Abstract
Emerging evidence suggests that endothelial cell-secreted factors contribute to the pathobiology of squamous cell carcinoma (SCC) by enhancing invasive migration and resistance to anoikis. Here we report that SCC cells within the perivascular niche have undergone epithelial to mesenchymal transition (EMT) in a primary human SCC of a patient that developed distant metastases. Endothelial cell-secreted EGF induced EMT of human SCC cells in vitro and also induced acquisition of a stem-like phenotype. In vivo, tumor xenografts vascularized with EGF-silenced endothelial cells exhibited a smaller fraction of cancer stem-like cells (ALDH+CD44+) and were less invasive than tumors vascularized with control endothelial cells. Collectively, these results demonstrated that endothelial cell-EGF induces EMT and an acquisition of stem-like properties by head and neck tumor cells. On this basis, we suggest that vascular endothelial cells contribute to tumor dissemination by secreting factors that endow carcinoma cells with enhanced motility and stemness.
Keywords: Epithelial mesenchymal transition, Cancer stem cells, Head and neck squamous cell carcinoma, Angiogenesis, Perivascular niche
Introduction
Epithelial to mesenchymal transition (EMT) enables the migration of epithelial cells into connective tissues, a critical process for embryonic development (1). In cancer, it has been shown that epithelial tumor cells exploit EMT to detach from the primary tumor mass and disseminate into the surrounding stroma (2). It has also been shown that epithelial cells can be endowed with stem-like properties through EMT (3). Such findings demonstrate that EMT plays a key role in the progression of epithelial tumors (4,5). However, the cellular source and the nature of the signals that turn on and off the EMT cascade in cancer remain largely unknown. Such knowledge is critical for the understanding of the pathobiology of local invasion and metastasis of epithelial tumors, and may unveil novel therapeutic targets for this type of malignancy.
Loss of E-cadherin, a hallmark of EMT, is an important step in the progression of papilloma to invasive carcinoma (6). It has been recently shown that EMT is modulated by transcription factors that regulate E-cadherin, e.g. Snail (3,7), Twist (8), and ZEB1 (9). For example, Snail controls EMT by repressing E-cadherin expression in development and cancer (10). Twist is required for EMT and breast cancer metastasis (8). In general, expression of these transcription factors is correlated with increased invasiveness-metastasis and poorer clinical prognosis (11).
EGF is a well-known mitogen that plays important roles in cell proliferation survival in physiological settings and in cancer (12-14). Continuous EGF treatment has been shown to downregulate E-cadherin expression and results in loss of cell-cell adherence junctions (15). EGF treatment enhances tumor progression and induces EMT in breast cancer cells and cervical cancer cells (13,14). Notably, a recent study showed that the overexpression of EGF receptor (EGFR) results in the enrichment of a subset of esophageal cells that is capable of undergoing EMT in response to TGF-β through ZEB transcription factor (16). However, the cellular source of EGF within the tumor microenvironment remains unclear.
Mounting evidence demonstrates that epithelial tumors contain a small sub-population of cells with stem-like and/or progenitor characteristics (17-19). These cells are highly tumorigenic, exhibit self-renewal, and are capable of differentiating into complex new tumors (20). The origin of these cancer stem cells remains unclear. However, exciting new evidence suggests that EMT allows for the generation of cells with stem cell properties in tumor models (3,21,22).
We have showed that EGF secreted by endothelial cells induces motility and protects human squamous cell carcinoma (SCC) against anoikis (23). In addition, we have shown that endothelial cell-secreted factors enhance the survival, self-renewal and tumorigenicity of cancer stem cells (24). Here, we hypothesized that EGF secreted by endothelial cells enables tumor cell motility by inducing EMT and by endowing SCC cells with stemness. This work showed that endothelial cell-secreted EGF induces Snail through the PI3k-Akt pathway and induces EMT of squamous cell carcinoma cells, as shown by downregulation of epithelial markers (E-cadherin, Desmoplakin), upregulation of mesenchymal markers (Vimentin, N-Cadherin), induction of cell motility and acquisition of stem-like properties (expression of ALDH and CD44) and growth as non-adherent orospheres. Notably, specific silencing of EGF in endothelial cells slowed down tumor growth and decreased the fraction of stem-like cells in xenograft models. Collectively, these data demonstrate that signals secreted by tumor-associated endothelial cells enhance the aggressive behavior (motility and stemness) of epithelial cancer cells.
Materials and Methods
Cell culture
University of Michigan squamous cell carcinoma (UM-SCC) cells, UM-SCC-1, UM-SCC-11A, UM-SCC-11B, UM-SCC-14A, UM-SCC-14B, UM-SCC-17A, UM-SCC-17B, UM-SCC-22A, UM-SCC-22B, UM-SCC-74A and UM-SCC-74B (Tissue Biorepository, University of Michigan Head and Neck SPORE) were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, Carlsbad, CA) supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. All cell lines were genotyped and authenticated prior to use in the experiments included in this manuscript. Pooled primary human dermal microvascular endothelial cells (HDMEC; Lonza, Walkersville, MD) were cultured in endothelial growth medium-2 for microvascular cells (EGM2-MV; Lonza). Cancer cells were serum starved overnight, and treated with 0-50 ng/ml rhEGF, anti-human EGF neutralizing antibody (Cat. MAB236; R&D Systems, Minneapolis, MN) for indicated time points. Alternatively, HDMEC were cultured in serum-free medium (EBM2, Lonza) for 24 hours, and the supernatants were collected as endothelial cell conditioned medium (EC CM). Notably, EGF levels in culture supernatants were normalized to cell number for all experiments included here. SCC were starved overnight and incubated with EC CM for 0-24 hours. In selected experiments, cells were pre-incubated with 0-20 μM Stattic V (Stat3 inhibitor; Calbiochem, San Diego, CA), 0-20 μM UO126 (MEK1/2 inhibitor; Cell Signaling Technology, Danvers, MA) or 0-20 μM Ly294002 (PI3 Kinase inhibitor; Cell Signaling Technology) for 1 hour, and then treated with 0-50 ng/ml EGF for 0-4 hours.
Western Blots
SCC cells were lysed in 1% Nonidet P-40 (NP-40) lysis buffer (50 mM Tris-HCL, PH 7.4, 10% glycerol, 200 mM NaCl and 2 mM MgCl2) containing protease inhibitors. Protein lysates were loaded onto 8-15% SDS-PAGE. Membranes were blocked with 5% non fat milk in 1X TBS containing 0.3% Tween-20, then incubated with the following primary antibodies overnight at 4°C: rabbit anti-human phosphor EGFR (Tyr 845, SC-23420-R), EGFR (SC-03), rabbit anti-human E-cadherin (SC-7870), rabbit anti-human Twist (SC-15393), rabbit anti-human Desmoplakin (SC-33555), rabbit anti-human pan-cytokeratin (SC-81714), mouse anti-human beta-actin conjugated with HRP (SC-47778 HRP) (Santa Cruz Biotechnology, Santa Cruz, CA); mouse anti-human phosphor STAT3 (Tyr 705, Cat. 9138), rabbit anti-human STAT3 (Cat. 9132), mouse anti-human ERK1/2 (Cat. 4696), rabbit anti-human phosphor-ERK1/2 (Thr 202/Tyr 204, Cat. 4376), rabbit anti-human phosphor-AKT (Ser 473, Cat. 9271), rabbit anti-human AKT (Cat. 9272), mouse anti-human Snail (Cat. 3895), rabbit anti human N-cadherin (Cat. 4061), mouse anti-human Vimentin (Cat. 2309), mouse anti-human CD44 (Cat. 3570) (Cell Signaling), mouse anti-human ALDH (Cat. 611195; BD Transduction Laboratories), mouse anti-GAPDH (MAB374) (Chemicon, Billerca, MA), rabbit anti-human Bmi-1 (Cat. SAB4200034; Sigma, St. Louis, MO). Affinity-purified second antibodies conjugated with horseradish peroxidase (Jackson Laboratories, West Grove, PA) were used, and immunoreactive proteins were visualized by SuperSignal West Pico chemiluminescent substrate (Thermo Scientific, Rockford, IL) and exposed to x-ray film.
Stem cell staining and flow cytometry analysis
Starved SCC cells were treated with 0-50 ng/ml EGF, EC CM or unconditioned medium for 24 hours. Stemness was assessed by ALDH (aldehyde dehydrogenase) activity and CD44 expression, as we showed (24). Briefly, cells were collected and stained with Aldefluor (Aldagen Inc, Durham, NC) using diethylaminobenzaldehyde (DEAB; specific inhibitor of ALDH activity) as negative control. Cells were also immunostained with mouse anti-human CD44 conjugated with APC (Cat. 559942; BD Biosciences, San Jose, CA) using IgG-APC as isotype control. Percent of ALDH and CD44 positive cells was determined by flow cytometry. 7AAD staining was used for elimination of dead cells.
Orosphere assay
UM-SCC-1, UM-SCC-22A or UM-SCC-22B cells (1,000-3,000) were cultured in ultra-low attachment plates (Corning Inc., Corning, NY) with serum-free medium containing 0-50 ng/ml EGF, EC CM, or unconditioned medium for 7-15 days, as we showed (25). Orospheres, defined as non-adherent spheres of ≤25 cells, were photographed and counted under light microscopy.
Immunohistochemistry
4 μm-thick sections were deparaffinized and rehydrated. Antigen retrieval was performed, and sections were incubated with primary antibodies for CD44 (clone EPR1013Y; Abcam, 1:100), ALDH (clone 44; BD Transduction Laboratories, 1:50), Vimentin (clone 3B4; Dako, 1:100), E-cadherin (clone 24E10; Cell Signaling, 1:400), Factor VIII related antigen/Von Willebrand Factor Ab-1 (RB-281-A; Thermo Scientific, 1:100), phospho-EGFR (Tyr 1068, Cat. 2236, Cell Signaling, 1: 100) overnight at 4°C. The EnVision™+ system (Dako) and 3,3-diamino benzidine (Dako) were utilized for visualization. Isotype-matched non-specific IgG was used as negative control.
ELISA
HDMEC and tumor cells were cultured with serum free medium (EBM and DMEM, respectively) for 24 hours. The supernatant was collected and ELISA for EGF (Cat. DEG00, R&D Systems), TGF-alpha (Cat. DTGA00, R&D Systems) and Amphiregulin (Cat. DAR00, R&D Systems) was performed.
EGF, EGFR silencing and EGFR over expression
HEK293T cells were transiently co-transfected with the lentiviral packaging vectors psPAX2, pMD2G and shRNA-EGF (3 clones: TAGCTGATAGGAGAGAATG, TTCAATCACAGAVTGCTTG, AACATCTTCACAGTACTTC, for endothelial cell infection), shRNA-EGFR (2 clones: TTTCCAAATTCCCAAGGAC, TTCCGTTACACACTTTGCG, for UM-SCC-14A infection) or scramble sequence control shRNA-C (Vector Core, University of Michigan) by the calcium phosphate method. Alternatively, we co-transfected HEK293T cells with retrovirus packaging vector pVpack-GP, pVpack-VSV-G and pBABE-EGFR (for UM-SCC-74B EGFR overexpression) or control vector pBABE-puro (Addgene, Cambridge MA) by the calcium phosphate method. Recipient cells were infected with supernatants containing lentivirus or retrovirus and selected with 1 μg/ml of puromycin (Sigma-Aldrich, St. Louis, MO) for at least 1 week. Here, and throughout this manuscript, EGF expression was evaluated by ELISA, EGFR expression was detected by Western blot.
SCID mouse model of human tumor angiogenesis
Xenograft human tumors vascularized with human blood vessels were generated under an UCUCA approved protocol. Briefly, highly porous poly-L(lactic) acid (Boehringer Ingelheim, Ingelheim, Germany) scaffolds were seeded with 9×105 HDMEC stably transduced with shRNA-EGF (or control shRNA-C) and 1×105 UM-SCC-22B cells. Immunodeficient mice (CB.17.SCID; Taconic, Germantown, New York) were anesthetized with ketamine and xylazine, and scaffolds were implanted in the subcutaneous space of the dorsal region (10 mice per experimental condition), as we showed (24). When tumors reached 0.5 cm3, mice were euthanized and tumors were removed, single cell suspensions were prepared and analyzed by flow cytometry, as described above.
Statistical Analysis
t-test or one-way ANOVA followed by appropriate post-hoc tests were performed with SigmaStat 2.0 software (SPSS, Chicago, IL, USA). Statistical significance was determined at p<0.05.
Results
Endothelial cell-secreted EGF induces EMT of squamous cell carcinoma cells
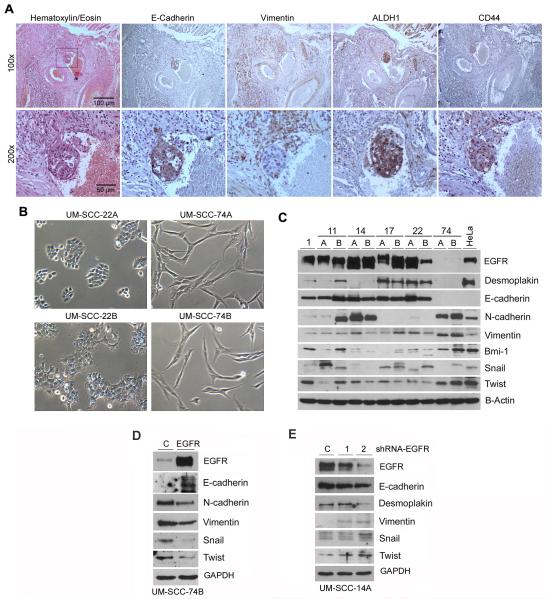
A hallmark of cells undergoing EMT is the loss of epithelial markers (e.g. E-cadherin, Desmoplakin) and concomitant acquisition of mesenchymal markers (e.g. Vimentin, N-cadherin) (26). We analyzed the primary head and neck squamous cell carcinoma (HNSCC) of a patient that presented with distant metastases 1 year after surgical removal of the primary tumor (Figure 1A). We observed a group of tumor cells approaching a blood vessel and a cluster of tumor cells in the lumen of this blood vessel (Figure 1A). Interestingly, the tumor cells approaching the blood vessel exhibited features of EMT, as characterized by low E-cadherin and high Vimentin expression. In contrast, the tumor cells within the lumen have reversed back to an epithelial phenotype characterized by high E-cadherin and low Vimentin, suggestive of mesenchymal to epithelial transition (MET) (Figure 1A). A link between EMT and acquisition of stemness has been proposed (3). Here, we observed that that many tumor cells in close proximity to the blood vessel express the stem cell markers ALDH and CD44, and that this expression is visibly enhanced in the tumor cells within the lumen of the blood vessel (Figure 1A). These data underline the plasticity of invasive epithelial tumor cells and suggest that once the epithelial cells have invaded through the connective tissue, and EMT is no longer beneficial, they quickly revert back to their original epithelial phenotype.
Figure 1.
EMT in human head and neck squamous cell carcinoma. (A) Photomicrographs of serial histological sections of a primary human HNSCC containing tumor cells inside the lumen of a blood vessel. This tumor was surgically excised from a Caucasian male, 72 years old, smoker, with a HNSCC in the floor of the mouth extending into base of the tongue, classified as stage T3N2M0 at time of diagnosis of the primary tumor. This patient was diagnosed with distant metastases 1 year after surgical removal of the primary tumor depicted here. E-Cadherin, Vimentin, ALDH1, CD44 expression was examined by immunohistochemistry (brown color). Low magnification (100×, upper row) and high magnification (200×, lower row) of the same area of the HNSCC (box), depicting in detail the tumor cell cluster within a blood vessel. (B) Photomicrographs (100×) of 2 representative pairs of HNSCC cell lines with distinctly different morphologies. The UM-SCC-22 pair exhibits epithelial, cobblestone-like morphology, while the UM-SCC-74 pair exhibits a more elongated, mesenchymal-like shape. (C) Western blots for analysis of constitutive expression of epithelial markers (E-cadherin, Desmoplakin), mesenchymal markers (N-cadherin, Vimentin), EMT-related transcription factors (Snail, Twist) and self-renewal marker (Bmi-1) in a series of established HNSCC cell lines. The origin of the human HNSCC cell lines is, as follows: UM-SCC-11A pretreatment biopsy, UM-SCC-11B post chemotherapy biopsy from same patient as UM-SCC-11A; UM-SCC-14A wide local excision after excisional biopsy, UM-SCC-14B recurrence after surgery and radiation; UM-SCC-17A primary tumor from the endolarynx, UM-SCC-17B from tumor extending outside the thyroid cartilage; UM-SCC-22A from primary site, UM-SCC-22B from lymph node metastasis; UM-SCC-74A surgical resection after chemotherapy and radiation, UM-SCC-74B second surgery for persistent cancer. (D and E) Western blots for EGFR, Vimentin, Snail, Twist, E-cadherin, Desmoplakin and N-cadherin in tumor cells (UM-SCC-74B) stably transduced with EGFR (D) or tumor cells (UM-SCC-14A) stably transduced with shRNA-EGFR (E).
To begin to understand the molecular mechanisms underlying this phenomenon, we screened a panel of head and neck squamous cell carcinoma cell lines for expression of markers and regulators of EMT and stemness (Figure 1B and C). This screen revealed a wide diversity of gene expression patterns. Noteworthy is the observation that one pair of cells lines, UM-SCC-74A (surgical resection after chemotherapy and radiation) and UM-SCC-74B (second surgical for persistent cancer) derived from the same patient (27), exhibit a gene expression profile consistent with constitutive EMT, as shown by mesenchymal-like morphology, low E-cadherin, Desmoplakin, and high N-cadherin, Vimentin and Twist (Figure 1B and C). In contrast, UM-SCC-22A (primary tumor of the hypopharynx) and UM-SCC-22B (neck metastasis) from the same patient (27) showed a gene expression profile that is more consistent with epithelial cells (i.e. high E-cadherin, Desmoplakin, and low N-cadherin, Vimentin and Twist). To determine whether EMT markers are correlated with EGFR expression in head and neck cancer cell lines, we performed EGFR overexpression in UM-SCC-74B cells (Figure 1D) and EGFR silencing with shRNA-EGF in UM-SCC-14A (Figure 1E). Overexpression of EGFR in UM-SCC-74B cells by stable transduction with lentivirus + selection correlated with increased expression of the epithelial marker E-cadherin, reduced expression of the mesenchymal markers N-cadherin and Vimentin, and reduced expression of the EMT transcriptional factors snail and twist (Figure 1D). On the other hand, knockdown of EGFR by shRNA in UM-SCC-14A cells correlated with downregulation of E-cadherin and Desmoplakin, and upregulation of Vimentin, snail and twist (Figure 1E).
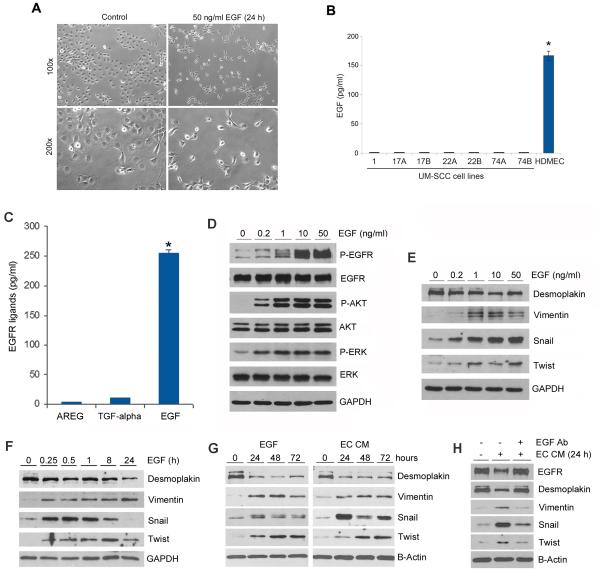
EGF has been strongly correlated with the aggressiveness of epithelial cancers (13,14). We observed that recombinant human EGF induces a change in HNSCC morphology, loosing their typical cobblestone appearance and assuming a more elongated, fibroblast-like, morphology (Figure 2A). Surprisingly, the expression levels of EGF in head and neck squamous cell carcinoma cells are significantly lower than in microvascular endothelial cells (Figure 2B), suggesting that the endothelial cells constitute a major source of EGF in the tumor microenvironment. To verify the relative expression of EGF in comparison with other EGFR ligands, we performed ELISA tests that demonstrated that the expression of EGF is 25-fold higher than transforming growth factor (TGF)-α and 62.5-fold higher than amphiregulin AREG (4 pg/ml) in endothelial cells (Figure 2C).
Figure 2.
Endothelial cell-secreted EGF induces phenotypic changes consistent with EMT in head and neck squamous cell carcinoma cells. (A) Photomicrographs of HNSCC cells cultured in presence or absence of EGF for 24 hours. (B) ELISA for EGF in the supernatant of a panel of UM-SCC cell lines and in pooled primary human microvascular endothelial cells (HDMEC). (C) ELISA for EGFR ligands (EGF, TGF-alpha, AREG) in the supernatant of HDMEC. (D and E) UM-SCC-22B cells were starved overnight and treated with 0-50 ng/ml EGF for 25 minutes (D) or 4 hours (E). Western blots for P-EGFR, EGFR, P-AKT, AKT, P-ERK, ERK (D), Desmoplakin, Vimentin, Snail and Twist (E). (F-H) UM-SCC-22B cells were starved overnight and treated with 50 ng/ml EGF (F,G) or endothelial cell conditioned medium (G,H) for indicated time points. (H) UM-SCC-22B cells were starved overnight and then treated with endothelial cell conditioned medium with or without 10 μg/ml EGF neutralizing antibody for 24 hours. Western blots were performed for Desmoplakin, Vimentin, Bmi-1, Snail and Twist. Asterisk depict p<0.05.
To explore a role of endothelial cells on the induction of EMT in head and neck cancer, we exposed HNSCC cells to EGF or to endothelial cell conditioned medium (EC CM). First, we checked dose response of cancer cells upon EGF stimulation (Figure 2D and E) and time dependent upon EGF and EC CM treatment (Figure 2F and G) and observed that not only P-EGFR, P-Akt and P-ERK of which related to proliferation and survival were up-regulated (Figure 2D), but also gene expression changes were consistent with EMT (Figure 2E-G). Both EGF and EC CM decreased expression of an epithelial marker (Desmoplakin), increased the expression of a mesenchymal marker (Vimentin), and activated expression of EMT transcriptional factors (Snail, Twist) in UM-SCC-22B cells (Figure 2E-G). To evaluate the role of EGF in the responses induced by endothelial cell-secreted factors, we used a neutralizing anti-EGF antibody and observed that EGF inhibition restored baseline levels of Desmoplakin, Vimentin and Twist, and partially inhibited upregulation of Snail (Figure 2H). As expected, both EGF and EC CM treatment mediated downregulation of total EGFR expression, which was rescued with the use of anti-EGF antibody (Figure 2H). These studies were repeated with a second tumor cell line (UM-SCC-1) to verify the nature of the responses across multiple tumor cell models (Suppl. Figure 1C-E).
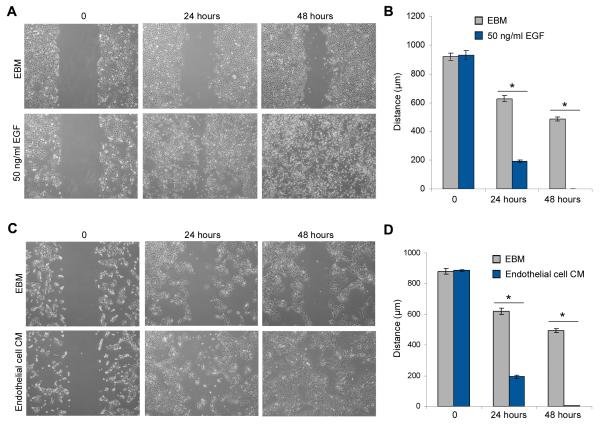
To further evaluate the effect of EGF on SCC motility, we performed the in vitro scratch assay (28). It revealed that EGF, or the full complement of endothelial cell-secreted factors, significantly induce tumor cell motility and speed up closure of the “scratch” (Figure 3; Suppl. Figure 2). Collectively, these data demonstrate that endothelial cell-secreted EGF induces EMT in squamous cell carcinoma cells.
Figure 3.
Endothelial cell-secreted factors enhance the motility of HNSCC. (A,C) HNSCC cells were grown on 6-well plates, starved overnight and scratched with a 1,000-μl loading tip, then incubated with 50 ng/ml EGF (A), or endothelial cell conditioned medium (C) for indicated time points. (B,D) Graphs depicting “scratch” width over time in response to EGF (B) or endothelial cell conditioned medium (D). Four independent experiments using triplicate wells/experimental condition were performed to verify reproducibility of the data. Asterisks depict p<0.05 within time point.
Endothelial cell-secreted EGF induces EMT through PI3K/Akt signaling
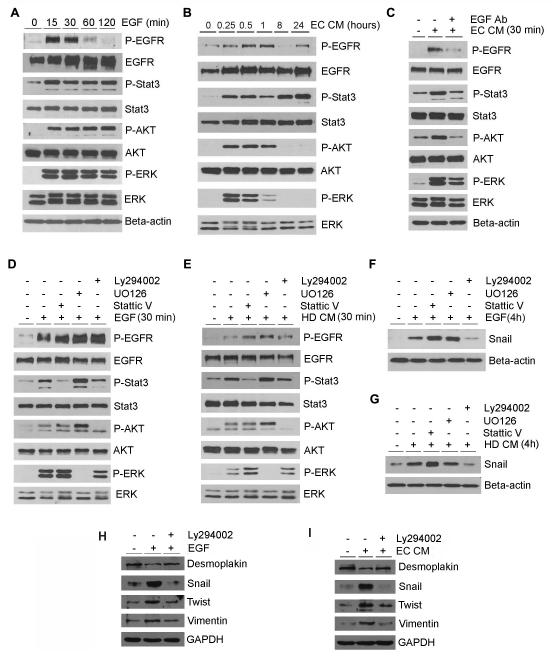
To evaluate the effect of endothelial cell-secreted EGF on the activity of signaling pathways that play a key role in HNSCC survival and motility (23), we evaluated signaling through STAT3, Akt and ERK in UM-SCC-22B (Figure 4A-C) and UM-SCC-1 (Suppl. Figure 3). We observed that EGF and endothelial cell-secreted factors potently induce activation of STAT3, Akt and ERK within 15 minutes (Figure 4A and B). While STAT3 phosphorylation was sustained for 24 hours, phosphorylation of Akt and ERK was more transient (Figure 4B). The use of a neutralizing anti-EGF antibody abolished endothelial cell-induced phosphorylation of Akt and STAT3, while partially inhibiting phosphorylation of ERK (Figure 4C). Inhibition of ERK with U0126 potentiated EGF-induced phosphorylation of STAT3 and Akt (Figure 4D and E). Notably, inhibition of PI3k/Akt signaling with LY294002 prevented EGF- and endothelial cell-induced Snail expression. These results were confirmed in a second cell line (UM-SCC-1) (Suppl. Figure 4). To further understand the effect of PI3K/Akt signaling on EMT, we performed Western blots that demonstrated that LY294002 inhibits rhEGF-induced or endothelial cell conditioned medium-induced upregulation of snail, twist and Vimentin and downregulation of Desmoplakin in tumor cells (Figure 4H and I). These data suggest that PI3K/Akt signaling plays an important role in the EMT mediated by EGF.
Figure 4.
Endothelial cell-secreted EGF induces Snail expression via PI3K/Akt signaling. (A-C) UM-SCC-22B cells were starved overnight and treated with 50 ng/ml EGF (A) or endothelial cell conditioned medium (EC CM) (B,C) for indicated time points. (C) UM-SCC-22B cells were starved overnight and then treated with endothelial cell conditioned medium with or without 10 μg/ml EGF neutralizing antibody for 30 minutes. Western blots were performed for P-Stat3, Stat3, P-Akt, Akt, P-ERK and ERK. (D-G) UM-SCC-22B cells were starved overnight, pre-incubated with 20 μM Stat3 inhibitor (Stattic V), 20 μM PI3K/Akt inhibitor (Ly294002), 20 μM MEK1/2 inhibitor (UO126) for 1 hour, and then exposed to 50 ng/ml EGF (F) or endothelial cell conditioned medium (G) for 4 hours. Western blots were performed for P-Stat3, Stat3, P-Akt, Akt, P-ERK and ERK (D,E) or Snail (F,G). (H and I) Western blots for Desmoplakin, Snail, Twist and Vimentin. UM-SCC-22B cells were starved overnight, pre-incubated with 20 μM LY294002 for 1 hour, then treated with 50 ng/ml EGF (H) or endothelial cell conditioned medium (EC CM) for 4 hours (I).
Endothelial cell-secreted EGF endows epithelial tumor cells with stem-like characteristics
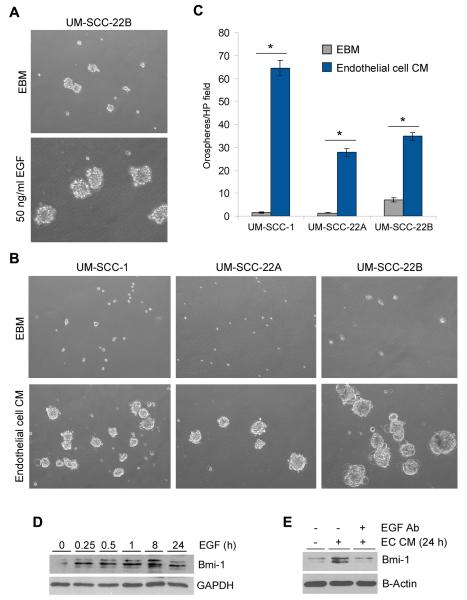
To evaluate a potential mechanistical link between endothelial cell-secreted EGF, EMT, and acquisition of stem cell features, we seeded SCC cells in ultra-low attachment plates and cultured them in serum-free medium with or without EGF, or in endothelial cell conditioned medium, for 7-14 days (Figure 5). EGF supplementation, or treatment with endothelial cell-secreted factors, induced a significant increase in the number of orospheres when compared to controls (Figure 5A-C). Notably, recombinant human EGF or the full milieu of growth factors secreted by endothelial cells induced expression of Bmi-1 (Figure 5D and E), an important regulator of self-renewal and stemness (29,30). The Bmi-1 activation in response to the endothelial cell growth factor milieu was strictly dependent on EGF (Figure 5E).
Figure 5.
Endothelial cell-secreted EGF induces expression of the self-renewal protein Bmi-1. (A) UM-SCC-22B were seeded in ultra-low attachment 6-well plates (3,000 cells/well) and cultured in serum-free medium with or without 50 ng/ml EGF (A) or endothelial cell conditioned medium (B) for 11 days. (C) Graph depicting the number of orospheres (defined as non-adherent colonies of ≤25 cells) generated by 3 different HNSCC cell lines (UM-SCC-1, UM-SCC-22A, UM-SCC-22B) exposed to endothelial cell conditioned medium. Three independent experiments using triplicate wells/experimental condition were performed to verify reproducibility of the data. (D,E) UM-SCC-22B cells were starved overnight and treated with 50 ng/ml EGF (D) or endothelial cell conditioned medium (E) for indicated time points. UM-SCC-22B cells were treated with endothelial cell conditioned medium with or without 10 μg/ml EGF neutralizing antibody for 24 hours. Western blots were performed for analysis of Bmi-1 expression. Asterisks depict p<0.05 within cell line.
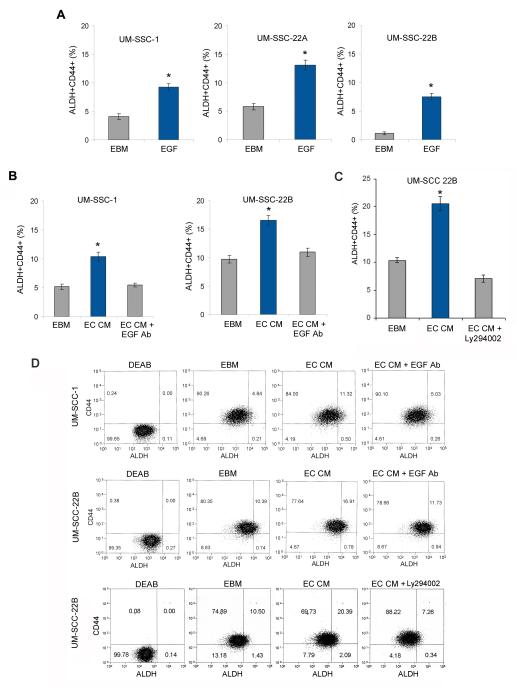
Next, we explored a possible role of endothelial cell-secreted EGF on the fraction of cancer stem-like cells in HNSCC, as determined by the activity of ALDH and expression of CD44. EGF increased significantly the fraction of ALDH+CD44+ cells (Figure 6A; Suppl. Figure 5). Notably, the increase in the fraction of ALDH+CD44+ cells mediated by endothelial cells was blocked by EGF neutralizing antibody (Figure 6B and D) and PI3K inhibitor LY294002 (Figure 6C and D). Collectively, these data suggest that endothelial cell-secreted factors enhanced the fraction of ALDH+CD44+ cells in an EGF-dependent manner via the PI3K/Akt signaling pathway.
Figure 6.
Endothelial cell-secreted EGF enhances the fraction of cancer stem-like cells. (A,B) Graphs depicting the proportion of cancer stem-like cells (ALDH+CD44+) when HNSCC cells (UM-SCC-1, UM-SCC-22A, UM-SCC-22B) were starved overnight and incubated with 50 ng/ml EGF for 24 hours (A); or HNSCC cells (UM-SCC-1, UM-SCC-22B) were cultured in endothelial cell conditioned medium with or without 10 μg/ml EGF neutralizing antibody (B) or 20 μM LY294002 (C) for 24 hours. ALDH activity was determined with the Aldefluor kit, CD44 expression by immunoreactivity and cells were analyzed by flow cytometry. (D) Graphs depicting representative flow cytometry plots for cells analyzed in (B and C). Three independent experiments using triplicate wells/experimental condition were performed to verify reproducibility of the data. Asterisks depict p<0.05.
Endothelial cell-secreted EGF enhances tumor growth and cancer stem cell fraction
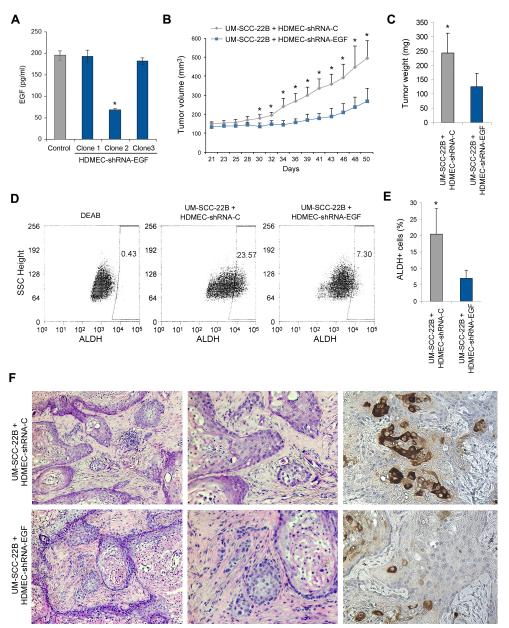
To evaluate the effect of endothelial cell-secreted EGF on the pathobiology of HNSCC, we engineered xenograft tumors vascularized with endothelial cells stably transduced with shRNA-EGF or vascularized with endothelial cells transduced with scramble sequence control lentiviral vectors. ELISA demonstrated that one of the shRNA sequences (Clone 2) produced significant and stable silencing of EGF expression in endothelial cells (Figure 7A). EGF silencing in the endothelial cells was sufficient to slow down tumor growth over a period of 50 days (Figure 7B and C). Mice were euthanized 50 days after cell transplantation, tumor were retrieved and single cell suspensions were prepared for ALDH analyses (Figure 7D and E). Tumors vascularized with EGF-silenced endothelial cells showed a significant reduction in the proportion of ALDH+ cells, as determined by flow cytometry (Figure 7D and E) and immunohistochemistry staining (Figure 7F). Notably, the tumors vascularized with EGF-silenced endothelial cells were more differentiated and less invasive, as compared to tumors vascularized with control endothelial cells (Figure 7F). Interestingly, we observed strong phosphorylation of EGFR in tumor cells near blood vessels in xenograft tumors vascularized with control endothelial cells (Suppl. Figure 6A). Nonetheless, there was no difference in microvascular density when xenograft tumors were vascularized with control or EGF-silenced endothelial cells (Suppl. Figure 6B and C). Collectively, these data demonstrate a direct correlation between the level of EGF secretion by endothelial cells and the fraction of stem-like cells in an animal model of HNSCC.
Figure 7.
Endothelial cell-secreted EGF enhances tumor growth and the fraction of cancer stem-like cells. (A) Pooled primary human endothelial cells (HDMEC) were stably transduced with scramble vector control (shRNA-C) or with shRNA-EGF and selected with 1 μg/ml puromycin. Graph depicting the expression of EGF, as determined by ELISA. Clone 2 showed a significant reduction in EGF expression, and was used for the remaining of the experiments of this figure. (B) 1×105 UM-SCC-22B cells were seeded with either 9×105 HDMEC-shRNA-EGF (clone 2) or 9×105 HDMEC-shRNA-C in biodegradable scaffolds that were transplanted in the dorsum of SCID mice (10 mice per experimental condition). Graph depicting tumor volume over time. (C-E) Mice were euthanized 50 days after transplantation, tumors were removed and weighed (C). Single cell suspensions were prepared from each tumor and ALDH activity was analyzed by flow cytometry (D,E). (F) A slice of each tumor was fixed, and analyzed by hematoxilin/eosin staining and by immunohistochemistry for ALDH1 (brown color). Asterisks depict p<0.05.
Discussion
The crosstalk between endothelial cells and tumor cells has been characterized as one of the key cell-cell interactions within the tumor microenvironment. For many years, the paradigm explaining this crosstalk has been centered on a dominant role for tumor cell-initiated signals (e.g. VEGF) that induce the recruitment and enhance the survival of blood vessels required for the influx of oxygen and nutrients necessary for the high metabolic demands of tumor cells. However, recent studies demonstrated that this crosstalk is a “two-way street”, where endothelial cell-initiated events have a profound impact on the behavior of tumor cells. Indeed, endothelial cell-initiated signaling enhances tumor cell proliferation, migration and anoikis resistance (23,31,32). And, more recently we showed that endothelial cell-initiated signaling promotes the survival and enhances the tumorigenic potential of cancer stem cells (24). Here, we demonstrated that endothelial cell-secreted EGF induces EMT and acquisition of stemness by epithelial tumor cells. Collectively, these studies showed that vascular endothelial cells play an active role in the pathobiology of cancer that is not limited to “blood vessel-making”. Rather, endothelial cells secrete factors that modulate the aggressiveness of epithelial tumor cells.
The rationale for this study is based on the following observations: A) Endothelial cells secrete significantly more EGF than squamous cell carcinoma cells; B) EGF secreted by endothelial cells induce EMT of squamous cell carcinoma cells; and C) Human epithelial tumor cells approaching a blood vessel undergo EMT (low E-Cadherin, high Vimentin). Surprisingly, once the tumor cells have entered the blood vessel, they revert back to an epithelial phenotype (high E-Cadherin, low Vimentin). This finding might be explained by MET (mesenchymal to epithelial transition) and underscores the plasticity of these tumor cells. Transitions between epithelial and mesenchymal states are involved in the acquisition of malignant and stem cell traits (33). Indeed, cell plasticity may allow the conversion of normal and neoplastic non-stem cells into a stem-like cell (34). MET often converts the disseminated mesenchymal cancer cells back to a more differentiated, epithelial cell state (35). We speculate that once the SCC cells have moved through the connective tissue, and EMT is no longer a favorable trait, these cells default back to their original epithelial phenotype.
ALDH is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome for patients with cancer (36,37). CD44-positive cells have the properties of cancer stem cells in human head and neck SCC (20). Notably, CD44 is a strong predictor of local recurrence after radiotherapy in larynx cancer (38). We have successfully used the combination of ALDH activity and CD44 expression to identify a small sub-population of highly tumorigenic cancer stem cells (24). Here, we observed that endothelial cell-secreted EGF determines the percentage of cancer stem cells (ALDH+CD44+) in vitro and in vivo. We also observed that endothelial cell-EGF levels correlate directly with the expression of the self-renewal marker Bmi-1 in epithelial tumor cells and with xenograft tumor growth. Interestingly, the high levels of expression of ALDH1 and CD44 observed in the cluster of tumor cells within the blood vessel of the primary human HNSCC presented here suggest that these cells have acquired stemness. Therefore, these cells may be highly tumorigenic and primed to establish metastatic foci at distant sites, as shown in breast cancer (36,37,39). Collectively, these observations suggest that endothelial cell-secreted factors (EGF) are likely involved in metastatic spread by attracting epithelial tumor cells towards blood vessels, allowing their transit through connective tissue via EMT, and enhancing their tumorigenic potential by endowing tumor cells with a stem cell-like phenotype.
We observed here that endothelial cell-secreted factors, and particularly EGF, activate major signaling pathways in squamous cell carcinoma cells, i.e. STAT3, ERK and PI3K/Akt. We have previously shown that these pathways regulate head and neck tumor cell proliferation, migration, and anoikis resistance (23). The induction of cell survival may be responsible, at least in part, for the ability of epithelial tumor cells to leave their nests and migrate through connective tissue. Notably, EGF signaling through PI3K/Akt appears to be the primary pathway for the induction of the Snail. This observation suggests that EGF-induced EMT is mediated by PI3K/Akt signaling and induction of Snail activity, a major transcriptional regulator of EMT and metastases (40,41). Collectively, these data suggest that EGF enables epithelial tumor cell invasion through the connective tissue via EMT while protecting these cells against anoikis.
The current paradigm for anti-angiogenic therapies for cancer is focused on the induction of endothelial cell death and disruption of the tumor microvascular network. The benefits of this approach to the survival of patients with cancer have remained largely evasive. Tumor cells are frequently able to overcome the effect of current anti-angiogenic therapies by: A) Activating alternative pro-angiogenic signaling pathways that reconstitute the tumor microvessel network; B) Inducing expression of potent pro-angiogenic molecules in response to the hypoxic conditions generated by effective disruption of tumor blood vessels; and/or C) Developing a process of evasive resistance (42), in which tumor cells migrate away from the primary tumor site in response to the unfriendly environment generated by anti-angiogenic drugs. Here, we showed a prominent role for endothelial cells as the source of signaling events that induce EMT and empower epithelial cells to move through connective tissues while endowing these cells them with stem-like properties. These data suggest that endothelial cells actively initiate signaling events that enable the movement of tumor-initiating cells towards the blood vessel, i.e. the first steps of the metastatic cascade. Collectively, these observations suggest a new treatment paradigm that is focused on the blockade of endothelial cell-initiated signaling mediating tumor cell dissemination, rather than on the killing of the endothelial cell. This could readily be tested by optimizing treatment regimens with existing targeted drugs to enable effective and sustained blockade of signaling events initiated by endothelial cells, likely by using a metronomic regimen (44,45) based on low dose/high frequency dosing. Such approach would not create the hypoxic and nutrient-deprived states that enhance the aggressiveness of the tumor cells that is frequently observed with standard anti-angiogenic therapies. Rather, it would inhibit tumor cell motility towards the blood vessel and metastatic spread, which is typically associated with poor patient outcome.
Supplementary Material
Acknowledgments
We thank Kristy Meyers, Carolina Nör, and Lisiane Bernardi for processing the tumor tissues for flow cytometric analyses; and thank Dr. Thomas Carey for the UM-SCC cell lines that were used here.
Grant Support: This work was supported by a grant from the Weathermax Foundation, University of Michigan Comprehensive Cancer Center; grant P50-CA97248 (University of Michigan Head and Neck SPORE) from the NIH/NCI; and grants R21-DE19279, R01-DE21139, R01-DE23220 from the NIH/NIDCR (JEN).
Footnotes
Disclosure of potential conflicts of interest.
The authors declare no conflict of interest
References
- 1.Shook D, Keller R. Mechanisms, mechanics and function of epithelial-mesenchymal transitions in early development. Mech Dev. 2003;120:1351–1383. doi: 10.1016/j.mod.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 3.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Scheel C, Weinberg RA. Phenotypic plasticity and epithelial-mesenchymal transitions in cancer and normal stem cells? Int J Cancer. 2011;129:2310–2314. doi: 10.1002/ijc.26311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–193. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- 7.Batlle E, Sancho E, Francí C, Domínguez D, Monfar M, Baulida J, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 8.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Sánchez-Tilló E, Lázaro A, Torrent R, Cuatrecasas M, Vaquero EC, Castells A, et al. ZEB1 represses E-cadherin and induces an EMT by recruiting the SWI/SNF chromatin-remodeling protein BRG1. Oncogene. 2010;29:3490–3500. doi: 10.1038/onc.2010.102. [DOI] [PubMed] [Google Scholar]
- 10.Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 11.Moreno-Bueno G, Portillo F, Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene. 2008;27:6958–6969. doi: 10.1038/onc.2008.346. [DOI] [PubMed] [Google Scholar]
- 12.Hackel PO, Zwick E, Prenzel N, Ullrich A. Epidermal growth factor receptors: critical mediators of multiple receptor pathways. Curr Opin Cell Biol. 1999;11:184–189. doi: 10.1016/s0955-0674(99)80024-6. [DOI] [PubMed] [Google Scholar]
- 13.Lo HW, Hsu SC, Xia W, Cao X, Shih JY, Wei Y, et al. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res. 2007;67:9066–9076. doi: 10.1158/0008-5472.CAN-07-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee MY, Chou CY, Tang MJ, Shen MR. Epithelial-mesenchymal transition in cervical cancer: correlation with tumor progression, epidermal growth factor receptor overexpression, and snail up-regulation. Clin Cancer Res. 2008;14:4743–4750. doi: 10.1158/1078-0432.CCR-08-0234. [DOI] [PubMed] [Google Scholar]
- 15.Lu Z, Ghosh S, Wang Z, Hunter T. Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of beta-catenin, and enhanced tumor cell invasion. Cancer Cell. 2003;4:499–515. doi: 10.1016/s1535-6108(03)00304-0. [DOI] [PubMed] [Google Scholar]
- 16.Ohashi S, Natsuizaka M, Wong GS, Michaylira CZ, Grugan KD, Stairs DB, et al. Epidermal growth factor receptor and mutant p53 expand an esophageal cellular subpopulation capable of epithelial-to-mesenchymal transition through ZEB transcription factors. Cancer Res. 2010;70:4174–4184. doi: 10.1158/0008-5472.CAN-09-4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 18.Kakarala M, Wicha MS. Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J Clin Oncol. 2008;26:2813–2820. doi: 10.1200/JCO.2008.16.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morel AP, Lièvre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santisteban M, Reiman JM, Asiedu MK, Behrens MD, Nassar A, Kalli KR, et al. Immune-induced epithelial to mesenchymal transition in vivo generates breast cancer stem cells. Cancer Res. 2009;69:2887–2895. doi: 10.1158/0008-5472.CAN-08-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neiva KG, Zhang Z, Miyazawa M, Warner KA, Karl E, Nör JE. Crosstalk initiated by endothelial cells enhances migration and inhibits anoikis of squamous cell carcinoma cells through STAT3/Akt/ERK signaling. Neoplasia. 2009;11:583–593. doi: 10.1593/neo.09266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krishnamurthy S, Dong Z, Vodopyanov D, Imai A, Helman JI, Prince ME, et al. Endothelial cell-initiated signaling promotes the survival and self-renewal of cancer stem cells. Cancer Res. 2010;70:9969–9978. doi: 10.1158/0008-5472.CAN-10-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krishnamurthy S, Nör JE. Orosphere assay: A method for propagation of head and neck cancer stem cells. Head Neck. 2013;35:1015–1021. doi: 10.1002/hed.23076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brenner JC, Graham MP, Kumar B, Saunders LM, Kupfer R, Lyons RH, et al. Genotyping of 73 UM-SCC head and neck squamous cell carcinoma cell lines. Head Neck. 2010;32:417–426. doi: 10.1002/hed.21198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 29.Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423:302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- 30.Park IK, Morrison SJ, Clarke MF. Bmi1, stem cells, and senescence regulation. J Clin Invest. 2004;113:175–179. doi: 10.1172/JCI20800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaneko T, Zhang Z, Mantellini MG, Karl E, Zeitlin B, Verhaegen M, et al. Bcl-2 orchestrates a cross-talk between endothelial and tumor cells that promotes tumor growth. Cancer Res. 2007;67:9685–9693. doi: 10.1158/0008-5472.CAN-07-1497. [DOI] [PubMed] [Google Scholar]
- 32.Warner KA, Miyazawa M, Cordeiro MM, Love WJ, Pinsky MS, Neiva KG, et al. Endothelial cells enhance tumor cell invasion through a crosstalk mediated by CXC chemokine signaling. Neoplasia. 2008;10:131–139. doi: 10.1593/neo.07815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 34.Chaffer CL, Brueckmann I, Scheel C, Kaestli AJ, Wiggins PA, Rodrigues LO, et al. Normal and neoplastic non-stem cells can spontaneously convert to a stem-like state. Proc Natl Acad Sci USA. 2011;108:7950–7955. doi: 10.1073/pnas.1102454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chaffer CL, Thompson EW, Williams ED. Mesenchymal to epithelial transition in development and disease. Cells Tissues Organs. 2007;185:7–19. doi: 10.1159/000101298. [DOI] [PubMed] [Google Scholar]
- 36.Charafe-Jauffret E, Ginestier C, Iovino F, Tarpin C, Diebel M, Esterni B, et al. Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clin Cancer Res. 2010;16:45–55. doi: 10.1158/1078-0432.CCR-09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher T, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Jong MC, Pramana J, van der Wal JE, Lacko M, Peutz-Kootstra CJ, de Jong JM, et al. CD44 expression predicts local recurrence after radiotherapy in larynx cancer. Clin Cancer Res. 2010;16:5329–5338. doi: 10.1158/1078-0432.CCR-10-0799. [DOI] [PubMed] [Google Scholar]
- 39.Ma I, Allan AL. The role of human aldehyde dehydrogenase in normal and cancer stem cells. Stem Cell Rev. 2011;7:292–306. doi: 10.1007/s12015-010-9208-4. [DOI] [PubMed] [Google Scholar]
- 40.Vincent T, Neve EP, Johnson JR, Kukalev A, Rojo F, Albanell J, et al. A SNAIL1-SMAD3/4 transcriptional repressor complex promotes TGF-beta mediated epithelial-mesenchymal transition. Nat Cell Biol. 2009;11:943–950. doi: 10.1038/ncb1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell. 2009;15:195–206. doi: 10.1016/j.ccr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 42.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bertolini F, Paul S, Mancuso P, Monestiroli S, Gobbi A, Shaked Y, et al. Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res. 2003;63:4342–6. [PubMed] [Google Scholar]
- 44.Imai A, Zeitlin BD, Visioli F, Dong Z, Zhang Z, Krishnamurthy S, et al. Metronomic dosing of BH3 mimetic small molecule yields robust antiangiogenic and antitumor effects. Cancer Res. 2012;72:716–25. doi: 10.1158/0008-5472.CAN-10-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.