishiura S, Nakase H. and Sugita H. (1983). Pathophysiology of muscle fiber necrosis induced by bupivacaine hydrochloride (Marcaine). Acta Neuropathol 60:167–174 [DOI] [PubMed] [Google Scholar]
- 45.Brooks CL. and Gu W. (2009). How does SIRT1 affect metabolism, senescence and cancer?. Nat Rev Cancer 9:123–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ. and Auwerx J. (2008). Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab 8:347–358 [DOI] [PubMed] [Google Scholar]
- 47.Pardo PS. and Boriek AM. (2011). The physiological roles of Sirt1 in skeletal muscle. Aging 3:430–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gauthier-Rouviere C, Vandromme M, Tuil D, Lautredou N, Morris M, Soulez M, Kahn A, Fernandez A. and Lamb N. (1996). Expression and activity of serum response factor is required for expression of the muscle-determining factor MyoD in both dividing and differentiating mouse C2C12 myoblasts. Mol Biol Cell 7:719–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.L'Honore A, Lamb NJ, Vandromme M, Turowski P, Carnac G. and Fernandez A. (2003). MyoD distal regulatory region contains an SRF binding CArG element required for MyoD expression in skeletal myoblasts and during muscle regeneration. Mol Biol Cell 14:2151–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lahoute C, Sotiropoulos A, Favier M, Guillet-Deniau I, Charvet C, Ferry A, Butler-Browne G, Metzger D, Tuil D. and Daegelen D. (2008). Premature aging in skeletal muscle lacking serum response factor. PLoS One 3:e3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sakuma K, Akiho M, Nakashima H, Akima H. and Yasuhara M. (2008). Age-related reductions in expression of serum response factor and myocardin-related transcription factor A in mouse skeletal muscles. Biochim Biophys Acta 1782:453–461 [DOI] [PubMed] [Google Scholar]
- 52.LaBarge MA. and Blau HM. (2002). Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell 111:589–601 [DOI] [PubMed] [Google Scholar]
- 53.Dezawa M, Ishikawa H, Itokazu Y, Yoshihara T, Hoshino M, Takeda S, Ide C. and Nabeshima Y. (2005). Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science 309:314–317 [DOI] [PubMed] [Google Scholar]
- 54.Le Blanc K. and Mougiakakos D. (2012). Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol 12:383–396 [DOI] [PubMed] [Google Scholar]
- 55.Cosgrove BD, Sacco A, Gilbert PM. and Blau HM. (2009). A home away from home: challenges and opportunities in engineering in vitro muscle satellite cell niches. Differentiation 78:185–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brack AS. and Rando TA. (2012). Tissue-specific stem cells: lessons from the skeletal muscle satellite cell. Cell Stem Cell 10:504–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guarente L. (2011). H Franklin. Epstein Lecture: Sirtuins, aging, and medicine. N Engl J Med 364:2235–2244 [DOI] [PubMed] [Google Scholar]
- 58.Backesjo CM, Li Y, Lindgren U. and Haldosen LA. (2006). Activation of Sirt1 decreases adipocyte formation during osteoblast differentiation of mesenchymal stem cells. J Bone Miner Res 21:993–1002 [DOI] [PubMed] [Google Scholar]
- 59.Tseng PC, Hou SM, Chen RJ, Peng HW, Hsieh CF, Kuo ML. and Yen ML. (2011). Resveratrol promotes osteogenesis of human mesenchymal stem cells by upregulating RUNX2 gene expression via the SIRT1/FOXO3A axis. J Bone Miner Res 26:2552–2563 [DOI] [PubMed] [Google Scholar]
- 60.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL. and Rando TA. (2005). Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433:760–764 [DOI] [PubMed] [Google Scholar]
- 61.Conboy IM. and Rando TA. (2005). Aging, stem cells and tissue regeneration: lessons from muscle. Cell Cycle 4:407–410 [DOI] [PubMed] [Google Scholar]
- 62.Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C. and Rando TA. (2007). Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317:807–810 [DOI] [PubMed] [Google Scholar]
- 63.Martin-Rendon E, Sweeney D, Lu F, Girdlestone J, Navarrete C. and Watt SM. (2008). 5-Azacytidine-treated human mesenchymal stem/progenitor cells derived from umbilical cord, cord blood and bone marrow do not generate cardiomyocytes in vitro at high frequencies. Vox Sang 95:137–148 [DOI] [PubMed] [Google Scholar]
- 64.Paquin J, Danalache BA, Jankowski M, McCann SM. and Gutkowska J. (2002). Oxytocin induces differentiation of P19 embryonic stem cells to cardiomyocytes. Proc Natl Acad Sci U S A 99:9550–9555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hatami L, Valojerdi MR. and Mowla SJ. (2007). Effects of oxytocin on cardiomyocyte differentiation from mouse embryonic stem cells. Int J Cardiol 117:80–89 [DOI] [PubMed] [Google Scholar]
- 66.Bossolasco P, Corti S, Strazzer S, Borsotti C, Del Bo R, Fortunato F, Salani S, Quirici N, Bertolini F, et al. (2004). Skeletal muscle differentiation potential of human adult bone marrow cells. Exp Cell Res 295:66–78 [DOI] [PubMed] [Google Scholar]
- 67.Liu Y, Yan X, Sun Z, Chen B, Han Q, Li J. and Zhao RC. (2007). Flk-1+ adipose-derived mesenchymal stem cells differentiate into skeletal muscle satellite cells and ameliorate muscular dystrophy in mdx mice. Stem Cells Dev 16:695–706 [DOI] [PubMed] [Google Scholar]
- 68.Huard J, Roy R, Guerette B, Verreault S, Tremblay G. and Tremblay J. (1994). Human myoblast transplantation in immunodeficient and immunosuppressed mice: evidence of rejection. Muscle Nerve 17:224–234 [DOI] [PubMed] [Google Scholar]
- 69.Swijnenburg RJ, Schrepfer S, Govaert JA, Cao F, Ransohoff K, Sheikh AY, Haddad M, Connolly AJ, Davis MM, Robbins RC. and Wu JC. (2008). Immunosuppressive therapy mitigates immunological rejection of human embryonic stem cell xenografts. Proc Natl Acad Sci U S A 105:12991–12996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM. and Morrison SJ. (2008). Efficient tumour formation by single human melanoma cells. Nature 456:593–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rosenzweig A. (2006). Cardiac cell therapy—mixed results from mixed cells. N Engl J Med 355:1274–1277 [DOI] [PubMed] [Google Scholar]
- 72.Segers VF. and Lee RT. (2008). Stem-cell therapy for cardiac disease. Nature 451:937–942 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.