Abstract
Background: We evaluated the risk factors and clinical course of Long QT syndrome (LQTS) in African‐American patients.
Methods: The study involved 41 African‐Americans and 3456 Caucasians with a QTc ≥ 450 ms from the U.S. portion of the International LQTS Registry. Data included information about the medical history and clinical course of the LQTS patients with end points relating to the occurrence of syncope, aborted cardiac arrest, or LQTS‐related sudden cardiac death from birth through age 40 years. The statistical analyses involved Kaplan‐Meier time to event graphs and Cox regression models for multivariable risk factor evaluation.
Results: The QTc was 29 ms longer in African‐Americans than Caucasians. Multivarite Cox analyses with adjustment for decade of birth revealed that the cardiac event rate was similar in African‐Americans and Caucasians with LQTS and that beta‐blockers were equally effective in reducing cardiac events in the two racial groups.
Conclusions: The clinical course of LQTS in African‐Americans is similar to that of Caucasians with comparable risk factors and benefit from beta‐blocker therapy in the two racial groups.
Ann Noninvasive Electrocardiol 2010;15(1):73–76
Keywords: long QT syndrome; risk stratification, African‐Americans
Long QT syndrome (LQTS) is a relatively rare, autosomal dominant genetic disorder affecting the heart's electrical activity with an estimated frequency of about 1 in 5000 people. 1 The disorder is caused mainly by mutations in genes that code for protein subunits of cardiac ion channels with resultant delay in ventricular repolarization from reduced ion‐channel currents. Patients with LQTS are at high risk for syncope, episodes of polymorphic ventricular tachycardia (torsades de pointes), and sudden cardiac death. 2
Clinically, LQTS is identified by abnormal prolongation of the QT interval on the ECG. Long QT syndrome has been studied in Caucasian, Japanese, and Chinese populations, but no previous study has examined African‐American patients with LQTS. In our study, we evaluated the risk factors for cardiac events and the clinical course of LQTS in African‐American patients and compared the findings to Caucasians with this disorder.
METHODS
The study population involved 3497 subjects who were enrolled in the U.S. portion of the International Long QT Registry and had a QTc ≥ 450 ms. 3 At the time of patient enrollment in the Registry, a 12‐lead electrocardiogram was obtained and measurements were made for the RR and QT intervals, and the QT interval was corrected for heart rate by the Bazett formula. At enrollment, a medical history was obtained, and the patient was followed prospectively at yearly intervals by phone contact; medical records were obtained from the patient's attending physician as previously reported. 2 Specific cardiac end points included syncope (defined as the transient loss of consciousness that was sudden in onset and offset), aborted cardiac arrest (requiring external defibrillation during resuscitation), or LQTS‐related sudden cardiac death (unexpected sudden death exclusive of a known cause). Only cardiac events occurring through age 40 were considered in our analysis to minimize the influence of age‐related disease other than LQTS on morbidity and mortality events. The age at onset and offset of medications used in the treatment of LQTS was recorded, as was the age of left cardiac sympathetic denervation therapy and the age at implantation of a pacemaker or cardiac defibrillator.
Statistical Analysis
Student's t‐test for continuous variables and chi‐square or Fishers exact test for categorical variables were used to compare the clinical characteristics in the patient subgroups. Kaplan‐Meier life‐table graphs 4 were constructed to evaluate the probability of survival to the first cardiac event by race, and the two groups were compared using the log‐rank test. The Cox proportional hazards regression model was used to evaluate the significant and independent contribution of various covariates including time‐dependent beta‐blocker therapy to specific outcome events. 5 All models were stratified by the decade in which the patient was born (before 1970, 1970–1980, 1980–1990, or after 1990) to account for differences in the baseline hazard function for historically different time periods in which different LQTS‐related therapies were used. The statistical software used for the analyses was SAS version 9.13 (SAS Institute Inc, Cary, NC). A 2‐sided 0.05 significance level was used for hypothesis testing.
RESULTS
In the study population of 3497 affected patients with LQTS (QTc ≥ 450 ms), 41 patients (1.2%) were African‐American. The clinical characteristics of the LQTS study population are presented in Table 1. A larger percentage of African‐Americans were enrolled in the U.S. portion of the LQTS Registry after 1950 than were Caucasians. The QTc was 29 ms longer in African‐Americans (P < 0.001), and cardiac events and LQTS therapies were more frequent in African‐Americans than in Caucasians. Only two African‐American subjects had genotype studies, and both had an LQT1 mutation. The Kaplan‐Meier graph for first cardiac events (syncope, aborted cardiac death, or LQTS‐related death, whichever comes first) is presented in Figure 1. The cardiac events were dominated by syncope, with the cardiac event rate somewhat higher in African‐Americans than in Caucasians especially after age 20.
Table 1.
Clinical Characteristics of the Study Population by Race
| Caucasians (n = 3456) | African‐Americans (n = 41) | P‐Value | |
|---|---|---|---|
| Females | 2140 (61%) | 26 (63%) | 0.9 |
| Born before 1950 | 892 (26%) | 4 (10%) | <0.01 |
| ECG data | |||
| RR interval (ms) | 809 | 820 | 0.73 |
| QT (ms) | 438 ± 75 | 468 ± 77 | 0.01 |
| QTc (ms) | 491 ± 45 | 520 ± 65 | <0.01 |
| Medical interventions | |||
| Beta‐blockers | 1573 (45%) | 30 (73%) | <0.01 |
| Left cardiac sympathectomy | 99 (33%) | 4 (10%) | 0.03 |
| Pacemaker | 210 (6%) | 6 (15%) | 0.03 |
| Implanted defibrillator | 323 (9%) | 6 (15%) | 0.30 |
| Cardiac event | |||
| Syncope | 1270 (37%) | 23 (56%) | 0.01 |
| Aborted cardiac arrest | 223 (6%) | 3 (7%) | 0.80 |
| LQTS‐related death | 124 (4%) | 2 (5%) | 0.70 |
Data are expressed as mean ± SD or as number (percentage).
Figure 1.
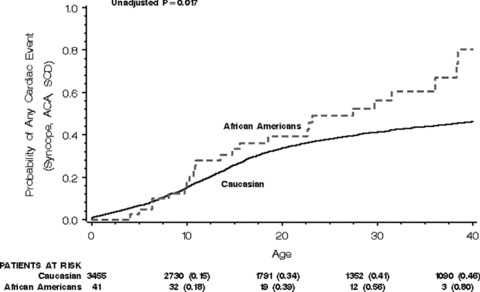
Kaplan‐Meier graph of the probability of a first cardiac event.
Multivariate analysis revealed that African‐Americans had a similar risk for cardiac events as Caucasians after adjustment for relevant covariates, both when QTc was excluded from the model (Table 2A) and when QTc was included in the model (Table 2B). Beta‐blockers were effective in reducing the cardiac event rate and there was no significant interaction of beta‐blocker therapy by race, a finding indicating that both racial groups obtained similar reduction in cardiac events with this therapy.
Table 2.
Multivariate Cox Model Hazard Ratios
| Factor | Hazard Ratio | 95% CI | P‐Value |
|---|---|---|---|
| (A) For cardiac events without the inclusion of QTc in the model | |||
| African‐American:Caucasian | 1.10 | 0.68–1.79 | 0.70 |
| Male:Female 0–13 years | 1.29 | 1.09–1.52 | <0.01 |
| Female:Male 14–40 years | 2.28 | 1.85–2.81 | <0.001 |
| Time‐dependent β‐blocker use (yes:no) | 0.62 | 0.48–0.78 | <0.001 |
| (B) For cardiac events with inclusion of QTc in the model | |||
| African‐American:Caucasian | 0.93 | 0.57–1.50 | 0.76 |
| QTc ≥ 500 ms | 2.10 | 1.87–2.37 | <0.001 |
| Male:Female 0–13 years | 1.28 | 1.08–1.50 | <0.01 |
| Female:Male 14–40 years | 2.32 | 1.88–2.85 | <0.001 |
| Time‐dependent beta‐blocker use (yes:no) | 0.55 | 0.44–0.70 | <0.001 |
DISCUSSION
This study highlights three important findings regarding LQTS in African‐Americans compared to Caucasians: (1) African‐Americans enrolled in the LQTS Registry have a significantly longer QTc interval than Caucasians; (2) African‐Americans have a similar risk for cardiac events as Caucasians after adjustment for relevant covariates; and (3) African‐Americans with LQTS receive the same benefit from beta‐blockers as Caucasians.
African‐Americans made up only a small percentage of subjects in our LQTS Registry‐based study population (1.2%), less that 10% of what might be expected since African‐Americans make up approximately 18% of the general U.S. population. In addition, African‐Americans included in the Registry had a more severe form of LQTS with longer QTc intervals and more frequent cardiac events than Caucasians. These findings may reflect a referral bias related in part to socioeconomic and medical care issues, such that only African‐Americans with more severe manifestations of LQTS were referred to the Registry. African‐Americans were enrolled later by birth decade into the LQTS Registry than Caucasians, and this might well explain their more severe form of LQTS that was observed in the univariate analyses. The Cox model with decade of birth stratification adjusts for this imbalance, and in the multivariate analysis, African‐Americans were compared to Caucasians born in the same decade. It is this birth stratification analysis that demonstrated a similar outcome by race (Table 2A and B).
Studies of healthy adult population have shown that African‐American men and women age 25–74 years have QTc intervals that are, on average, about 2–5 ms shorter than Caucasians. 6 However, hypertension is more frequent in African‐Americans than in Caucasians and hypertension is one of the leading causes of left ventricular hypertrophy. 7 Oikarinen, et al. showed that ECG evidence of left ventricular hypertrophy in hypertensive patients is associated with a longer QT interval than in patients without ventricular hypertrophy. 8 We do not have reliable measures of blood pressure in our study population, so we cannot say anything about the frequency of hypertension in the two racial groups. Unrecognized hypertension and left ventricular hypertrophy may have contributed to the longer QTc interval in African‐Americans subjects with LQTS.
It is well known that gene variants have different frequency distributions by race. Splawski, et al. showed that 13.2% of African‐Americans carry the Y1102 allele variant of the SCN5A cardiac sodium channel gene, and this allele was not observed in a control group of 511 Caucasians. 9 The Y1102 variant was associated with subtle electrophysiologic changes in channel gating, yet African‐Americans carrying this variant had normal QTc values but an increased susceptibility to QTc prolonging medications. 9 Some of the African‐American patients in our LQTS study may have carried the Y1102 gene variant, but in the absence of genetic screening for this polymorphism, we are not able to make any comment on the influence that this gene variant may have had on the clinical course of LQTS in our African‐American study patients.
The major limitations in this observational retrospective study are the relatively small number of African‐Americans with LQTS and the paucity of genetic data. Nevertheless, the study involves the largest number of African‐Americans subjects with LQTS reported to date. The current findings highlight the need for larger studies to more fully evaluate the phenotype‐genotype aspects of LQTS in African‐Americans.
The U.S. Portion of the International Long QT Syndrome Registry Investigators includes: Michael J. Ackerman, M.D., Ph.D. (Mayo Clinic College of Medicine, Rochester, MN), Elizabeth Kaufman, M.D., (MetroHealth Campus, Case Western Reserve University, Cleveland, OH), Jeffrey Towbin, M.D. (Cincinnati Children's Hospital Medical Center, Cincinnati, OH), and G. Michael Vincent, M.D. (University of Utah Medical School, Salt Lake City, UT.
This work was supported in part by research grants HL‐33843 (Moss) and HL‐51618 (Moss) from the National Institutes of Health, Bethesda, Maryland and by by an unrestricted research grant from BioReference Labs, Inc., Elmwood Park, New Jersey.
REFERENCES
- 1. Goldenberg I, Moss AJ. Long QT syndrome. J Am Coll Cardiol 2008;51:2291–2300. [DOI] [PubMed] [Google Scholar]
- 2. Moss AJ, Schwartz PJ, Crampton RS, et al The long QT syndrome. Prospective longitudinal study of 328 families. Circulation 1991;84:1136–1144. [DOI] [PubMed] [Google Scholar]
- 3. Moss AJ. Long QT Syndrome. JAMA 2003;289:2041–2044. [DOI] [PubMed] [Google Scholar]
- 4. Kaplan EL, Meier P. Non‐parametric estimation from incomplete observations. J Am Stat Association. 1958;53:457–481. [Google Scholar]
- 5. Cox DR. Regression models and life‐tables. J Stat Soc [B] 1972;34:187–220. [Google Scholar]
- 6. Ferdinand KC, Saunders E. Hypertension‐related morbidity and mortality in African Americans—Why we need to do better. J Clin Hyperten (Greenwich, Conn) 2006;8(1 Suppl 1):21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vitelli LL, Crow RS, Shahar E, et al Electrocardiographic findings in a healthy biracial population. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Am J Cardiol 1998;81:453–459. [DOI] [PubMed] [Google Scholar]
- 8. Oikarinen L, Nieminen MS, Viitasalo M, et al Relation of QT interval and QT dispersion to echocardiographic left ventricular hypertrophy and geometric pattern in hypertensive patients. The LIFE study. The Losartan Intervention For Endpoint Reduction. J Hyperten 2001;19:1883–1891. [DOI] [PubMed] [Google Scholar]
- 9. Splawski I, Timothy KW, Tateyama M, et al Variant of SCN5A sodium channel implicated in risk of cardiac arrhythmia. Science 2002;297:1333–1336. [DOI] [PubMed] [Google Scholar]


