Abstract
Genetically encoded fluorescent sensors can be valuable tools for studying the abundance and flux of molecules in living cells. We recently developed a novel class of sensors composed of RNAs that can be used to detect diverse small molecules and untagged proteins. these sensors are based on spinach, an RNA mimic of GFP, and they have successfully been used to image several metabolites and proteins in living bacteria. Here we discuss the generation and optimization of these spinach-based sensors, which, unlike most currently available genetically encoded reporters, can be readily generated to any target of interest. We also provide a detailed protocol for imaging ADP dynamics in living Escherichia coli after a change from glucose-containing medium to other carbon sources. the entire procedure typically takes ~4 d including bacteria transformation and image analysis. the majority of this protocol is applicable to sensing other metabolites and proteins in living bacteria.
Introduction
The ability to monitor changes in abundance of molecules in living cells is crucial for studying cellular physiology. We recently described a generalizable, fluorescence-based approach for sensing small molecules and proteins in vitro and in living bacteria. This approach involves fusing an RNA aptamer, which is selective for a target ligand, to the Spinach aptamer, which is an RNA mimic of GFP1–3.
Spinach is a 98-nt-long RNA aptamer that binds to and switches on the fluorescence of 3,5-difluoro-4-hydroxybenzylidene imidazolinone (DFHBI), a small molecule that resembles the chromophore of GFP1. Importantly, both Spinach and DFHBI are essentially nonfluorescent when unbound, whereas the Spinach-DFHBI complex is brightly fluorescent both in vitro and in living cells1.
We extended the Spinach technology to develop a modular platform for generating sensors to small-molecule metabolites2. To do this, we fused previously published RNA aptamers for target metabolites into stem loop 3 of Spinach via a transducer stem (Fig. 1). In the absence of a ligand, the aptamer region (recognition module) and Spinach are unfolded, and thus nonfluores-cent. However, in the presence of a ligand, the aptamer region folds, which induces the folding, dye binding and fluorescence of Spinach.
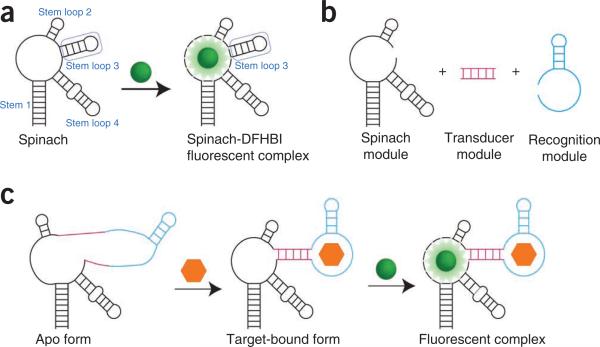
Figure 1.
Modular strategy for generating Spinach-based sensors. (a) Spinach is an RNA aptamer that binds a small-molecule dye called DFHBI (green ball). Both DFHBI and Spinach are nonfluorescent until binding occurs and activates the fluorescence of the Spinach-DFHBI complex. Stem loop 3 of Spinach can tolerate insertion of additional sequences, and it is the region that is modified to generate sensors. (b) In Spinach-based sensors, Spinach is modified to include a transducer region (magenta) and a recognition module (cyan). Recognition molecules are typically aptamers generated against a target ligand by SELEX2,3, but they can also be composed of riboswitch regions10,11 and naturally occurring RNAs3. Transducers of varied length and composition can be generated in order to optimize sensor function. (c) In the absence of DFHBI and ligand (orange hexagon), the Spinach-based sensor displays minimal fluorescence. However, upon target binding, the recognition module of the sensor folds and induces folding of the Spinach portion of the sensor. The Spinach-based sensor is then able to bind DFHBI and activate fluorescence.
A major advantage of the modular, Spinach-based sensor design strategy over other genetically encoded sensors is that it is readily adaptable to monitor a wide array of target molecules4. In principle, a sensor can be made for any ligand that has a corresponding aptamer, such as an aptamer generated using the systematic evolution of ligands by exponential enrichment (SELEX) approach. SELEX has been used to generate highly specific aptamers for a diverse range of targets5,6.
This generalizable approach to sensor construction is in contrast to the generation of FRET-based sensors. Current FRET-based sensors are composed of a fluorescent protein FRET pair fused to either side of a protein recognition element specific for a ligand of interest7,8. In the presence of a ligand, the protein undergoes a conformational change that results in a change in FRET signal. Such sensors are useful because they are genetically encoded, and they have been successfully used to monitor multiple cellular metabolites in living cells in real time. However, these FRET-based sensors are not easily generalizable, as they rely on the existence of a ligand-binding protein that binds specifically to the target and undergoes enough conformational change upon binding to alter FRET efficiency. Many proteins and metabolites lack such a protein, which makes the development of FRET-based sensors very challenging.
Several other fluorescence intensity–based protein sensors have been successfully used to monitor ions, small molecules and proteins9. These methods typically involve bifluorescence complementation of a single fluorescent protein upon ligand binding or modulation of the intrinsic fluorescence of a fluorescent protein by a target ligand9. However, none of these approaches is easily generalizable to monitor any ligand of interest. It is important to note, however, that a shared weakness of all fluorescence intensity– based sensors, including Spinach-based sensors, is that they require coexpression of another fluorescent protein to which to normalize the fluorescence signal. This is particularly important in order to control for variations in cellular volume. Fluorescence intensity–based sensors tend to be most useful for monitoring changes in ligand concentrations rather than for precise measurements of absolute ligand concentrations.
To date, we have generated Spinach-based sensors to study the small-molecule metabolites adenosine, ADP,S-adenosylmethionine (SAM), guanine and GTP2, and the proteins streptavidin, thrombin and MS2 coat protein (MCP)3. Spinach-based sensors for the second messengers cyclic di-GMP10,11 and cyclic AMP-GMP10 were also recently reported. A detailed list of currently available sensors is provided in Table 1. In this protocol, we describe the method developed in our laboratory for using Spinach-based sensors to image ADP dynamics in living E. coli cells in response to varying carbon sources. We also discuss the use of Spinach-based sensors to monitor dynamic changes in target protein expression in E. coli.
TABLE 1.
Available Spinach-based sensors.
| Sensor | Ligand | EC50 for ligand (μM) | KD (nM) | Fold activation | Tested in bacteria |
|---|---|---|---|---|---|
| Ade sensora | Adenosine | 44 | 16 | No | |
| ADP sensora | Adenosine diphosphate | 270 | 18 | Yes | |
| Gua sensora | Guanine | 1.5 | 32 | No | |
| GTP sensora | Guanosine triphosphate | 7.7 × 103 | 15 | No | |
| SAM sensora | S-adenosylmethionine | 120 | 25 | Yes | |
| Vc2-Spinachb | c-di-GMP | 230 | ND | Yes | |
| G20A Vc2-Spinachb | c-di-GMP | 1,000 | ND | Yes | |
| Camp-GMP | 4,200 | ND | Yes | ||
| SP_2c | c-di-GMP | ND | ND | No | |
| Streptavidin sensord | Streptavidin | ND | 10 | Yes | |
| Thrombin sensord | Thrombin | ND | 7 | No | |
| MCP sensord | MS2 coat protein | ND | 42 | Yes |
Experimental design
Generation of Spinach-based sensors
Spinach-based sensors can be developed rapidly against a wide variety of ligands, because they take advantage of the widely used SELEX approach for aptamer generation5,6. The SELEX technique can be carried out in 3–4 weeks, and it involves the screening of a large library (typically >1013 unique molecules) to enrich for sequences that bind a target ligand. This approach has been successfully used to generate aptamers against a range of molecules including ions, small molecules, metabolites, proteins and protein complexes6.
An important aspect of aptamer design for use in a Spinach-based sensor is ligand-binding affinity (EC50, Box 1). For proper sensing, a sensor should be tuned to the physiological range of the target molecule. This means that aptamers should be chosen with an EC50 that will allow relevant changes in concentration to result in linear changes in fluorescence. To obtain such aptamers, SELEX protocols can be modified to enrich for aptamers with a desired binding affinity6. The ADP aptamer used in the Spinach-based ADP sensor described in this protocol has an EC50 of 270 μM (Fig. 2a)2, which is appropriate for monitoring ADP levels in E. coli, as ADP is present at concentrations well below the EC50 value12.
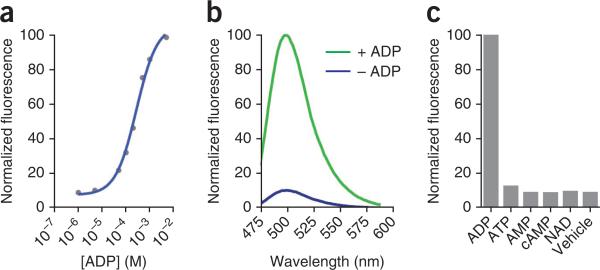
Figure 2.
Characteristics of the ADP sensor. (a) Dose-response curve of fluorescence of the ADP sensor with increasing concentrations of ADP. (b) Emission spectra of the ADP sensor in the presence or absence of ADP. (c) Molecular discrimination of the ADP sensor. Fluorescence brightness values for the ADP sensor in the presence of 1 mM ADP, ATP, AMP, cyclic AMP (cAMP), nicotinamide adenine dinucleotide (NAD) or vehicle are shown.
Another key consideration is ligand specificity, as some small metabolite molecules differ from other cellular molecules by as little as a methyl or phosphate group. SELEX has been used to generate highly specific RNA aptamers13. For example, SELEX was used to discover the aptamer fused to Spinach in the ADP sensor, which specifically binds ADP, even in the presence of high concentrations of ATP14.
Once an appropriate aptamer has been found, a fusion is generated, in which the aptamer is inserted into stem loop 3 of Spinach via a short transducer domain (Fig. 1). Our studies have demonstrated that the precise sequence of this transducer domain can have a strong effect on the activity of the sensor2,3. For this reason, we test several transducer domains during the development of each sensor. These test transducer domains vary in both length and complementarity, typically ranging from 1 to 8 bp in length and containing 0–4 mismatches. The optimal transducer domain is chosen on the basis of in vitro fluorescence activation experiments, with the optimal transducer being the one that produces the highest fold-activation signal in the presence of the ligand coupled with the lowest background signal in the absence of the ligand. In the case of ADP sensing, the optimal ADP sensor is composed of the ADP aptamer fused to Spinach via a 4-bp transducer domain2 (Table 2).
TABLE 2.
Spinach-based sensor sequences.
| Name | Sequencea |
|---|---|
| Spinach | GACGCAACTGAATGAAATGGTGAAGGACGGGTCCAGGTGTGGCTGCTTCGGCAGTGCAGCTTGTTGAGTAGAGTGTGAGCTCCGTAACTAGTCGCGTC |
| ADP sensor | GACGCAACTGAATGAAATGGTGAAGGACGGGTCCAGCACGAGGGGGAAACCCCGGACAATCAGACACGGTGCTTGTTGAGTAGAGTGTGAGCTCCGTAACTAGTCGCGTC |
The Spinach module of the sensor (top row) is shown in normal font; underlined is the sequence of its stem-loop 3, which is to be replaced by the molecule-specific module. The molecule-specific module is shown inserted into the Spinach sequence in the bottom row, in bold, and comprises the transducer module (underline) and the molecule recognition module (italics). See Figure 1 for more information.
Synthesis and cloning of Spinach-based sensors
A panel of Spinach-based sensors with different transducer domains can be ordered as single-stranded DNA oligonucleotides or as short double- stranded synthetic DNA molecules. These can then be used in PCR to generate template for in vitro transcription reactions, as previously described1–3. The resulting RNA can be used for screening the sensors by using the in vitro assays described below.
After determining the optimal sensor based on in vitro analyses, the DNA corresponding to the optimal sensor can then be cloned into a bacterial expression vector under the control of an inducible promoter. To do this, the desired DNA template should be amplified by PCR using primers that encode appropriate restriction sites for cloning into the target vector. Next, the PCR product should be digested with the specified restriction enzymes and it should cloned into the target vector that has also been subjected to the appropriate restriction digest. For the experiments described in this protocol, the ADP sensor was cloned into the pET-28c vector under the control of the inducible T7 promoter for strong expression2. It is notable that for all in vivo experiments, we express the Spinach-based sensors in the context of a tRNA2,3. This tRNA acts as a scaffold15 and improves the folding of Spinach-based sensors, and it has resulted in fluorescence signals that are sufficient to image all sensors tested.
Testing Spinach-based sensors
All Spinach-based sensors should be characterized for function in vitro before their use in live-cell imaging. First, the level of fold activation for the sensor should be measured. To determine fold activation, prepare the sensor by in vitro transcription, and incubate it with DFHBI in the presence or absence of a ligand. The fold activation is represented by the fluorescence value in the presence of a ligand divided by the fluorescence value in the absence of a ligand. Useful sensors will display near background fluorescence in the absence of a ligand, and 10-to-40-fold activation in the presence of a ligand. We observed an 18-fold activation for the ADP sensor in the presence of ADP (Fig. 2b)2.
In addition, the EC50 for ligand binding should be determined by measuring the sensor signal in the presence of increasing concentrations of the ligand. This measurement will also demonstrate the linear range of the sensor and serve as a standard curve for signal measurement. Ideally, the changes in cellular ligand concentration will be within the linear range of the sensor. However, fitting the fluorescence data to generate a standard curve for sensor signal can allow for concentrations outside the linear range of the sensor to be determined mathematically16.
The specificity of the sensor should also be tested by determining fold activation by relevant molecules that are chemically similar to the ligand. For example, the ADP sensor shows no activation in the presence of 1 mM ATP, although it differs from ADP by only a single phosphate (Fig. 2c)2.
In addition, the kinetics of fluorescence activation and deactivation of a sensor should be established. These experiments are typically performed in vitro. Ideally, a sensor will respond instantaneously to changes in ligand concentration. However, in practice, some time is necessary for ligand binding to induce the conformation changes in the sensor that lead to fluorescence2,3. Thus, it is important to measure the kinetics of each sensor to ensure that the rates of activation and deactivation are suitable for the processes being monitored. For example, if a sensor requires minutes to achieve maximal signal or lose fluorescence after ligand depletion, it may not be suitable to monitor processes that occur on the order of seconds. The ADP sensor reaches maximal fluorescence after 12 min of incubation with ADP, and it is >90% deactivated after 8 min (ref. 2), making it suitable for monitoring changes in ADP levels that occur on the scale of tens of minutes. For detailed protocols for each aspect of sensor validation, please refer to Paige et al.2 and Song et al.3. After testing and validation, sensors can be used to monitor ligand concentration dynamics in living bacteria.
Validated sensors should then be tested in bacterial cells. Ideally, use control conditions in which the ligand of interest is present at the limits of its normal concentration range; this allows for the dynamic range of fluorescence signal change to be determined empirically2,3,10. For some ligands, such as ions, these conditions can be artificially generated by perforating the cells and incubating them with buffers that either contain or lack the ligand of interest17. At the highest concentrations, fluorescence signal should be high, whereas at the lowest concentrations, fluorescence signal should be near background. For example, in our studies of S-adenosylmethionine (SAM) levels in E. coli, we measured fluorescence signals in cells deprived of methionine2, a treatment known to markedly reduce cellular levels of SAM18. In the case of our studies measuring MS2 coat protein (MCP) levels, we established our background in E. coli that do not express MCP, whereas the maximal signal was determined by overexpression of MCP3. These kinds of control experiments are especially important to demonstrate proper sensor function in cases where no independent measure of ligand concentration is available.
If the sensor signal is constitutively high at both high and low ligand concentrations, it indicates that the ligand concentration is too high to be accurately measured by using the sensor, or that the sensor is being activated nonspecifically. If the sensor signal is low or near background level at both high and low concentrations, it indicates that either the ligand concentration is too low to be measured with the sensor or that the sensor is misfolded in cells.
For the sensors that we have tested, we have observed that the high specificity needed for sensor activation observed in vitro was maintained in living bacteria2,3. Once a sensor has been validated in bacteria, it can be used to monitor changes in ligand concentration over time.
Additional considerations
An additional aspect of sensor design and use that should be considered is sensor occupancy. Sensors can be developed that have either high or low occupancy of ligand binding. High occupancy refers to sensors that are always bound to their ligand in normal cellular conditions. Thus, the fluorescence signal is directly proportional to the amount of ligand. This binding mode typically occurs when EC50 values for ligand binding are low relative to cellular ligand concentrations. We observed this sort of high-occupancy binding for the MCP sensor3. This sensor used an MCP-binding domain that was measured to interact with MCP with a KD of around 200 pM (ref. 19). As a result, each MCP protein, which is present at a concentration of 0–60 μM cells, is bound to a sensor RNA. A potential advantage of high-occupancy binding is a large dynamic range in signal. A drawback is that the function of the ligand may be compromised by the bound sensor. The sensor RNA concentration needs to exceed the concentration of the target molecule to ensure linear increases in fluorescence.
In contrast, low-occupancy binding occurs when the ligand concentration is lower than the EC50 value for the sensor, and hence only a fraction of the sensor molecules are bound by the ligand. Low-occupancy ligand binding is observed for the ADP sensor2. In this case, the EC50 is 270 μM, whereas the cellular concentrations of ADP vary between ~100 [.proportional]M and ~200 μM (refs. 2,20). The amount of the sensor that is bound and emitting fluorescence can be approximated by the following equation, where F is the fraction of sensor molecules bound to the ligand, [L] is the cytosolic ligand concentration and EC50 is the EC50 for fluorescence activation of the sensor:
A potential advantage of low-occupancy binding is that some fraction of the ligand is free to participate in normal cellular function.
Imaging ADP dynamics in E. coli
Ligand levels may vary in response to growth conditions in cells, and they can be a useful readout of the cellular metabolic state. For example, the levels of ADP rise rapidly under conditions of glucose deprivation, as ATP molecules are consumed by cells.
To monitor this conversion of ATP to ADP in E. coli, we express the ADP sensor from the pET28c vector under the control of an inducible, strong T7 promoter. After induction, cells are added to medium supplemented with various carbon sources and DFHBI and imaged over time. The fluorescence intensity in a given E. coli can then be monitored over time to determine ADP concentration dynamics within an individual cell, as well as a population of cells (Fig. 3). For these experiments, cells expressing pET28c-tRNA with no sensor are used as controls to determine background fluorescence levels in the presence of DFHBI. Cells induced to express Spinach from pET28ctRNA-Spinach are also imaged under all conditions to provide a positive control for fluorescence with varying carbon sources.
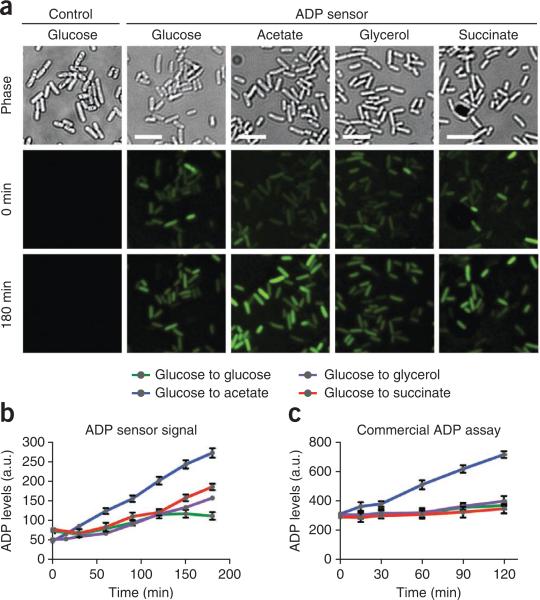
Figure 3.
Live-cell imaging and analysis of endogenous ADP levels. (a) E. coli cells expressing the ADP sensor grown in various carbon sources. Cells grown in minimal medium containing 200 μM DFHBI and glucose were switched to alternate carbon sources as indicated. Images shown were obtained at 0 and 180 min after medium change, respectively. Scale bars, 5 μm. (b) Quantification of ADP levels on the basis of ADP sensor fluorescence signal. Individual cells were imaged over 3 h, and fluorescence was measured and normalized to cellular area by using ImageJ software. Results shown represent mean and s.e.m. values for 150 cells per condition. (c) Quantification of ADP levels by using a commercially available ADP assay. Cells grown under identical conditions to those used for the ADP sensor assay were subjected to a bulk fluorometric ADP assay. Shown are mean and s.e.m. values for three independent experiments. The results of this assay correlate well to signal changes observed using the ADP sensor. a.u., arbitrary units.
When available, secondary assays such as commercial kits and HPLC are useful for corroborating results obtained using Spinach-based sensors2,3,10. Although our control experiments demonstrated that sensor expression is highly uniform among cells2,3, additional controls can include coexpression of Spinach-based sensors with either a blue or red fluorescent protein. If coexpression of a fluorescent protein is used to control for variability in sensor expression, the sensor fluorescence can be normalized to the expression of the fluorescent protein. The fluorescent protein and sensor should be expressed from separate promoters on the same plasmid in order to control for plasmid copy number variability. These types of normalizations may reduce experimental noise3.
Additional optimizations may be necessary when you are imaging other sensors. For example, sensor expression level may be adjusted on the basis of promoter strength or length of expression. Lowering sensor expression could enhance the signal- to-noise ratio in situations where background fluorescence is high. Conversely, increasing sensor expression could enhance total observable signal for some target molecules. These levels must be optimized individually for each sensor and ligand pair.
Adapting methodologies to protein sensing in bacteria
We recently described Spinach-based sensors to measure protein concentration of untagged proteins in living E. coli3. The imaging strategy for monitoring protein levels is analogous to that of monitoring the levels of small molecules described in this protocol. As discussed above, protein sensors must be tested for fold activation and validated for specificity before use. Our experiments with the MCP sensor demonstrated that the sensor signal increased linearly with protein expression in E. coli, and can provide unique insight into cell-to-cell variability in expression levels3.
The use of Spinach sensors in mammalian systems
To date, the use of Spinach-based sensors has been limited to in vitro analyses and studies in bacteria. This is due to low signal from Spinach-based sensors in mammalian cells. This low signal is probably caused by the relatively low abundance and poor stability of these small RNAs in mammalian cells. In contrast, bacterial cells produce high concentrations of Spinach-based sensors, which provide readily detectable fluorescence readouts of ligand levels. The use of Spinach-based sensors in mammalian cells will require methods for improved expression and stability in such cells.
MATERIALS
REAGENTS
Poly-l lysine (PLL; Trevigen, cat. no. 3438-100-01)
Tryptone (Sigma-Aldrich, cat. no. T9410)
Yeast extract (Fisher, cat. no. BP9727)
Agar (VWR, cat. no. 90000)
Sodium chloride (NaCl; US Biological, cat. no. S5000)
Kanamycin sulfate (Sigma-Aldrich, cat. no. K4000)
Chloramphenicol (Sigma-Aldrich, cat. no. C0378)
Potassium phosphate monobasic (KH2PO4; Sigma-Aldrich, cat. no. P9791)
Sodium phosphate dibasic (Na2HPO4; Sigma-Aldrich, cat. no. 71643)
Magnesium sulfate (MgSO4; Sigma-Aldrich, cat. no. M7506)
Calcium chloride (CaCl2; Sigma-Aldrich, cat. no. C5080)
Ammonium chloride (NH4Cl; Sigma-Aldrich, cat. no. A9434)
Super optimum culture medium (SOC; Invitrogen, cat. no. 15544)
Glucose (Sigma-Aldrich, cat. no. G7528)
Potassium acetate (Sigma-Aldrich, cat. no. P1190)
Glycerol (Sigma-Aldrich, cat. no. G9012)
Succinate (Sigma-Aldrich, cat. no. W327700)
IPTG (Sigma-Aldrich, cat. no. I6758)
DMSO (Sigma-Aldrich, cat. no. D2650)
Chemically competent E. coli BL21 Star (DE3) cells (Invitrogen, cat. no. C6010-03)
DNase, RNase-free water (Gibco, cat. no. 10977)
Plasmids: pET28c-tRNA, pET28c-tRNA-Spinach and pET28c-tRNAADP sensor (or any other sensor). Plasmids were generated as previously described1,2, and they are freely available to the scientific community by contacting the Jaffrey laboratory (srj2003@med.cornell.edu)
DFHBI (Lucerna, cat. no. 400)
EQUIPMENT
Glass-bottom 24-well plates (Mattek Corporation, cat. no. P24G-1.5-13-F)
Petri dishes, 10 cm (VWR International, cat. no. 25384-094)
Culture tubes (VWR, cat. no. 60818)
Erlenmeyer flasks with baffles, 25 ml (Corning, cat. no. 355115)
Microcentrifuge tubes, 1.5 ml (USA Scientific, cat. no. 1615-5500)
Microcentrifuge (Eppendorf, 5424)
Water bath or heat block, 42 °C
Air incubator, 37 °C
Inverted wide-field fluorescent microscope (Nikon, TE2000)
Motorized stage (Ludl, BioPrecision motorized flat-top stage) or similar
High-numerical-aperture (high-NA) fluorescence objective (Nikon, Plan Apo, ×60/1.4, oil)
CoolSnap HQ2 CCD camera (Photometrics) or similar
Filter cube suitable for green fluorescence imaging such as FITC or GFP filter sets (e.g., with a sputter-coated excitation filter 470/40, dichroic mirror 495 (long pass) and emission filter 525/50 (Chroma Technology))
Temperature control environmental chamber for microscope. Our microscope is housed in a custom-built environmental chamber that maintains a temperature of 35–37 °C
NIS Elements (Nikon) or similar image acquisition software
Fiji (http://fiji.sc/Fiji), ImageJ or similar image analysis software
REAGENT SETUP
LB medium Combine 10 g of tryptone, 5 g of yeast extract and 10 g of NaCl; dissolve the mixture in a final volume of 1 liter of deionized water and sterilize it by autoclaving for 15 min at 121 °C. The medium can be stored indefinitely at room temperature (25 °C) until antibiotic addition, at which point it can be stored for up to 2 months at 4 °C. Prewarm the medium to desired temperature (37 or 25 °C) before use.
LB solid medium (plates) Combine 10 g of tryptone, 5 g of yeast extract, 10 g of NaCl and 20 g of agar; dissolve the mixture in a final volume of 1 liter of deionized water, and sterilize it by autoclaving for 15 min at 121 °C. Allow the medium to cool before supplementing it with 50 γ]g γl −1 kanamycin and 34 μg μl −1 chloramphenicol, and then pour it into 10-cm Petri dishes. Plates can be stored for up to 2 months at 4 °C. Prewarm to the desired temperature (37 or 25 °C) before use.
M9 salts solution, 5× Combine 64 g of Na2HPO4, 15 g of KH2PO4, 2.5 g of NaCl and 5 g of NH4Cl, and then dissolve the mixture in a final volume of 1 liter of deionized water and sterilize it in an autoclave. M9 salts can be stored indefinitely at room temperature.
M9 medium Combine 200 ml of 5× M9 salts solution, 2 ml of 1 M MgSO4 (sterile), 100 μl of 1 M CaCl2 (sterile) and an appropriate concentration of the desired carbon source. Adjust the pH of the solution to 6.0 and adjust the final volume to 1 liter with sterile deionized water. For this medium, carbon sources should be added as powder to the appropriate final concentration of 2 mg ml −1 for glucose, 6.65 mg ml −1 for potassium acetate, 4.05 mg ml −1 for glycerol or 5.51 mg ml −1 for succinate, and the final medium should be sterilized by vacuum filtration through a 0.22-μm-pore filter. The medium can be stored indefinitely at room temperature until the addition of 50 μg μl −1 kanamycin and 34 [.proportional]g μl −1 chloramphenicol, at which point it can be stored for up to 2 months at 4 °C. Prewarm the medium to the desired temperature (37 or 25 °C) before use.
DFHBI Create a 40 mM stock solution by dissolving 5 mg of DFHBI in 500 μl of DMSO. Store the solution at 4 °C in the dark. ▲ CRITICAL DFHBI should be shielded from light. All stock solutions of DFHBI should be maintained in dark tubes or wrapped in foil. Plates containing cultures incubated with DFHBI should be kept in the dark by using a foil overwrap.
Imaging medium To the M9 medium prepared as described above, add DFHBI to a final concentration of 200 μM.
EQUIPMENT SETUP
Microscope Allow the microscope light source to warm up for 30 min before imaging by prewarming the environmental chamber to 37 °C.
PROCEDURER
Generation of E. coli strains for imaging ● TIMING 12–24 h
▲ CRITICAL All steps must be performed using proper sterile technique.
Thaw three tubes of competent E. coli BL21 Star (DE3) cells on ice.
To the first tube, add 40 ng of pET28c-tRNA-Spinach (positive control). To the second tube, add 40 ng of pET28c-tRNA (negative control). To the third tube, add 40 ng of pET28c-tRNA-ADP Sensor. Mix the DNA with the cells by gently flicking the tubes, and then incubate the mixtures on ice for 20 min.
Heat-shock the cells in a 42 °C water bath for 30 s. Place the tubes back on ice immediately after heat-shock treatment and chill them for 2 min.
Add 300 μl of SOC medium to each tube and incubate the tubes at 37 °C with rotation for 45 min.
Plate 50 μl of each cell mixture onto a Petri dish containing LB medium supplemented with 50 μg μl −1 kanamycin and 34 μg μl −1 chloramphenicol. Incubate plates at 37 °C for 12–14 h to grow individual colonies harboring the desired plasmids.
□ PAUSE POINT Plates containing transformed E. coli cells can be sealed with Parafilm and stored at 4 °C for up to 2 weeks.
Preparation of 24-well plates ● TIMING 4 h
-
6
Remove a glass-bottomed 24-well plate from packaging. Add 300 μl of poly-l-lysine solution to each well in the first three rows (12 wells total). Incubate the plate at 37 °C for 3 h.
-
7
Remove the poly-l-lysine solution by aspiration. Rinse each well twice with 500 μl of sterile water to remove excess poly-l-lysine. Add 500 μl of water and store the plate at room temperature until it is ready to use.
□ PAUSE POINT The prepared 24-well plate can be stored for 24 h at room temperature or for up to 1 week at 4 °C. Unused wells can be used in future experiments.
Preparation of E. coli for imaging ● TIMING 2 d
▲ CRITICAL DFHBI should be shielded from light. All stock solutions of DFHBI should be maintained in dark tubes or wrapped in foil. Plates containing cultures incubated with DFHBI should be kept in the dark by using a foil overwrap.
-
8
Grow an overnight culture for each transformation reaction (Step 5) by inoculating a single colony into a sterile culture tube containing 5 ml of LB medium + 50 μg μl −1 kanamycin and 34 μg [μl −1 chloramphenicol. Grow the cultures at 37 °C with shaking for 12–14 h.
-
9
Measure the optical density at 600 nm (OD600) for a 1:10 dilution of each overnight culture.
-
10
Start fresh cultures by diluting the cells to a starting concentration of 0.05 OD units ml −1 OD600 units ml−1 in a 25-ml baffled Erlenmeyer flask containing 10 ml of LB medium + 50 μg μl −1 kanamycin and 34 μg μl −1 chloramphenicol.
-
11
Grow the cells at 37 °C with shaking to OD600 = 0.4 (~2 h).
-
12
Add IPTG to a 1-mM final concentration. Continue to grow the cells at 37 °C with shaking for 2 h.
▲ CRITICAL STEP IPTG induction can be carried out for 30 min–4 h. However, if induction is carried out for 4 h, the cells should be monitored for concentration and diluted if the OD600 exceeds 2. In our experience, no change in fluorescence intensity is observed between 2- and 4-h induction times.
-
13
Take a 200-μl aliquot from each flask and transfer each to a fresh microcentrifuge tube. Spin the cells at 5,000g for 2 min at room temperature to pellet. Remove the medium from the pellet by aspiration.
-
14
Resuspend each pellet in 1 ml of M9 medium supplemented with 2 mg ml −1 glucose.
▲ CRITICAL STEP M9 minimal medium must be used in imaging experiments for two reasons. The first is that components of LB medium will prevent adherence of E. coli to PLL-treated dishes. The second is that M9 medium has low green fluorescence background.
-
15
Turn on the environmental chamber to prewarm the microscope to 37 °C for imaging.
? TROUBLESHOOTING
-
16
Remove any remaining water from the wells in the PLL-treated 24-well dish (Step 7). Add 200 μl of each cell suspension sample to 4 wells (12 wells total), and incubate the cells at 37 °C for 45 min to allow E. coli cells to adhere to the glass bottom.
-
17
Wash the wells twice with 500 μl of M9 medium to remove unattached cells.
-
18
Replace M9 medium with 200 μl of M9 medium supplemented with 2 mg ml −1 glucose and 200 μM DFHBI, and incubate the cells at 37 °C for 30 min.
Imaging the ADP sensor ● TIMING 4 h
-
19
Move the 24-well plate to the microscope in the prewarmed environmental chamber.
-
20
Use a ×60 objective under phase illumination to focus on adhered E. coli.
? TROUBLESHOOTING
-
21
Move the objective to the well containing cells expressing tRNA-Spinach in imaging medium. In this well, focus on a field containing 50–100 evenly adhered E. coli cells.
-
22
Switch from phase to fluorescence acquisition with FITC filter sets in place. Determine the proper exposure time by acquiring images from 100-ms to 2-s exposure times. This optimal exposure time will allow for the highest possible signal in which no pixels are saturated.
? TROUBLESHOOTING
-
23
By using NIS Elements or your image acquisition software, find appropriate fields of cells in every well, and save the x, y and z positions for your microscope stage so that you can return to the same optical field.
? TROUBLESHOOTING
-
24
Each of the three samples must be imaged using each carbon source: 2 mg ml −1 glucose, 6.65 mg ml −1 potassium acetate, 4.05 mg ml −1 glycerol or 5.51 mg ml −1 succinate. To change carbon sources, wash each well with 500 [.proportional]l of M9 supplemented with the appropriate carbon source twice. Immediately before imaging, replace the medium with imaging medium (M9 + appropriate carbon source + 200 μM DFHBI).
-
25
Set up your software to acquire phase and fluorescence images at time zero (immediately after changing the medium) and every 10 min for 3 h for every well.
Data analysis ● TIMING 1–2 h
-
26
By using NIS Elements or your equivalent image analysis software, open the first image (t = 0) for the cells expressing tRNA alone in M9 + glucose + DFHBI. This frame will be used to subtract the background fluorescence value from autofluorescence and free DFHBI (Fig. 4). In NIS Elements, subtract background by opening the lookup table (LUT) and by adjusting the lower bar until the average pixel intensity within the cells is zero (Fig. 4a). Save this LUT setting and reuse it for all samples grown in glucose. Rename the files and save them as TIFF images. Repeat this step for each carbon source.
-
27
Use Fiji or ImageJ software to open all background-subtracted, time-point images for cells expressing tRNA-Spinach in glucose.
-
28
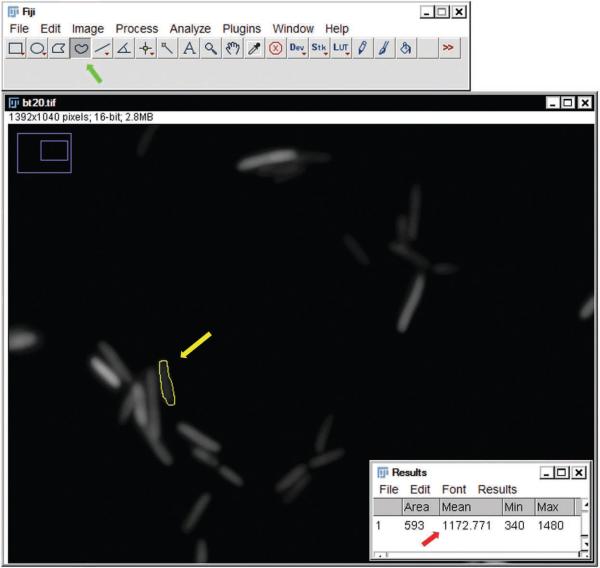
Use the Freehand selection tool to circle an E. coli cell. Open the Analyze menu and select ‘set measurements’. Select the mean gray value. Now select ‘measure’ from the Analyze menu (Fig. 5). Record this value for the same 20 cells in the frame for each time point.
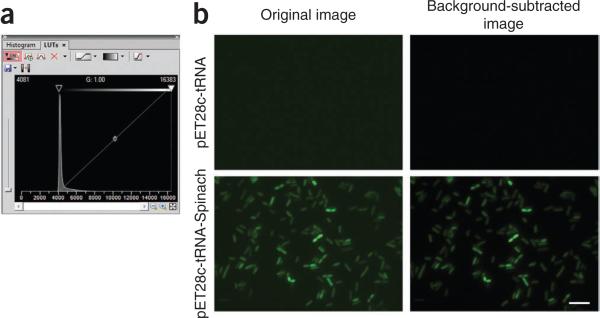
Figure 4.
Background subtraction using the NIS Elements software. (a) Screenshot of the LUT panel of the NIS Elements software. Background subtraction should be carried out by adjusting the lower threshold (yellow arrow) such that the mean intensity within an E. coli is zero. The mean intensity within a cell is typically zero when the lower threshold is set adjacent to the intensity peak as shown. (b) Examples of background-subtracted images. Images are shown before (left column) and after (right column) background subtraction. The upper row shows negative control cells, expressing tRNALys alone. The lower row shows cells expressing Spinach. Scale bar, 5 μ.
Figure 5.
Quantification of mean fluorescence in E. coli. Screenshot of image analysis with Fiji software. The free drawing tool (green arrow in toolbar) can be selected in order to draw a circle around an E. coli cell of interest (yellow circle, yellow arrow). Once this object is selected, the software will calculate the average intensity within the cell. This value will be displayed in a pop-up window, as indicated by the red arrow.
▲ CRITICAL STEP Choose cells that are fully adhered and entirely in focus by using phase images to avoid bias toward the brightest fluorescent cells. Carefully track which cells have been recorded, and use the same cells in each frame.
-
29
Calculate the mean fluorescence value of a field per time point by adding all the mean gray values of the different cells and dividing by the number of cells (20). This mean value represents the maximum fluorescence value for each time point. This value will be used to normalize fluorescence values from the ADP sensor in order to adjust for changes in Spinach signal due to differences in carbon sources over time. Repeat this step for each carbon source.
▲ CRITICAL STEP A tRNA-Spinach control should always be used to adjust for condition-dependent changes in fluorescence signal that are not caused by ligand concentration.
? TROUBLESHOOTING
-
30
Repeat Steps 27–29 for 25–50 cells in wells expressing the tRNA-ADP sensor. By using Microsoft Excel or other spreadsheet software, create a table tracking the fluorescence value of an individual cell relative to time.
-
31
Normalize these values to the tRNA-Spinach signal from the corresponding time point with the appropriate carbon source. These normalized values represent the change in signal fluorescence due to changes in ADP concentration, and they can be compared across carbon sources. To determine fold changes, divide all fluorescence values by the values observed at time zero of treatment.
Generation of heat maps ● TIMING 1 h
-
32
For a pictorial representation of fold change in signal, open each background-subtracted, time-point image (Step 26) in NIS Elements software. Right-click the lower left corner tab labeled ‘FITC’, select ‘channel color’ and choose ‘dark rainbow’.
-
33
By using the image with the most intense ADP sensor signal (Step 26), adjust the upper LUT arrow until the brightest cell is red. Save these LUT settings and apply to all other images from the same time course.
-
34
To show the intensity scale bar as part of the image, select ‘View LUT intensity’ from the side panel and ‘accept’.
-
35
Save false-colored images by creating full-view snapshots. First, show the original image at 100% zoom. Then, select ‘create full view snapshot’ from the Edit menu. Save this file as the heat map image of ADP sensor fluorescence.
? TROUBLESHOOTING
Troubleshooting advice can be found in Table 3.
TABLE 3.
Troubleshooting table.
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 15 | No environmental chamber available | Carry out all steps after Step 18 at room temperature | |
| 20 | Difficulty focusing on cells | Small size of E. coli cells or low cell density | Focus on cells expressing Spinach using fluorescence in the FITC channel |
| Poor magnification of microscope | Make sure the miscroscope in use has an objective capable of imaging E. coli (i.e., ×60–×100 with >1.4 NA) | ||
| 20 | No adhered cells | Residual LB medium in samples can inhibit cell adhesion | Wash cells in M9 media twice before adding them to wells (Step 24) |
| Incomplete poly-lysination | Check freshness and concentration of PLL stock. Carry out second PLL coating (Step 7) | ||
| 22 | No fluorescence signal | Improper concentration of DFHBI | Check stock solutions and remake imaging medium |
| No Spinach expression | Check for Spinach expression by reverse transcription (RT)-PCR or by other means | ||
| 23 | Cannot save and move between x, y and z positions | No automated stage | Carry out time-course measurements on a per-sample basis |
| 29 | Greater than 1.5-fold changes in Spinach signal over time | Incorrect pH of media | Check that all media solutions were made properly and that the pH is adjusted to 6.0 |
● TIMING Steps 1–5, generation of E. coli strains for imaging: 12–24 h
Steps 6 and 7, preparation of 24-well plates: 4 h
Steps 8–18, preparation of E. coli for imaging: 2 d
Steps 19–25, imaging the ADP sensor: 4 h
Steps 26–31, data analysis: 1–2 h
Steps 32–35, generation of heat maps: 1 h
ANTICIPTED RESULTS
Representative images of experimental results are shown in Figure 3a. In our studies, we observed an overall increase in fluorescence of the ADP sensor in cells switched from glucose- to acetate-containing medium, but we observed no substantial change in response to glycerol or succinate. These changes were observable as early as 30 min after changing the medium and increased throughout the 3-h time course of the experiment (Fig. 3b). No change in signal intensity is anticipated for Spinach signal, regardless of carbon source. In the case of small changes in sensor signal, the average fluorescence values obtained during experimental analysis can be subjected to statistical analysis to reveal whether the changes are significant. As a control for our sensor's activity, we carried out an independent analysis of ADP concentrations by using an ADP fluorometric assay kit (Abcam) in cells grown under identical conditions and switched from glucose to acetate, glycerol and succinate. We obtained results (Fig. 3c) that were consistent with the observed changes in fluorescence measured with our ADP sensor, indicating that the ADP sensor accurately reflects changes in ADP levels2. The use of independent assays for ADP levels can allow for the generation of a concentration curve based on fluorescence levels, which can then be used to determine the precise concentration of ADP in every individual E. coli in a field of cells.
Box 1 | Definitions.
EC50: the EC50 is the concentration of the ligand necessary for half-maximal activation of the sensor. This value is related to, but not necessarily identical to, the KD for ligand binding, as the EC50 value is dependent upon sensor concentration. EC50 values for Spinachbased sensors are determined by measuring the concentration of the ligand necessary for achieving half-maximal fluorescence signal of the sensor in the presence of excess DFHBI. EC50 values are obtained for sensors when the KD value cannot be accurately determined. This occurs when the KD value is substantially lower than the concentration of sensor needed for signal detection.
KD: the KD is the dissociation constant of a ligand to its receptor, and it can be determined as the concentration at which half of all receptor molecules are bound to the ligand.
ACKNOWLEDGEMENTS
We thank J.S. Paige and T. Nguyen-Duc for helpful suggestions and Jaffrey lab members for constructive comments on the manuscript. This work was supported by the US National Institutes of Health (NIH) National Institute of General Medical Sciences (NIGMS) grant nos. F32GM106683 (R.L.S.) and R01EB010249 (S.R.J.).
Footnotes
AUTHOR CONTRIBUTIONS R.L.S., W.S. and S.R.J. conceived the experiments. W.S. had a major role in the development of the Spinach-based sensors. R.L.S. carried out imaging experiments and optimized the imaging protocol. R.L.S. and S.R.J. wrote the manuscript.
COMPETING FINANCIAL INTERESTS The authors declare competing financial interests: details are available in the online version of the paper.
References
- 1.Paige JS, Wu KY, Jaffrey SR. RNA mimics of green fluorescent protein. Science. 2011;333:642–646. doi: 10.1126/science.1207339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paige JS, Nguyen-Duc T, Song W, Jaffrey SR. Fluorescence imaging of cellular metabolites with RNA. Science. 2012;335:1194. doi: 10.1126/science.1218298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song W, Strack RL, Jaffrey SR. Imaging bacterial protein expression using genetically encoded RNA sensors. Nat. Methods. 2013;10:873–875. doi: 10.1038/nmeth.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strack RL, Jaffrey SR. New approaches for sensing metabolites and proteins in live cells using RNA. Curr. Opin. Chem. Biol. 2013;17:651–655. doi: 10.1016/j.cbpa.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 6.Stoltenburg R, Reinemann C, Strehlitz B. SELEX—a (r)evolutionary method to generate high-affinity nucleic acid ligands. Biomol. Eng. 2007;24:381–403. doi: 10.1016/j.bioeng.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Campbell RE, Ting AY, Tsien RY. Creating new fluorescent probes for cell biology. Nat. Rev. Mol. Cell Biol. 2002;3:906–918. doi: 10.1038/nrm976. [DOI] [PubMed] [Google Scholar]
- 8.Frommer WB, Davidson MW, Campbell RE. Genetically encoded biosensors based on engineered fluorescent proteins. Chem. Soc. Rev. 2009;38:2833–2841. doi: 10.1039/b907749a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ibraheem A, Campbell RE. Designs and applications of fluorescent protein-based biosensors. Curr. Opin. Chem. Biol. 2010;14:30–36. doi: 10.1016/j.cbpa.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 10.Kellenberger CA, Wilson SC, Sales-Lee J, Hammond MC. RNA-based fluorescent biosensors for live-cell imaging of second messengers cyclic di-GMP and cyclic AMP-GMP. J. Am. Chem. Soc. 2013;135:4906–4909. doi: 10.1021/ja311960g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakayama S, Luo Y, Zhou J, Dayie TK, Sintim HO. Nanomolar fluorescent detection of c-di-GMP using a modular aptamer strategy. Chem. Commun. (Camb) 2012;48:9059–9061. doi: 10.1039/c2cc34379g. [DOI] [PubMed] [Google Scholar]
- 12.Buckstein MH, He J, Rubin H. Characterization of nucleotide pools as a function of physiological state in Escherichia coli. J. Bacteriol. 2008;190:718–726. doi: 10.1128/JB.01020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jenison RD, Gill SC, Pardi A, Polisky B. High-resolution molecular discrimination by RNA. Science. 1994;263:1425–1429. doi: 10.1126/science.7510417. [DOI] [PubMed] [Google Scholar]
- 14.Srinivasan J, et al. ADP-specific sensors enable universal assay of protein kinase activity. Chem. Biol. 2004;11:499–508. doi: 10.1016/j.chembiol.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 15.Ponchon L, Dardel F. Recombinant RNA technology: the tRNA scaffold. Nat. Methods. 2007;4:571–576. doi: 10.1038/nmeth1058. [DOI] [PubMed] [Google Scholar]
- 16.Gottschalk PG, Dunn JR. The five-parameter logistic: a characterization and comparison with the four-parameter logistic. Anal. Biochem. 2005;343:54–65. doi: 10.1016/j.ab.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 17.McCombs JE, Palmer AE. Measuring calcium dynamics in living cells with genetically encodable calcium indicators. Methods. 2008;46:152–159. doi: 10.1016/j.ymeth.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cozzone AJ. Regulation of acetate metabolism by protein phosphorylation in enteric bacteria. Annu. Rev. Microbiol. 1998;52:127–164. doi: 10.1146/annurev.micro.52.1.127. [DOI] [PubMed] [Google Scholar]
- 19.Lowary PT, Uhlenbeck OC. An RNA mutation that increases the affinity of an RNA-protein interaction. Nucleic Acids Res. 1987;15:10483–10493. doi: 10.1093/nar/15.24.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lowry OH, Carter J, Ward JB, Glaser L. The effect of carbon and nitrogen sources on the level of metabolic intermediates in Escherichia coli. J. Biol. Chem. 1971;246:6511–6521. [PubMed] [Google Scholar]