Abstract
Bcl-2 is overexpressed in about a half of human cancers and 50–70% of breast cancer patients, thereby conferring resistance to conventional therapies and making it an excellent therapeutic target. Small interfering RNA (siRNA) offers novel and powerful tools for specific gene silencing and molecularly targeted therapy. Here, we show that therapeutic silencing of Bcl-2 by systemically administered nanoliposomal (NL)-Bcl-2 siRNA (0.15 mg siRNA/kg, intravenous) twice a week leads to significant antitumor activity and suppression of growth in both estrogen receptor-negative (ER(−)) MDA-MB-231 and ER-positive (+) MCF7 breast tumors in orthotopic xenograft models (P < 0.05). A single intravenous injection of NL-Bcl-2-siRNA provided robust and persistent silencing of the target gene expression in xenograft tumors. NL-Bcl-2-siRNA treatment significantly increased the efficacy of chemotherapy when combined with doxorubicin in both MDA-MB-231 and MCF-7 animal models (P < 0.05). NL-Bcl-2-siRNA treatment-induced apoptosis and autophagic cell death, and inhibited cyclin D1, HIF1α and Src/Fak signaling in tumors. In conclusion, our data provide the first evidence that in vivo therapeutic targeting Bcl-2 by systemically administered nanoliposomal-siRNA significantly inhibits growth of both ER(−) and ER(+) breast tumors and enhances the efficacy of chemotherapy, suggesting that therapeutic silencing of Bcl-2 by siRNA is a viable approach in breast cancers.
Keywords: apoptosis, autophagy, Bcl-2, breast cancer, chemotherapy, estrogen receptor, gene silencing, liposome, nanoparticles, nanotherapeutics, programmed cell deathsiRNA
Introduction
The Bcl-2 oncogene is overexpressed in 50–70% of all human cancers, including breast cancers, and is associated with an aggressive clinical course and poor survival.1,2,3,4,5,6,7 The Bcl-2 family comprises prosurvival antiapoptotic proteins (Bcl-2, Bcl-xL, Mcl-1, Bcl-w, and A-1) and proapoptotic proteins (Bax, Bak, Bik, Bad, Bid, HRK, BMF, NOXA, and PUMA).1,2 The Bcl-2 family can be defined by the presence of conserved motifs known as Bcl-2 homology domains (BH1 to BH4). Bcl-2 contains all four BH domains, whereas the other prosurvival members contain at least BH1 and BH2.1 The Bcl-2 gene codes for a 25-kDa antiapoptotic protein that promotes cell survival and neoplastic cell expansion.3,4,5,6,7,8 Inhibition of Bcl-2 enhances the sensitivity of cancer cells to standard therapies,8,9 thereby indicating the importance of this gene as a potential therapeutic target in various human cancers.
RNA interference, a recently discovered natural process of gene silencing, emerged as an important tool for sequence-specific gene knockdown and is considered to hold great promise for developing targeted molecular therapies for cancer and other diseases associated with increased gene expression as well as viral infections.10 RNA interference mediated by small interfering RNA (siRNA) can specifically knock down target gene expression via DICER and the RNA-induced silencing complex, causing degradation of the mRNA and preventing the corresponding protein expression.10 Although siRNA has been shown to target and silence genes, in vivo delivery of siRNA to tumors remains as a great challenge. The major limitations to translate siRNA-based therapies into the clinic include the degradation of siRNA by nucleases after systemic administration, poor cellular uptake, and a lack of effective systemic delivery methods that are safe and nontoxic.11 Although nanocarriers, including liposomes, are not specifically targeted to tumor cells, they passively accumulate in tumor tissues owing to an enhanced permeability and retention effect and the leaky and disorganized nature of angiogenic tumor vasculature. Typically tumor-associated endothelium contains openings varying in size from 50 to >500 nm, in contrast to normal endothelium with pores (fenestra) of 10–50 nm.12 Cationic (positively charged) liposomes have traditionally been used in in vitro and in vivo gene transfections. However, applications of cationic liposomes in humans are limited because of their toxicity to mammalian cells owing to reactive oxygen species induction and lung inflammation.13 If successfully and safely administered, siRNA-based therapies have advantages in drug development over small molecules, biological agents, antisense oligonucleotides and antibodies because they can target “undruggable” targets, which comprise more than two-thirds of the oncogenic targets. Furthermore, siRNA is highly specific, easily synthesized, and cost effective.11,12 In addition, siRNA-mediated target gene silencing is significantly more potent (more than 100-fold difference in the half maximal inhibitory concentration) and efficient than antisense oligonucleotides or ribozymes.14
Autophagy is a lysosomal degradation pathway that is a major cellular process for degradation of cytoplasmic organelles and long-lived, misfolded, or damaged proteins.15 Autophagy is mediated by a set of conserved genes called ATG, including Beclin 1 (ATG6), ATG5 and ATG8 (LC3), and others.15 Autophagy is induced by nutrient and energy deprivation and metabolic stress and may function as a protective and prosurvival mechanism.16 Autophagy induction can lead to cell death, also known as autophagic cell death (type II programmed cell death), depending on the cellular context and stimulus.15,16,17,18,19,20 Bcl-2 inhibits the autophagic process by physically binding to Beclin-1, an autophagy-promoting protein, and limiting its function.21 Inhibition of Bcl-2 leads to autophagic cell death in MCF7 breast cancer cells.17 Furthermore, recent data suggest that the oncogenic effect of Bcl-2 arises from its ability to inhibit autophagy but not apoptosis, thereby indicating that modulating autophagy may be important in designing anticancer therapies.22
In this study, we sought to determine whether therapeutic silencing of Bcl-2 by systemic i.v. administration of nanoliposomal siRNA provides effective gene silencing, inhibits tumor growth and further enhances the efficacy of the most commonly used chemotherapeutic agents (doxorubicin and paclitaxel) in both estrogen receptor-negative (ER (−)) and ER-positive (+) orthotopic breast tumors in nude mice. To our knowledge, our findings are the first evidence that in vivo targeting of Bcl-2 suppresses the growth of ER(−) and ER(+) breast tumors in orthotopic xenografts via the induction of both apoptotic and autophagic cell death, thereby suggesting that in vivo inhibition of Bcl-2 is a viable clinically therapeutic approach and may prevent disease progression.
Results
In vitro Bcl-2 silencing leads to inhibition of cell growth and colony formation in ER(−) breast cancer cells
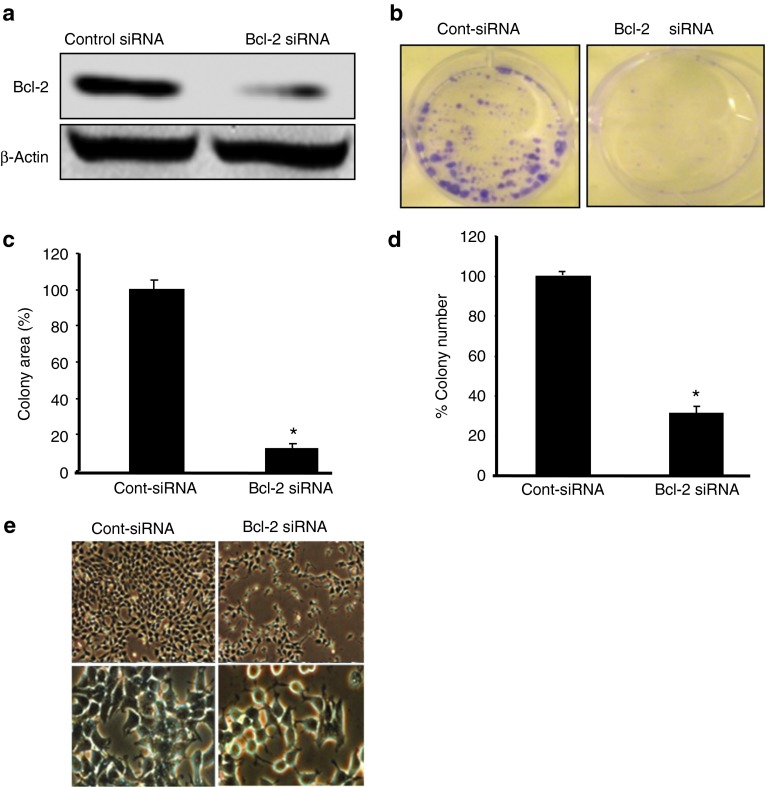
Bcl-2 positivity is associated with poor survival and tumor aggression in ER(−) and triple-negative breast cancer patients,7 indicating that Bcl-2 may be a potential therapeutic target in these tumors. We previously showed that in vitro silencing of Bcl-2 by siRNA inhibited the proliferation and colony formation of ER(+) MCF7 breast cancer cells.17 Thus, in the present study, we sought to determine the effects of Bcl-2 silencing on the proliferation and colony formation of ER(−) MDA-MB-231 cells. The clonogenic assay is an in vitro cell survival assay that is based on the ability of a single cell to grow into a colony in 2–3 weeks.18 Using a specific Bcl-2 siRNA,17 we first showed that Bcl-2 siRNA (50 nmol/l, 48 hours) significantly inhibits Bcl-2 expression in MDA-MB-231 cells by western blot analysis (Figure 1a). Furthermore, Bcl-2 silencing significantly reduced the total colony area (88%) (Figure 1b) and the number (69%) of MDA-MB-231 colonies (Figure 1c) compared with cells treated with nonsilencing control siRNA (P < 0.0049 and P < 0.006, respectively). Bcl-2 siRNA treatment also resulted in the detachment of cells from the surface of the cell culture flask, and cell death was detected via phase-contrast light microscopy (Figure 1d).
Figure 1.
Silencing of Bcl-2 by a specific siRNA inhibits proliferation and colony formation of ER(−) breast cancer cells. (a) MDA-MB-231 cells were treated with control or Bcl-2 siRNA for 48 hours and analyzed using anti-Bcl-2 monoclonal antibody by western blot analysis. (b) Silencing of Bcl-2 by siRNA inhibits size and number of colonies formed by MDA-MB-231 cells. Cells were treated with Bcl-2 or control siRNA once a week and colonies were detected 2 weeks later. Bcl-2 silencing significantly reduced colony size and area (88%, P < 0.0049) (c) and the colony number (69%, P < 0.006) (d) of MDA-MB-231 colonies as compared with nonsilencing control siRNA-treated cells (*P < 0.05). (e) Morphological appearance of breast cancer cells treated with Bcl-2 siRNA by phase contrast microscopy (72 hour-MCF7) at 10 and 40× magnification.
Dose- and time-dependent kinetics of Bcl-2 downmodulation in ER(−) MDA-MB-231 breast tumor xenografts after systemic i.v. administration of nanoliposomal (NL)-Bcl-2-siRNA
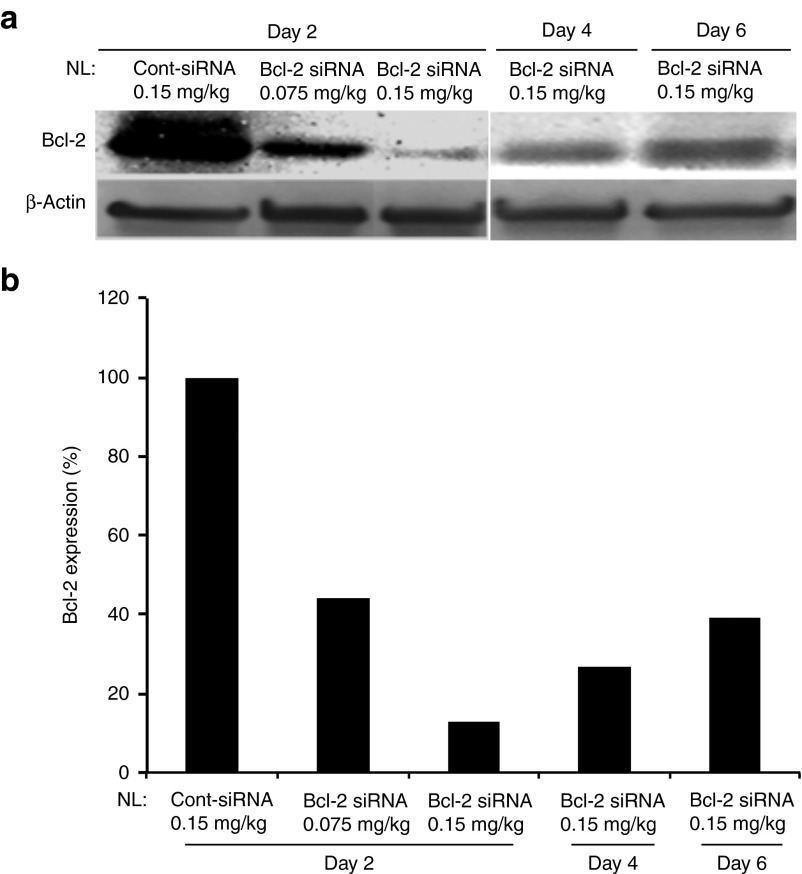
Before determining the effects of in vivo therapeutic silencing of Bcl-2 by siRNA in breast tumors, we first evaluated the in vivo kinetics of Bcl-2 downmodulation in MDA-MB-231 tumors in an orthotopic xenograft model in mice following systemically administered NL-Bcl-2 siRNA. Mice were injected with a single i.v. dose of NL-Bcl-2-siRNA at 0.075, 0.15, 0.30, and 0.60 mg/kg from tail vein as described in “Materials and Methods.” Tumors were collected at 2, 4, and 6 days after injections. Western blot analysis revealed a significant reduction in Bcl-2 protein expression in tumors treated with 0.15 mg/kg or more of NL-Bcl-2 siRNA (Figure 2a, b). The higher Bcl-2 siRNA doses (0.30 and 0.60 mg/kg) resulted in slightly better downmodulation of Bcl-2 after a single injection (Supplementary Figure 1A, online). NL-Bcl-2 siRNA at 0.15 mg/kg provided robust target inhibition on days 2, 4, and 6 (94, 83, and 64.8%, respectively) compared with control siRNA treatment. Therefore, 0.15 mg siRNA/kg was selected as an optimal lowest dose of NL-Bcl-2 siRNA for the subsequent in vivo experiments.
Figure 2.
Time- and dose-dependent kinetics of Bcl-2 inhibition by systemically administered nanoliposomal (NL)-Bcl-2-siRNA in MDA-MB-231 orthotopic xenograft model. (a) Mice-bearing MDA-MB-231 tumors were injected with a single i.v. dose of NL-Control-siRNA or NL-Bcl-2-siRNA (0.075 or 0.15 mg siRNA/kg from tail vein) and tumors were removed on days 2, 4 and 6. Inhibition of Bcl-2 protein expression was detected by western blot analysis of tumor lysates. (b) Inhibition of Bcl-2 protein expression by densitometric analysis of bands shown in 1A tumors.
Systemic administration of NL-Bcl-2-siRNA twice a week inhibits the growth of ER(−) MDA-MB-231 breast tumors in nude mice
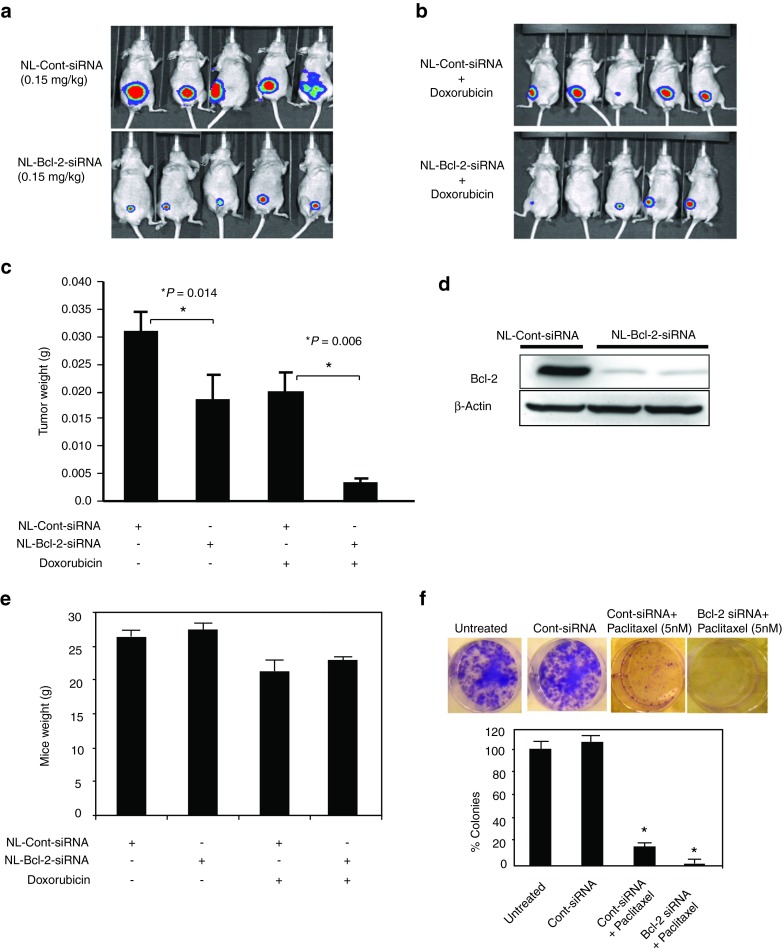
The antitumor efficacy of therapeutic Bcl-2 gene silencing by systemic administration of siRNA in ER(−) breast tumors is currently unknown. Thus, we investigated the effects of NL-Bcl-2-siRNA therapy in an MDA-MB-231 model. About 2 weeks after orthotopic injection of tumor cells into their mammary fat pads, mice-bearing equally sized MDA-MB-231 tumors were randomly assigned to two groups (n = 5).23 Mice were injected with either NL-Bcl-2-siRNA or NL-nonsilencing control siRNA (0.15 mg/kg, i.v., from tail vein, twice a week) for 4 weeks. Mice treated with NL-Bcl-2-siRNA had significantly smaller tumors than the mice that received NL-control-siRNA (P = 0.014; Figure 3a, c). Even three i.v. injections of NL-Bcl-2 siRNA (0.15 mg/kg) resulted significantly inhibited the growth of MDA-MB-231 tumors compared with NL-control siRNA treatment (P < 0.05; Supplementary Figure 2, online).
Figure 3.
Therapeutic silencing of Bcl-2 by NL-Bcl-2 siRNA inhibits in vivo tumor growth of ER(−) MDA-MB-231 xeonografts in nude mice. (a) Mice were orthotopically injected into mammary fat pat with luciferase-expressing MDA-MB-231 cells. Mice-bearing tumors with equal size were randomly assigned into control and treatment groups (n = 5 mice per group). Mice received either NL-Bcl-2 siRNA or NL-control siRNA treatments (0.15 mg siRNA/kg or 4 µg/mouse, i.v, twice a week) from tail vein for 4 weeks (total of eight injections). After 4-weeks of treatments, mice were imaged following luciferin injection (i.p.) by Xenogen-IVIS live-imaging system under anesthesia. (b) Therapeutic silencing of Bcl-2 by NL-Bcl-2 siRNA enhances the antitumor efficacy of doxorubicin. Mice were treated with NL-Bcl-2-siRNA or NL-control siRNA (0.15 mg siRNA/kg, i.v, twice a week) and also received doxorubicin (4 mg/kg, i.p, once a week) for 4 weeks and imaged. (c) Mice that were treated with NL-Bcl-2 siRNA had significantly reduced tumor weight compared with the control group that was treated with NL-Control siRNA. Enhanced the antitumor efficacy of doxorubicin was observed when combined with NL-Bcl-2 siRNA. The tumor weights were measured 4 weeks of treatments shown in Figure 3a, b. (d) Bcl-2 protein expression after 4 weeks of treatments in MDA-MB-231 tumors. After sacrificing mice, tumors were removed (48 hours after the last treatment) and tumor lysates were analyzed for Bcl-2 expression by western blot. (e) NL-Bcl-2-siRNA treatment was well tolerated and did not cause weight lose in mice, compared with those that received NL-control siRNA. Mice that received doxorubicin had slight reduced weight loss compared with those that did not receive chemotherapy. (f) In vitro silencing of Bcl-2 by siRNA treatment increases the antiproliferative effects of paclitaxel and inhibit colony formation of MDA-MB-231 cells.
Therapeutic silencing of Bcl-2 by NL-Bcl-2-siRNA enhances the antitumor efficacy of chemotherapy in an ER(−) MDA-MB-231 model
To evaluate the in vivo effects of siRNA-induced Bcl-2 silencing on the antitumor efficacy of chemotherapy, we also combined NL-Bcl-2 siRNA with weekly doxorubicin (4 mg/kg, i.p.), one of the most commonly used chemotherapeutic agents. Mice that received the combination of NL-Bcl-2-siRNA and doxorubicin had significantly smaller tumors than the control group that received NL-control siRNA and doxorubicin (P = 0.006; Figure 3b, c). As expected, a marked inhibition of Bcl-2 protein expression was observed in MDA-MB-231 tumors after 4 weeks of NL-Bcl-2 siRNA treatment (Figure 3d). No toxicity was observed in mice exposed to NL-Bcl-2 siRNA for 4 weeks (Figure 3e). Mice appeared healthy and active and showed no apparent side effects after treatment with NL-Bcl-2 siRNA (Figure 3e). The mean weight in the NL-Bcl-2 siRNA-treated group was 27.5 ± 0.7 g and did not statistically differ from that in the NL-control-siRNA group (28.6 ± 0.5 g). However, as expected, mice that received doxorubicin were slightly smaller after treatment.
Furthermore, we also sought to determine whether the silencing of Bcl-2 by siRNA can increase the activity of chemotherapeutic agents other than doxorubicin and assessed the effects of paclitaxel in combination with Bcl-2 siRNA. The combination of Bcl-2 silencing with paclitaxel significantly reduced the growth and colony formation of MDA-MB-231 cells in vitro, suggesting that siRNA-mediated Bcl-2 silencing can enhance the efficacy of other commonly used chemotherapeutic agents.
Therapeutic targeting of Bcl-2 by NL-Bcl-2-siRNA inhibits tumor growth of ER(+) MCF-7 breast tumors and increases the efficacy of chemotherapy
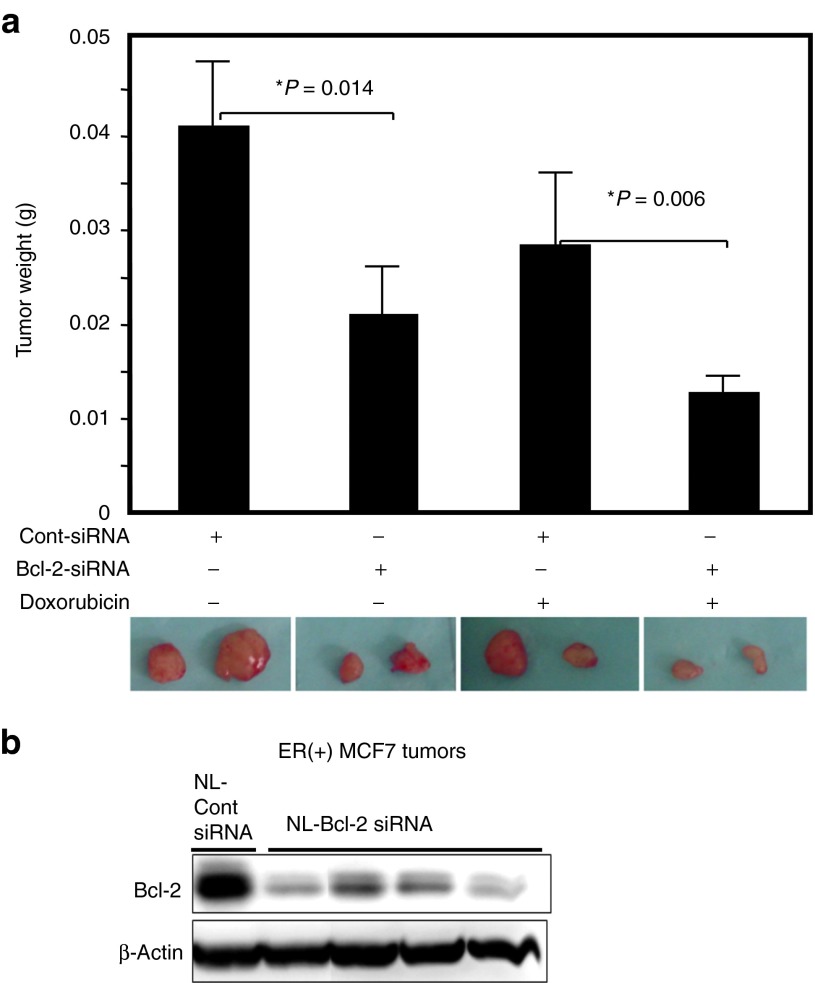
Because no published study has assessed the in vivo effects of siRNA-mediated therapeutic Bcl-2 silencing in ER(+) breast tumors, we also investigated the antitumor efficacy of NL-siRNA treatment in an MCF-7 orthotopic tumor model in nude mice. About 2 weeks after tumor cells were injected into their mammary fat pads, mice with equally sized tumors were randomly split into groups and given either NL-Bcl-2 siRNA or NL-control siRNA (0.15 mg siRNA/kg, i.v. tail vein, twice a week) for 4 weeks. Tumor growth was significantly inhibited in mice treated with NL-Bcl-2 siRNA (Figure 4a). The mean tumor weight in the NL-Bcl-2 siRNA-treated group was significantly lower than that in the control group (P = 0.034). When with weekly doxorubicin (4 mg/kg, i.p.) was added, NL-Bcl-2 siRNA-treated mice had significantly smaller tumors than NL-control siRNA-treated mice (P = 0.006; Figure 4a; Supplementary Figure 3, online (treatment plan)). However, compared with the ER(−) model, this effect was slightly less observed in ER(+) model. Western blot analysis using lysates from MCF-7 tumors collected at the end of 4 weeks of treatment with NL-Bcl-2 siRNA revealed a significant reduction in Bcl-2 expression (Figure 4b). These data suggest that therapeutic silencing of Bcl-2 by NL-siRNA is an effective strategy for inhibiting tumor growth and increasing the efficacy of chemotherapy for ER(+) breast tumors.
Figure 4.
In vivo therapeutic targeting of Bcl-2 by nanoliposomal siRNA inhibits growth of ER(+) MCF-7 tumors and increases the activity of chemotherapy in an orthotopic xenograft model in mice. (a) About 2 weeks after tumor cell injection, mice-bearing equal size of MCF-7 tumors were randomly assigned to groups (n = 6) and treated with either NL-Bcl-2-siRNA or NL-control siRNA alone (0.15 mg siRNA/kg, i.v, twice a week) or in combination with doxorubicin (3 mg/kg, i.p, once a week) for 4 weeks. Mice treated with NL-Bcl-2 siRNA alone and NL-Bcl-2 siRNA and doxorubicin had significantly smaller tumor xenografts when compared with the control group (P = 0.014 and P = 0.006, respectively) (*P < 0.05). The representative tumors from each treatment group is shown below the chart. (b) Mice treated with NL-Bcl-2 siRNA (4 weeks) showed marked inhibition of Bcl-2 protein in MCF-7 tumors. Tumors were collected at the end of 4 weeks of treatment (a) and analyzed by western blot.
In vivo therapeutic targeting of Bcl-2 induces autophagy in ER(−) and ER(+) breast tumor xenografts
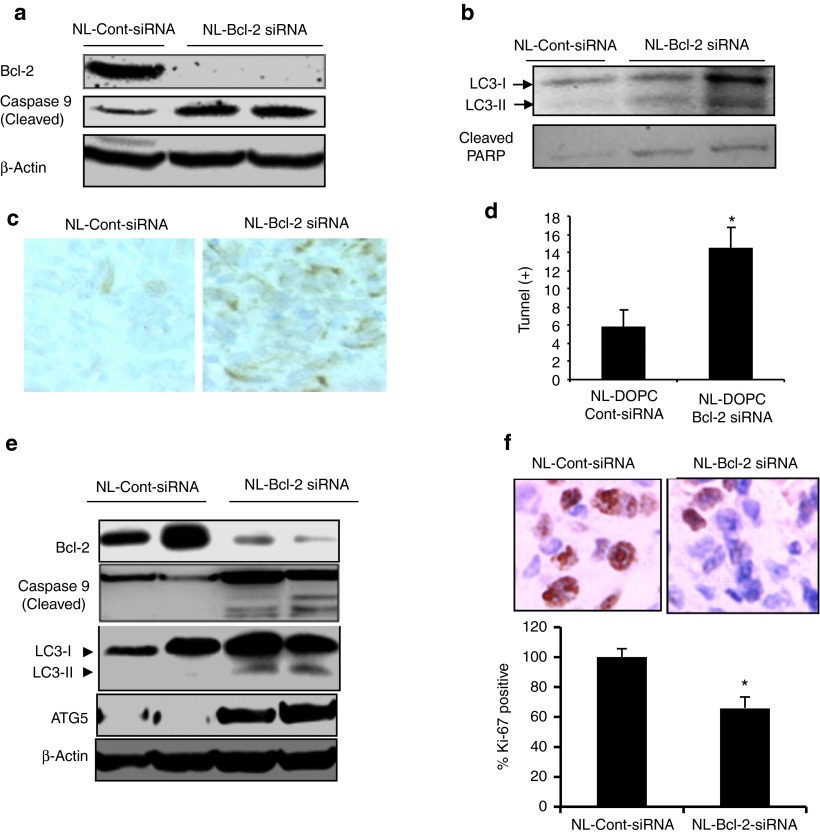
In a recent study, Oh et al. demonstrated that the oncogenic effect of Bcl-2 is related to its inhibition of autophagy rather than apoptosis.22 We previously demonstrated that in vitro silencing of Bcl-2 by siRNA induces autophagy in ER(+) MCF7 breast cancer cells.17 However, whether in vivo silencing of Bcl-2 leads to autophagy ER(+) and ER(−) breast tumors remains unknown. Considering the significant differences in gene expression and treatment response between tumors growing three dimensionally in a highly acidic, metabolically challenged, and hypoxic tumor microenvironment compared with cells growing in monolayers in tissue culture flasks, demonstration of autophagy in tumors in vivo is highly important for designing novel molecularly targeted therapies.16 Therefore, we first sought to determine the induction of autophagy in addition to apoptosis following therapeutic Bcl-2 silencing in MDA-MB-231 and MCF7 tumors in mice. We found marked induction of apoptosis, as evidenced by increased expression of cleaved caspase 9 and PARP, and autophagy, as indicated by increased expression of autophagy marker microtubule-associated protein-1 light chain 3 (LC-3 II) and ATG5 (Figure 5a, b) in NL-Bcl2 siRNA-treated tumor samples. TUNEL assay further confirmed the induction of apoptosis in MDA-MB-231 tumors collected after 4 weeks of NL-Bcl-2siRNA treatment (Figure 5c). NL-Bcl-2 siRNA induced a threefold increase in the number of TUNEL-positive apoptotic cells compared with NL-control-siRNA (P < 0.05) (Figure 5d). Western blot analysis of MCF-7 tumors treated with NL-Bcl-2 siRNA also revealed the induction of autophagy, as evidenced by increased expression of LC3-II protein and ATG5 (Figure 5e). We also evaluated cell proliferation by evaluating the expression of the proliferation marker Ki-67 and found that its expression was significantly inhibited in MDA-MB-231 tumors after NL-Bcl-2 siRNA treatment (P < 0.05; Figure 5f).
Figure 5.
In vivo silencing of Bcl-2 induces autophagy and apoptosis in ER(−) MDA-MB-231 tumors. (a) After 4 weeks treatment with NL-control siRNA or NL-Bcl-2 siRNA treatments, MDA-MB-231 tumors shown in Figure 3a were analyzed by western blot for detection activated/cleaved caspase-9 and PARP for evaluation of apopotosis. (b) Autophagy induction in MDA-MB-231 tumors was evidenced by detection of autophagy marker LC3-II in. (c) NL-Bcl-2-siRNA treatment-induced apoptosis was also shown by TUNEL staining of MDA-MB-231 tumors. (d) Quantification of TUNEL-positive cells in (c) shows that inhibition of Bcl-2 led to a threefold increase in apoptotic cells (P < 0.05). (e) Silencing of Bcl-2 expression by NL-Bcl-2-siRNA induced apoptosis and autophagy in MCF7 tumors. MCF tumors shown in Figure 4a were analyzed by western blot using specific antibodies to cleaved/activated caspase-9 for detection of apoptosis and LC3-II and ATG5 for detection of autophagy as described in the “Materials and Methods.” (f) NL-Bcl-2-siRNA treatment inhibited Ki-67 proliferation marker expression as indicated by immunohistochemistry (IHC). Ki-67 positive cells stained by IHC were quantified by counting five field from each tumor, indicating significant reduction of Ki-67 expression (*P < 0.05).
Autophagy contributes to cell death induced by Bcl-2 silencing in breast cancer cells
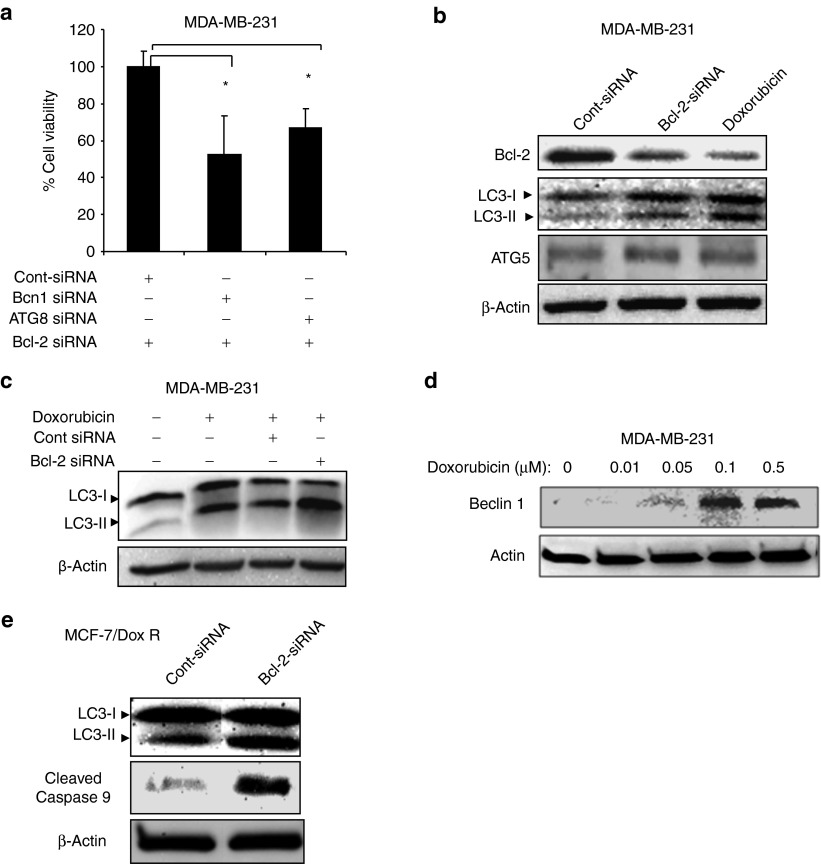
We previously demonstrated for the first time to our knowledge that siRNA-mediated Bcl-2 downregulation induces autophagic cell death in ER(+) MFC-7 breast cancer cells.17 However, the role of autophagy induced in response to Bcl-2 knockdown in ER(−) breast cancer cells is not known. To determine whether autophagy is involved in the induction of cell death after Bcl-2 inhibition, we knocked down autophagy genes, including Beclin-1 (BCN1) or ATG8 by specific siRNAs. Knockdown of either ATG8 or Beclin-1 significantly reduced Bcl-2 siRNA-induced cell death in MDA-MB-231 cells (P < 0.05; Figure 6a), suggesting that autophagy plays a role in the induction of cell death in ER(−) breast cancer cells.
Figure 6.
Autophagy contributes to cell death induced by Bcl-2 silencing in breast cancer cells. (a) Inhibition of autophagy by knocking down autophagy genes, including Beclin-1 or ATG8 inhibits cell death induced by Bcl-2-siRNA in MDA-MB-231 cells. Bcl-2 siRNA treatment was started 48 hours after control, Beclin1 or ATG8 siRNA transfections in MDA-MB-231 cells and cell death was assessed about 48 hours after Bcl-2 siRNA treatment. (b) Doxorubicin-induced autophagy is mediated by Bcl-2 downregulation in MDA-MB231 breast cancer cells. Doxorubicin treatment leads to Bcl-2 downregulation, which leads to autophagy induction as evidenced by increased expression of LC3-II autophagy marker. (c) Silencing of Bcl-2 by siRNA increased doxorubicin-induced autophagy in MDA-MB-231 cells. Cells were treated Bcl-2 siRNA for 24 hours and later incubated with doxorubicin for 48 hours. Western blot analysis shows that combination therapy (Bcl-2 siRNA and doxorubicin) induces more potent authophagy as evidenced by LC3-II and ATG5 expression. (d) Silencing of Bcl-2 by siRNA leads to autophagy as indicated by upregulation of Beclin-1 autophagy promoting protein in MDA-MB-231 cells. (e) Silencing of Bcl-2 by siRNA also induces autophagy MCF7/DoxR breast cancer cells as evidenced by LC3-II induction and apoptosis.
Doxorubicin-induced autophagy is mediated by downregulation of Bcl-2 and induction of Beclin-1 and ATG5
We previously reported that doxorubicin induces autophagy in ER(+) MCF7 breast cancer cells in vitro.17 However, the mechanism by which doxorubicin induces autophagy in breast cancer cells is not known. Therefore, we first sought to determine the doses of doxorubicin that induce growth inhibition, autophagy, and apoptosis in MDA-MB-231 cells by MTS assay, acridine orange and Annexin V staining followed by FACS analysis, respectively (Supplementary Figure 4A–C, online). We found that doxorubicin treatment led to the induction of autophagy, as evidenced by increased expression of autophagy marker LC3-II and upregulation of autophagy-promoting proteins such as ATG5 and Beclin-1 in MDA-MB-231 cells (Figure 6b–d). Because Bcl-2 physically binds and inhibits Beclin-1,21 we further sought to determine whether doxorubicin treatment leads to inhibition of Bcl-2 expression. Doxorubicin induced marked Bcl-2 downregulation in MDA-MB-231 cells (Figure 6b). Inhibition of Bcl-2 expression by siRNA also induced autophagy, as indicated by LC3-II induction, suggesting that doxorubicin-induced autophagy is mediated by Bcl-2 downregulation. This finding was further supported by an observation that specific inhibition of either Beclin-1 or ATG5 by siRNA inhibited doxorubicin-induced autophagy (Supplementary Figure 4D, online). Bcl-2 silencing also induced autophagy and apoptosis in doxorubicin-resistant breast cancer cells (MCF7-DoxR; Figure 6e). Overall, these results suggest that Bcl-2 downregulation by doxorubicin contributes to the induction of autophagy in breast cancer cells.
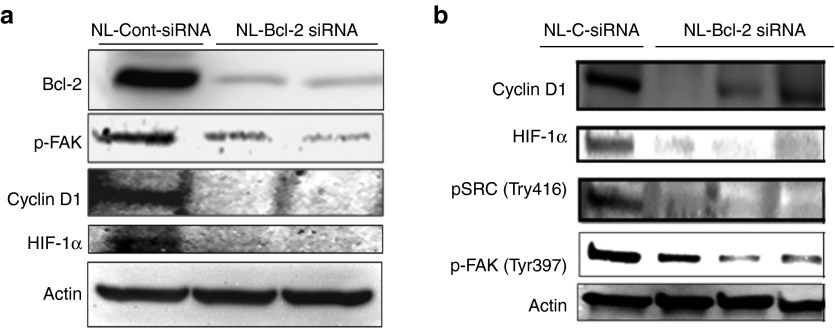
Targeting of Bcl-2 inhibits cyclin D1, HIF-1α, and Src/Fak activity in tumor xenografts
Recent studies of various cancers suggested that Bcl-2 promotes cancer progression by enhancing cell invasion, cell cycle, and angiogenesis.20,24,25,26,27,28,29 We also investigated expression of these factors in MDA-MB-231 tumors after the NL-Bcl-2 siRNA treatments. We found that Bcl-2 downregulation reduced the activity (phosphorylation) of focal adhesion kinase (FAK) (Tyr397) and Src (Tyr416) and the expression of hypoxia induced factor-1α (HIF1α) and cyclin D1 (Figure 7a,b) in tumor xenografts after 4 weeks of Bcl-2 siRNA treatment, suggesting that Bcl-2 silencing may provide antitumor effects other than the induction of autophagy and apoptosis in breast tumors.
Figure 7.
In vivo therapeutic silencing of Bcl-2 by nanoliposomal siRNA treatment inhibits activation of focal adhesion kinase (p-FAK), cyclin D1, HIF1α in tumors. Tumors shown in Figure 4a were analyzed after 4 weeks of treatments with NL-Bcl-2-siRNA or NL-control siRNA alone (0.15 mg siRNA/kg, i.v, twice a week). Mice treated with NL-Bcl-2 siRNA had reduced activity of Src and FAK signaling pathways and expression of Cyclin D1 and HIF1α in tumor xenografts when compared with corresponding control groups for 4 weeks of treatment.
Discussion
In this study, we demonstrated for the first time that in vivo therapeutic targeting of Bcl-2 by i.v. nanoliposomal Bcl-2-siRNA significantly inhibits tumor growth in preclinical models of both ER(−) and ER(+) breast cancers. We also provide the first evidence that therapeutic targeting of Bcl-2 induces autophagy and apoptosis in both ER(−) and ER(+) breast tumors in vivo. Furthermore, silencing of Bcl-2 also significantly increased the efficacy of chemotherapy in both models in vivo.
Bcl-2 is one of the most important and common mediators of survival and drug resistance in most human cancers.1,2,3,30 Bcl-2 expression leads to aggressive disease course poor survival in patients with different cancers.7 Therefore, Bcl-2 is considered an excellent molecular target for therapies for breast and other cancers. However, therapeutic silencing of Bcl-2 in tumors remains a great challenge. Although siRNA-based gene silencing has great potential for molecularly targeted therapies, clinical applications of siRNA-based therapies are hampered by challenges to systemic administration and delivery into tumors.31,32 When injected systemically, siRNA is rapidly degraded by nucleases in serum and body fluids and cleared from plasma with a half-life of minutes. Therefore, the development of safe and effective in vivo systemic delivery systems for successful clinical applications of siRNA-based therapies is critical.10,33,34 To therapeutically silence Bcl-2 in breast tumors in vivo, we used liposomes incorporating Bcl-2-specific siRNA that led to significant and robust target gene knockdown in tumors (Figure 2a). A single injection of a small dose of liposomal siRNA (0.15 mg/kg) provided a potent (~80–90%) inhibition of Bcl-2. It is also important to note that the siRNA doses used in our study were about 60- to 120-fold less compared with other reports that used 10 mg/kg siRNA in cationic liposomes,35 and Bcl-2 siRNA was well tolerated in mice. The neutral lipid-based delivery system was safe and effective and produced no obvious toxic effects in the animals during treatment in the current and previous studies.36 However, most commonly used cationic liposomes are highly toxic in vitro and in vivo in mice, thereby limiting their clinic applications.13,37
The other important finding was that NL-Bcl-2 siRNA treatment significantly enhanced the antitumor efficacy of chemotherapy (Doxorubicin), especially in the ER(−) animal model. However, compared with ER(−) model this effect was slightly less pronounced compared with ER(+) model. This could be related the intrinsic balance between pro- and antiapoptotic proteins (e.g., Bcl-2 vs. Bax) as well as the activity of other signaling pathways such as PI3K/Akt and Ras/Raf/Erk in the ER(−) and ER(+) cancer cells. Although ER(−) cells tend to express less Bcl-2, p53, and K-Ras are mutated in MDA-MB-231 cells compared with ER(+) MCF7 cells.
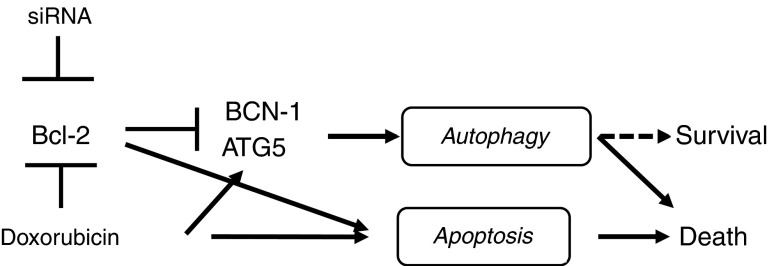
Autophagy is one of the novel mechanisms of cell death.16,38,39 Autophagy may function as a survival pathway during nutrient deprivation or starvation.15,16,19 More importantly, reduced or defective autophagy in mammary tumors activates DNA damage response and synergizes with defective apoptosis to accelerate tumorigenesis.34 We previously showed that inhibition of Bcl-2 induces autophagic cell death in ER(+) MCF-7 breast cancer cells in vitro.17 The findings of the present study demonstrated that Bcl-2 silencing in ER(+) and ER(−) breast tumors induces autophagy and apoptosis, leading to the suppression of tumor growth (Figure 8). The induction of autophagy by doxorubicin was also mediated by Bcl-2 downmodulation, leading to Beclin-1, ATG5 and LC3-II induction (Figure 8). More importantly, Bcl-2 siRNA contributes to cell death, as knockdown of authophagy genes prevented the induction of cell death and increased cell survival (Figures 6a, 8).
Figure 8.
Proposed mechanism of Bcl-2 silencing and doxorubicin-induced events in breast cancer cells. Bcl-2 silencing by specific siRNA and doxorubicin induce apoptosis and autophagy that is mediated by downregulation Bcl-2 and induction of ATG5 and Beclin1. Inhibition of autophagy genes prevents cell death by Bcl-2 silencing suggest that autophagy contributes to cell death in MDA-MB-231 breast cancer cells.
The induction of autophagy following Bcl-2 silencing may be mediated by two different mechanisms in breast cancer cells: (i) inhibition of Bcl-2 relieves its suppressor activity on Beclin-1, which is physically bound and blocked by Bcl-221 and (ii) the higher apoptotic threshold owing to the lack of caspase 3 and p53 mutations in MCF-7 and MDA-MB231 cells, respectively, may favor the induction of autophagy as a default cell death mechanism.40 In fact, studies of Bax/Bak double-knockout MEF cells showed that cells undergo autophagy-mediated cell death in response to a death stimulus, suggesting that autophagy may function as an alternative cell death mechanism when the apoptotic threshold is increased or blocked; thus, this type of cell death may be used in eliminating cancer cells that are resistant to apoptosis.6,41 More important, a recent study Oh et al. demonstrated that the antiautophagic property of Bcl-2 is the key feature of Bcl-2-induced tumorigenesis.22 They reported that the growth-promoting activity of Bcl-2 is strongly correlated with its suppression of autophagy in xenograft tumors. MCF-7 cells expressing mutated Bcl-2 that was defective in inhibiting apoptosis but competent for suppressing autophagy grew in vitro and in vivo as efficiently as wild-type Bcl-2-expressing cells, indicating that the oncogenic effect of Bcl-2 arises from its ability to inhibit autophagy but not apoptosis.22
Tumors derived from cells that overexpress Bcl-2 grow more aggressively in vivo. This could be attributed to events other than the antiapoptotic and antiautophagic properties of Bcl-2. In fact, emerging studies suggest that Bcl-2 promotes cancer progression by enhancing cell invasion, cell migration, and the metastatic potential of various cancer types.27,28,29 We observed that Bcl-2 downregulation reduced the activity (phosphorylation) of FAK/SRC, HIF-1α, and cyclin D1 in tumor xenografts (Figure 7). FAK is known to play a major role in cell migration, invasion/metastasis, and drug resistance by activating the Ras/MEK/ERK5 and PI3K/Akt survival pathways.42,43,44 Future studies should investigate in detail how Bcl-2 regulates cell migration, invasion, and angiogenesis and cell cycle in breast tumors in vivo. HIF-1α is a mediator of cellular response to hypoxia and is associated with increased angiogenesis, metastasis, treatment resistance, and poor prognosis.20 Anai et al. recently showed that inhibition of Bcl-2 leads to reduced angiogenesis in human prostate tumor xenografts.24 In addition, Bcl-2 overexpression increases vascular endothelial growth factor promoter activity through the HIF-1α transcription factor,25 thereby providing a link between Bcl-2 and angiogenesis.20,26 Breast cancer patients with a higher Ki-67 have been shown to have significantly poorer prognosis, early recurrence, and reduced overall survival rates.45 Inhibition of Ki-67 expression in tumors after Bcl-2 siRNA treatment suggests that overall treatment response and antitumor effects may be due to multiple mechanisms, including apoptosis and autophagy.
Pretreatment with Bcl-2 antisense enhanced the antitumor activity of various chemotherapeutic agents, such as cyclophosphamide, dacarbazine, and docetaxel, in several cancers in vitro.46 George et al. reported that in vitro treatment of human glioma cells with Bcl-2 siRNA and taxol (100 nmol/l) increased the apoptotic cells in a TUNEL assay up to 70% compared with 30% in those treated with taxol alone (100 nmol/l).47 Our in vitro and in vivo findings suggest that targeting Bcl-2 is a highly effective therapeutic strategy for enhancing the efficacy of standard chemotherapeutic agents in breast cancer.
In conclusion, our study suggests that highly specific targeting of Bcl-2 by siRNA-based therapies provides efficient and potent target silencing, inhibits tumor growth, and enhances the efficacy of standard chemotherapies. Thus, targeting Bcl-2 by systemically administered siRNA is a viable approach and may be beneficial to breast cancer patients with Bcl-2-overexpressing tumors.
Materials and methods
Cell lines, culture conditions, and reagents. ER(−) MDA-MD-231 and ER (+) MCF-7 human breast cancer cell lines were purchased from ATCC. Doxorubicin-resistant MCF7 cells were obtained from Dr. Kapil Mehta (The University of Texas MD Anderson Cancer Center, Houston, Texas, USA). MDA-MB-231/luciferase cells were transduced with luciferase-expressing lentiviral particles that were prepared in our laboratory.23 Transfection of MDA-MB-231 cells was performed at a multiplicity of infection virus particle concentration of 5. Five days after transfection, the cells were treated with 5 µg of puromycin concentration to select stably luciferase expressing cells. All cell lines were cultured at 37 °C in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum in a humid incubator with 5% CO2. For the cell proliferation experiments, cells were seeded at a density of 1–2 × 105 cells in T-25 tissue culture flasks or 1.25–3 × 103 in 96-well plates. Cells were collected via trypsin exposure, and cell numbers were determined using a Neubauer cell-counting chamber. All experiments were repeated at least three-times.
Cell viability and growth assays. Cell viability in response to siRNA treatments or doxorubicin was evaluated using a tetrazolium/formazan (MTS)-based CellTiter 96 AQueous One Solution cell proliferation assay (Promega, Madison, WI).17 Cells were seeded in 96-well plates at a density of 3 × 103 cells per well in 100 µl of medium. The next day, the medium was removed, and cells were transfected with siRNA (50 nmol/l) in 100 µl of medium plus transfection mix or treated with doxorubicin for 72 hours. Plates were read at wavelength of 490 nm in a VMax kinetic enzyme-linked immunosorbent assay microplate reader (Molecular Devices Corporation, Sunnyvale, CA, USA). The dead and viable cells were also detected via a trypan blue exclusion assay in which viable cells are able to exclude the dye and remain unstained while dead cells take up the blue coloring agent.
Clonogenic assay. This assay is an in vitro cell survival and proliferation assay based on the ability of a single cell to grow into a colony.18,36 Briefly, 500 cells were mixed gently and plated on a 6-well plate. After being incubated for 24 hours, the cells were transfected with control and Bcl-2 siRNA every 5 days, and about 2 weeks later, the cells were washed with phosphate-buffered saline and stained with crystal violet. Colonies with a diameter of more than 50 cells were counted. The experiment was repeated three-times.
siRNA transfections. Exponentially growing untreated MCF-7 and MDA-MB-231 cells were collected and plated (2 and 1.5 × 105/flask in 4 ml, respectively) 24 hours before transfection. Plated cells were transfected with either Bcl-2 siRNA or control siRNA (50 nmol/l). siRNA sequences targeting Bcl-2 and nonsilencing control siRNA 5′-AACATCGCCCTGTGGATGACT-3′ and 5′-AATTCTCCGAACGTGTCACGT-3′, respectively.17 Beclin-1 siRNA and ATG8 siRNA22 were used. The siRNAs were dissolved in sterile buffer provided by the manufacturer (all from Qiagen Inc, Valencia, CA, USA). On the day of transfection, 1.5 µg of siRNA was mixed with HiPerFect transfection reagent according to the manufacturer's instructions (Qiagen) and added to the cells in each well.
Western blot analysis. After treatment, the cells were trypsinized and collected by centrifugation, and whole-cell lysates were obtained using a lysis buffer as described previously.48 Total protein concentration was determined using a protein assay kit (Bio-Rad, Hercules, CA, USA). Aliquots containing 30 µg of total protein from each sample were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis with a 4–20% gradient gel and transferred to polyvinylidene difluoride membranes. The membranes were blocked with 5% dry milk in Tris-buffered saline with Tween 20 (TBST) and probed with primary antibodies of human specific Bcl-2 monoclonal antibody (Dako Cytomation California Inc., Carpinteria, CA, USA), Beclin-1, ATG5 (Santa Cruz, CA, USA), LC3 (Axorra LLC, San Diego, CA, USA), human specific monoclonal cleaved poly(ADP-ribose polymerase (PARP; #9546), and cleaved caspase 9 (#9590, Cell Signaling Technology, Beverly, MA, USA). The antibodies were diluted in TBST containing 2.5% dry milk and incubated at 4 °C overnight. After the membranes were washed with TBST, they were incubated with horseradish peroxidase-conjugated antirabbit or antimouse secondary antibody (Amersham Life Science, Cleveland, OH, USA). Mouse anti-β-actin and donkey antimouse secondary antibodies (Sigma–Aldrich, St. Louis, MO, USA) were used to monitorβ -actin expression to ensure equal loading of proteins. Chemiluminescent detection was performed with ChemiGlow detection reagents (Alpha Innotech, San Leandro, CA, USA). The blots were visualized with a FluorChem 8900 imager and quantified with a densitometer using an AlphaImager system (Alpha Innotech).
In vivo detection of apoptosis via TUNEL assay. Apoptotic cells in tumor tissue were detected by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining using an apoptotic cell detection kit following the manufacturer's directions (Promega, Madison, WI, USA).36 Images of the sections were captured by a microscope (Nikon, Tokyo, Japan). The apoptotic index was calculated by dividing the number of TUNEL-positive cells by the total number of cells in the field. Light microscopy was used to count the number of TUNEL-positive cells on ten randomly selected fields for each section.
Evaluation of autophagy via detection of acidic vesicular organelles. Cells were stained with acridine orange as described previously18 to detect and quantify acidic vesicular organelles. The number of acridine orange-positive cells was determined via fluorescence-activated cell sorting (FACS) analysis. Cell morphology was examined using a phase-contrast microscope (Nikon, Melville, NY, USA) while the cells remaining in their culture flasks.
Nanoliposomal siRNA preparation. Control siRNA and Bcl-2 siRNA were encapsulated using 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine-lipid–based nanoliposomal particles. Briefly, siRNA was mixed with the lipid at a ratio of 1:10 (w/w). Tween 20 was added to the mixture at a ratio of 1:19 Tween 20: siRNA/lipid in the presence of excess tertiary butanol.36 After being vortexed, the mixture was frozen in an acetone/dry ice bath and lyophilized. Before animals were injected, the lyophilized lipid-siRNAs were reconstituted with 0.9% saline to form liposomes and sonicated for 3 minutes. The mean size of the liposomes incorporating the siRNAs was measured using a Zetasizer Nano ZS (Malvern, Worcestershire, UK) and found to be about 65 nm with zeta potential of 1.9 ± 0.24 for NL-empty and −2.7 ± 0.33 for NL-cont siRNA in phosphate-buffered saline. Free siRNA was separated from liposomes using filter units with a 30,000 nominal molecular weight limit (Millipore Corp., Billerica, MA, USA). The liposomal suspension was added to the filters and centrifuged at 5,000×g for 40 minutes at room temperature. Fractions were collected, the material trapped in the filter was reconstituted with 0.9% saline, and the siRNA of the collected fraction and the elute were measured via spectrophotometry.
Tumor models in mice. Athymic female nude mice (NCr nu/nu) mice 5–6-weeks old were obtained from the Department of Experimental Radiation Oncology at MD Anderson. The mice were housed three per cage in standard acrylic glass cages in a room maintained at a constant temperature and humidity with a 12-hour light-dark cycle. They were fed a regular autoclaved chow diet with water ad libitum. All studies were conducted according to an experimental protocol approved by the MD Anderson Institutional Animal Care and Use Committee. ER(−) MDA-MB-231 cells (1.5 × 106) and ER(+) MCF7 cells (7.0 × 106) were orthotopically injected into the right mammary fat pat of each mouse. For the experiments using MCF-7 cells, mice were primed with 17β-estradiol applied subcutaneously (1.7 mg estradiol/pellet) under the left shoulder to promote tumor growth. When tumor size reached 3–5 mm about 2 weeks later, mice were administered liposomal siRNA and doxorubicin once a week.
Evaluation of in vivo growth of tumors after systemic liposomal siRNA treatments. MDA-MB-231 and MCF-7 cells were implanted orthotopically in the mammary fat pads of athymic nude mice (NCr nu/nu) that were 5–6-weeks old. Two weeks tumor cell injection, luciferase activity was measured by injecting d-luciferin potassium salt (Molecular Probes, Eugene, OR, USA) using an IVIS imaging system (Xenogen, Alemeda, CA, USA) as previously described.23 Briefly, the mice were anesthetized, and d-luciferin was injected at 100 mg/kg mouse body weight. Ten minutes after d-luciferin injection, the mice were imaged with an IVIS Imaging System 2000 coupled with data acquisition controlled by a computer running LivingImage software (Xenogen, Alameda, CA, USA).23 Mice with equally sized tumors were randomly assigned to one out of four treatment groups: group I received nanoliposomal (NL)-control siRNA (0.15 mg siRNA/kg) twice weekly via intravenous (i.v.) injection; group II received NL-Bcl-2-siRNA (0.15 mg siRNA/kg) twice weekly via i.v. injection; group III received both control NL-siRNA (0.15 mg siRNA/kg) and doxorubicin (4 mg/kg) weekly via intraperitoneal (i.p.) injection; and group IV received both NL-Bcl-2-siRNA (0.15 mg siRNA/kg) twice weekly via i.v. injection and doxorubicin (4 mg/kg) weekly via i.p. injection.36 The resulting tumor growth was assessed after 4 weeks (eight doses) of treatment using the IVIS imaging system. The mice were euthanized 48 hours after the final injection, and primary tumors were excised and weighed. A portion of the tumors was in liquid nitrogen for molecular analysis and another portion was formalin fixed and paraffin embedded. In any instance, please clarify how liquid nitrogen was used for immunohistochemistry for routine hematoxylin and eosin staining and TUNEL assay as described previously.36 The remaining tumor tissue was stored at −80 °C until use.
Statistical analysis. The data were expressed as the means ± SD of three or more independent experiments, and statistical analysis was performed using the two-tailed and paired Student's t test. P < 0.05 was considered statistically significant and indicated by an asterisk.
SUPPLEMENTARY MATERIAL Figure S1. Dose-dependent downregulation of Bcl-2 protein in MDA-MB231 tumors after single NL-Bcl-2 siRNA injection (iv. tail vein). Figure S2. Therapeutic silencing of Bcl-2 by only three i.v. injections of NL-Bcl-2 siRNA inhibits in vivo tumor growth of ER(-) MDA-MB-231 xenografts in nude mice (*p<0.05). Figure S3. Treatment schedules with siRNA and chemotherapy in mice bearing tumors. Figure S4. A) Dose-dependent inhibition of MDA-MB-231 cells by doxorubicin (72h). B) Doxorubicin induces autophagy in MDA-MB-231 cells as indicated by acridine orange staining and FACS analysis (48h). C) Doxorubicin induces apoptosis and autophagy in MDA-MB-231 cells as indicated by Annexin V/PI and acridine orange staining and FACS analysis (48h). D) Knockdown of autophagy genes including ATG5 and Beclin 1 inhibits doxorubicin-induced autophagy in MDA-MB-231 cells.
Acknowledgments
This work was funded by a Susan Komen Breast Cancer Award (BO) and, in part, by the NIH (grants U54 CA096300, U54 CA151668, P50 CA083639, the DOD (grant BC085265) and An NCI institutional Core Grant (CA16672).
Supplementary Material
Dose-dependent downregulation of Bcl-2 protein in MDA-MB231 tumors after single NL-Bcl-2 siRNA injection (iv. tail vein).
Therapeutic silencing of Bcl-2 by only three i.v. injections of NL-Bcl-2 siRNA inhibits in vivo tumor growth of ER(-) MDA-MB-231 xenografts in nude mice (*p<0.05).
Treatment schedules with siRNA and chemotherapy in mice bearing tumors.
A) Dose-dependent inhibition of MDA-MB-231 cells by doxorubicin (72h). B) Doxorubicin induces autophagy in MDA-MB-231 cells as indicated by acridine orange staining and FACS analysis (48h). C) Doxorubicin induces apoptosis and autophagy in MDA-MB-231 cells as indicated by Annexin V/PI and acridine orange staining and FACS analysis (48h). D) Knockdown of autophagy genes including ATG5 and Beclin 1 inhibits doxorubicin-induced autophagy in MDA-MB-231 cells.
References
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Yip KW, Reed JC. Bcl-2 family proteins and cancer. Oncogene. 2008;27:6398–6406. doi: 10.1038/onc.2008.307. [DOI] [PubMed] [Google Scholar]
- Korsmeyer SJ. BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 1999;59 7 Suppl:1693s–1700s. [PubMed] [Google Scholar]
- Buchholz TA, Davis DW, McConkey DJ, Symmans WF, Valero V, Jhingran A, et al. Chemotherapy-induced apoptosis and Bcl-2 levels correlate with breast cancer response to chemotherapy. Cancer J. 2003;9:33–41. doi: 10.1097/00130404-200301000-00007. [DOI] [PubMed] [Google Scholar]
- Patel MP, Masood A, Patel PS, Chanan-Khan AA. Targeting the Bcl-2. Curr Opin Oncol. 2009;21:516–523. doi: 10.1097/CCO.0b013e328331a7a4. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB, et al. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol. 2004;6:1221–1228. doi: 10.1038/ncb1192. [DOI] [PubMed] [Google Scholar]
- Tawfik K, Kimler BF, Davis MK, Fan F, Tawfik O. Prognostic significance of Bcl-2 in invasive mammary carcinomas: a comparative clinicopathologic study between “triple-negative” and non-“triple-negative” tumors. Hum Pathol. 2012;43:23–30. doi: 10.1016/j.humpath.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Tabuchi Y, Matsuoka J, Gunduz M, Imada T, Ono R, Ito M, et al. Resistance to paclitaxel therapy is related with Bcl-2 expression through an estrogen receptor mediated pathway in breast cancer. Int J Oncol. 2009;34:313–319. [PubMed] [Google Scholar]
- Tanabe K, Kim R, Inoue H, Emi M, Uchida Y, Toge T. Antisense Bcl-2 and HER-2 oligonucleotide treatment of breast cancer cells enhances their sensitivity to anticancer drugs. Int J Oncol. 2003;22:875–881. [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Pecot CV, Calin GA, Coleman RL, Lopez-Berestein G, Sood AK. RNA interference in the clinic: challenges and future directions. Nat Rev Cancer. 2011;11:59–67. doi: 10.1038/nrc2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- Filion MC, Phillips NC. Major limitations in the use of cationic liposomes for DNA delivery. Int J Pharm. 1998;162:159–170. [Google Scholar]
- Bertrand JR, Pottier M, Vekris A, Opolon P, Maksimenko A, Malvy C. Comparison of antisense oligonucleotides and siRNAs in cell culture and in vivo. Biochem Biophys Res Commun. 2002;296:1000–1004. doi: 10.1016/s0006-291x(02)02013-2. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalby KN, Tekedereli I, Lopez-Berestein G, Ozpolat B. Targeting the prodeath and prosurvival functions of autophagy as novel therapeutic strategies in cancer. Autophagy. 2010;6:322–329. doi: 10.4161/auto.6.3.11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akar U, Chaves-Reyez A, Barria M, Tari A, Sanguino A, Kondo Y, et al. Silencing of Bcl-2 expression by small interfering RNA induces autophagic cell death in MCF-7 breast cancer cells. Autophagy. 2008;4:669–679. doi: 10.4161/auto.6083. [DOI] [PubMed] [Google Scholar]
- Ozpolat B, Akar U, Mehta K, Lopez-Berestein G. PKC delta and tissue transglutaminase are novel inhibitors of autophagy in pancreatic cancer cells. Autophagy. 2007;3:480–483. doi: 10.4161/auto.4349. [DOI] [PubMed] [Google Scholar]
- Shen HM, Codogno P. Autophagic cell death: Loch Ness monster or endangered species. Autophagy. 2011;7:457–465. doi: 10.4161/auto.7.5.14226. [DOI] [PubMed] [Google Scholar]
- Trisciuoglio D, Gabellini C, Desideri M, Ziparo E, Zupi G, Del Bufalo D. Bcl-2 regulates HIF-1alpha protein stabilization in hypoxic melanoma cells via the molecular chaperone HSP90. PLoS ONE. 2010;5:e11772. doi: 10.1371/journal.pone.0011772. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Oh S, Xiaofei E, Ni D, Pirooz SD, Lee JY, Lee D, et al. Downregulation of autophagy by Bcl-2 promotes MCF7 breast cancer cell growth independent of its inhibition of apoptosis. Cell Death Differ. 2011;18:452–464. doi: 10.1038/cdd.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akar U, Ozpolat B, Mehta K, Lopez-Berestein G, Zhang D, Ueno NT, et al. Targeting p70S6K prevented lung metastasis in a breast cancer xenograft model. Mol Cancer Ther. 2010;9:1180–1187. doi: 10.1158/1535-7163.MCT-09-1025. [DOI] [PubMed] [Google Scholar]
- Anai S, Goodison S, Shiverick K, Hirao Y, Brown BD, Rosser CJ. Knock-down of Bcl-2 by antisense oligodeoxynucleotides induces radiosensitization and inhibition of angiogenesis in human PC-3 prostate tumor xenografts. Mol Cancer Ther. 2007;6:101–111. doi: 10.1158/1535-7163.MCT-06-0367. [DOI] [PubMed] [Google Scholar]
- Iervolino A, Trisciuoglio D, Ribatti D, Candiloro A, Biroccio A, Zupi G, et al. Bcl-2 overexpression in human melanoma cells increases angiogenesis through VEGF mRNA stabilization and HIF-1-mediated transcriptional activity. FASEB J. 2002;16:1453–1455. doi: 10.1096/fj.02-0122fje. [DOI] [PubMed] [Google Scholar]
- Li X, Fan Z. The epidermal growth factor receptor antibody cetuximab induces autophagy in cancer cells by downregulating HIF-1alpha and Bcl-2 and activating the beclin 1/hVps34 complex. Cancer Res. 2010;70:5942–5952. doi: 10.1158/0008-5472.CAN-10-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricca A, Biroccio A, Del Bufalo D, Mackay AR, Santoni A, Cippitelli M. bcl-2 over-expression enhances NF-kappaB activity and induces mmp-9 transcription in human MCF7(ADR) breast-cancer cells. Int J Cancer. 2000;86:188–196. doi: 10.1002/(sici)1097-0215(20000415)86:2<188::aid-ijc7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Wick W, Wagner S, Kerkau S, Dichgans J, Tonn JC, Weller M. BCL-2 promotes migration and invasiveness of human glioma cells. FEBS Lett. 1998;440:419–424. doi: 10.1016/s0014-5793(98)01494-x. [DOI] [PubMed] [Google Scholar]
- Takaoka A, Adachi M, Okuda H, Sato S, Yawata A, Hinoda Y, et al. Anti-cell death activity promotes pulmonary metastasis of melanoma cells. Oncogene. 1997;14:2971–2977. doi: 10.1038/sj.onc.1201147. [DOI] [PubMed] [Google Scholar]
- Reed JC. Bcl-2: prevention of apoptosis as a mechanism of drug resistance. Hematol Oncol Clin North Am. 1995;9:451–473. [PubMed] [Google Scholar]
- Fernández Y, Gu B, Martínez A, Torregrosa A, Sierra A. Inhibition of apial optosis in human breast cancer cells: role in tumor progression to the metastatic state. Int J Cancer. 2002;101:317–326. doi: 10.1002/ijc.10628. [DOI] [PubMed] [Google Scholar]
- Ozpolat B, Sood AK, Lopez-Berestein G. Nanomedicine based approaches for the delivery of siRNA in cancer. J Intern Med . 2010;267:44–53. doi: 10.1111/j.1365-2796.2009.02191.x. [DOI] [PubMed] [Google Scholar]
- Dorsett Y, Tuschl T. siRNAs: applications in functional genomics and potential as therapeutics. Nat Rev Drug Discov. 2004;3:318–329. doi: 10.1038/nrd1345. [DOI] [PubMed] [Google Scholar]
- Rubinsztein DC, Gestwicki JE, Murphy LO, Klionsky DJ. Potential therapeutic applications of autophagy. Nat Rev Drug Discov. 2007;6:304–312. doi: 10.1038/nrd2272. [DOI] [PubMed] [Google Scholar]
- Sonoke S, Ueda T, Fujiwara K, Sato Y, Takagaki K, Hirabayashi K, et al. Tumor regression in mice by delivery of Bcl-2 small interfering RNA with pegylated cationic liposomes. Cancer Res. 2008;68:8843–8851. doi: 10.1158/0008-5472.CAN-08-0127. [DOI] [PubMed] [Google Scholar]
- Tekedereli I, Akar U, Lopez-Berestein G, Ozpolat B. Therapeutic silencing of nanoliposomal EF2K by siRNA in primary and metastic breast cancer in animal models. PLoS One. 2012;e41171;7 7 [Google Scholar]
- Lv H, Zhang S, Wang B, Cui S, Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J Control Release. 2006;114:100–109. doi: 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005;5:726–734. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- Hannon GJ, Rossi JJ. Unlocking the potential of the human genome with RNA interference. Nature. 431. 2004. pp. 371–378. [DOI] [PubMed]
- Devarajan E, Sahin AA, Chen JS, Krishnamurthy RR, Aggarwal N, Brun AM, et al. Down-regulation of caspase 3 in breast cancer: a possible mechanism for chemoresistance. Oncogene. 2002;21:8843–8851. doi: 10.1038/sj.onc.1206044. [DOI] [PubMed] [Google Scholar]
- Moretti L, Attia A, Kim KW, Lu B. Crosstalk between Bak/Bax and mTOR signaling regulates radiation-induced autophagy. Autophagy. 2007;3 2:142–144. doi: 10.4161/auto.3607. [DOI] [PubMed] [Google Scholar]
- van Nimwegen MJ, Huigsloot M, Camier A, Tijdens IB, van de Water B. Focal adhesion kinase and protein kinase B cooperate to suppress doxorubicin-induced apoptosis of breast tumor cells. Mol Pharmacol. 2006;70:1330–1339. doi: 10.1124/mol.106.026195. [DOI] [PubMed] [Google Scholar]
- Bouchard V, Harnois C, Demers MJ, Thibodeau S, Laquerre V, Gauthier R, et al. B1 integrin/Fak/Src signaling in intestinal epithelial crypt cell survival: integration of complex regulatory mechanisms. Apoptosis. 2008;13:531–542. doi: 10.1007/s10495-008-0192-y. [DOI] [PubMed] [Google Scholar]
- Lee BH, Ruoslahti E. alpha5beta1 integrin stimulates Bcl-2 expression and cell survival through Akt, focal adhesion kinase, and Ca2+/calmodulin-dependent protein kinase IV. J Cell Biochem. 2005;95:1214–1223. doi: 10.1002/jcb.20488. [DOI] [PubMed] [Google Scholar]
- Nishimura R, Osako T, Okumura Y, Hayashi M, Toyozumi Y, Arima N. Ki-67 as a prognostic marker according to breast cancer subtype and a predictor of recurrence time in primary breast cancer. Exp Ther Med. 2010;1:747–754. doi: 10.3892/etm.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahta R, Esteva FJ. Bcl-2 antisense oligonucleotides: a potential novel strategy for the treatment of breast cancer. Semin Oncol. 2003;30 5 Suppl 16:143–149. doi: 10.1053/j.seminoncol.2003.08.016. [DOI] [PubMed] [Google Scholar]
- George J, Banik NL, Ray SK. Bcl-2 siRNA augments taxol mediated apoptotic death in human glioblastoma U138MG and U251MG cells. Neurochem Res. 2009;34:66–78. doi: 10.1007/s11064-008-9659-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozpolat B, Akar U, Steiner M, Zorrilla-Calancha I, Tirado-Gomez M, Colburn N, et al. Programmed cell death-4 tumor suppressor protein contributes to retinoic acid-induced terminal granulocytic differentiation of human myeloid leukemia cells. Mol Cancer Res. 2007;5:95–108. doi: 10.1158/1541-7786.MCR-06-0125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dose-dependent downregulation of Bcl-2 protein in MDA-MB231 tumors after single NL-Bcl-2 siRNA injection (iv. tail vein).
Therapeutic silencing of Bcl-2 by only three i.v. injections of NL-Bcl-2 siRNA inhibits in vivo tumor growth of ER(-) MDA-MB-231 xenografts in nude mice (*p<0.05).
Treatment schedules with siRNA and chemotherapy in mice bearing tumors.
A) Dose-dependent inhibition of MDA-MB-231 cells by doxorubicin (72h). B) Doxorubicin induces autophagy in MDA-MB-231 cells as indicated by acridine orange staining and FACS analysis (48h). C) Doxorubicin induces apoptosis and autophagy in MDA-MB-231 cells as indicated by Annexin V/PI and acridine orange staining and FACS analysis (48h). D) Knockdown of autophagy genes including ATG5 and Beclin 1 inhibits doxorubicin-induced autophagy in MDA-MB-231 cells.