Abstract
Background
Men and women with type-2 long QT syndrome (LQT2) exhibit time-dependent differences in the risk for cardiac events. We hypothesized that data regarding the location of the disease-causing mutation in the KCNH2 channel may affect gender-specific risk in LQT2
Objectives
To risk stratify LQT2 patients for life-threatening cardiac events based on clinical and genetic information.
Methods
The risk for life-threatening cardiac events from birth through age 40 (comprising aborted cardiac arrest [ACA] or sudden cardiac death [SCD]) years was assessed among 1,166 LQT2 males (n=490) and females (n=676) by the location of the LQTS-causing mutation in the KCNH2 channel (pre-specified in the primary analysis as pore-loop vs. nonpore-loop).
Results
During follow-up, the cumulative probability of life-threatening cardiac events years was significantly higher among LQT2 women (26%) as compared with men (14%; p<0.001). Multivariate analysis showed that the risk for life-threatening cardiac events was not significantly different between women with and without pore-loop mutations (HR=1.20; p=0.33). In contrast, men with pore-loop mutations displayed a significant >2-fold higher risk of a first ACA or SCD as compared with those with nonpore-loop mutations (HR=2.18; p=0.01). Consistently, women experienced a high rate of life-threatening events regardless of mutation-location (pore-loop: 35%, nonpore-loop: 23%), whereas in men the rate of ACA or SCD was high among those with pore-loop mutations (28%) and relatively low among those with nonpore-loop mutations (8%).
Conclusion
Combined assessment of clinical and mutation-specific data can be used for improved risk stratification for life-threatening cardiac events in type-2 long QT syndrome.
Keywords: long-QT syndrome, pore-loop mutations, sudden cardiac death, gender
Long QT syndrome (LQTS) is an inherited arrhythmogenic disorder caused by mutations in several cardiac ion channel genes.1 Clinically, LQTS is identified by abnormal QT interval prolongation on the electrocardiogram (ECG) and is associated with arrhythmogenic syncope and sudden arrhythmic death (SCD).1,2 Type 2 long QT (LQT2), the second most common variant of LQTS, is characterized by mutations in the α subunit of the KCNH2 channel which conducts the rapid delayed rectifier potassium current (IKr) in cardiac myocytes.1,2–4 Recent data show that mutations in the KCNH2 pore-loop region, which is responsible for forming the ion conduction pathway of the channel, are associated with a significantly higher risk of cardiac events as compared with mutations that are located in other regions of the channel.5,6 Furthermore, the clinical course of LQT2 patients was shown to be associated with major time-dependent gender differences, wherein women display a significantly higher risk for cardiac events than men after the onset of adolescence.7 Prior studies in LQT2 patients, however, evaluated mainly the combined end point of any cardiac event during follow-up (comprising mostly nonfatal syncopal episodes) and did not relate gender-specific risk to mutation-location in this population. Accordingly, the present study was carried out in a population of 1,166 genetically-confirmed LQT2 patients from Multinational LQTS Registries and was designed to: 1) evaluate time-dependent gender differences in the risk of life-threatening cardiac events (comprising aborted cardiac arrest [ACA] or SCD) in LQT2 patients; and 2) relate gender-specific risk for life-threatening events in this population to the location of the LQT2-causing mutation in the KCNH2 channel; and 3) develop a risk stratification scheme among LQT2 patients that combines clinical and mutation specific data.
Methods
Study Population
The study population was comprised of 1,166 subjects derived from (n=263) proband identified families with genetically confirmed KCNH2 mutations. Patients were drawn from the Rochester, NY enrolling center (center #1) of the International LQTS Registry (n=761), the Netherlands LQTS Registry (n=214), and the Japanese LQTS Registry (n=95), as well as from data submitted by other investigators specifically for this collaborative mutation analysis project: Denmark (n=62), Israel (n=24), and Sweden (n=10). The proband in each family had otherwise unexplained, diagnostic QTc prolongation or experienced LQTS-related symptoms. Patients were excluded from the study if they had > 1 LQTS-causing mutation (n=11).
Data Collection and Management
For each patient, personal history including cardiac events, ECGs, and therapies, as well as family history, were obtained at enrollment. Clinical data were then collected yearly on prospectively designed forms with information on demographic characteristics, personal and family medical history, ECG findings, medical therapies, left cardiac sympathetic denervation, implantation of a pacemaker or an implantable cardioverter defibrillator (ICD), and the occurrence of LQTS-related cardic events. The QT interval was corrected for heart rate using Bazett’s formula.8 Data common to all LQTS registries involving genetically tested individuals were electronically merged into a common database for the present study.
Genotype Characterization
KCNH2 mutations were identified with the use of standard genetic tests performed in academic molecular genetic research laboratories and/or in commercial laboratories. Genetic alterations of the amino acid sequence were characterized by location in the channel protein and by the type of mutation (missense, splice site, in-frame insertions/deletions, nonsense [stop codon], and frameshift).9 The transmembrane (TM) region of the KCNH2 encoded protein was defined as the coding sequence involving amino acid residues from 404 through 659 (pore-loop region: 548–659), with the N-terminus region defined before residue 404, and the C-terminus region after residue 659.
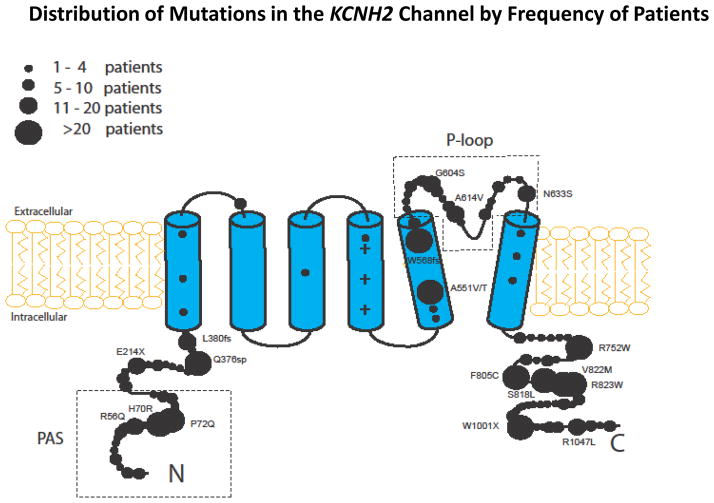
Pore-loop mutations disrupt normal channel gating10 and were shown to be associated with a significantly higher risk of cardiac events as compared with mutations in each of the other regions of the KCNH2 channel.5,6 Accordingly, mutation-location was categorized in the primary analysis of the present study as pore-loop vs. nonpore-loop. In a secondary analysis, nonpore-loop mutations were further subcategorized into those located in the transmembrane (nonpore-loop) region and in the C/N-terminus regions. Mutation-type was categorized as missense vs. non-missense. The specific mutations included in the present study, by location, type, and number of patients, are detailed in the Supplementary Appendix Table. The distribution of study mutations in the KCNH2 channel, by the relative number of patients, is shown in Figure 1.
Figure 1.
Distribution of mutations in the KCNH2 potassium channel among study patients
End Point
The primary end point of the study was the occurrence of a first life-threatening cardiac event, comprising ACA (requiring defibrillation as part of resuscitation), or LQTS-related SCD (abrupt in onset without evident cause, if witnessed, or death that was not explained by any other cause if it occurred in a non-witnessed setting such as sleep). To further validate the consistency of the results among patients who received an ICD during follow-up, we also assessed a secondary end point comprising the first occurrence of ACA, SCD, or appropriate ICD shock during follow-up.
Statistical Analysis
The baseline and follow up clinical characteristics of the study population were evaluated using the chi-square test for categorical variables, and the t-test and the Mann-Whitney-Wilcoxon test for continuous variables. The cumulative probability of a first ACA or SCD by gender and by mutation-location was assessed by the Kaplan-Meier method, and significance was tested by the log-rank test. Follow-up data was censored at age 40 to avoid confounding by acquired cardiovascular disease. Multivariate Cox proportional-hazards regression models were used to evaluate the independent contribution of clinical and genetic factors to the first occurrence of ACA or SCD. Prespecified covariates in the total population model included gender, QTc duration (categorized as ≥500 msec vs. <500 msec), mutation-location and type (as defined above), the occurrence of syncope during follow-up, and medical therapy with blockers. Syncope and beta-blocker therapy were assessed as time-dependent covariates in the multivariate models. The effect of each covariate in males and females was assessed by interaction-term analysis (i.e. by including a gender-by-risk factor interaction-term in the multivariate models), with interactions tested one at a time. To avoid violation of the proportional hazards assumption due to gender-risk crossover during adolescence, we employed an age-gender interaction-term in the multivariate models. Patients without available baseline QTc data (n=150) were included as a separate (QTc-missing) covariate in the multivariate models.
Using the Cox model that included interactions among gender, mutation-location, QTc duration, and time-dependent syncope, covariate patterns with similar estimated hazard ratios were unioned to form time-dependent risk groups.
Because almost all the subjects were first- and second-degree relatives of probands, the effect of lack of independence between subjects was evaluated in the Cox model with grouped jackknife estimates for family membership.11 All grouped jackknife standard errors for the covariate risk factors fell within 3% of those obtained from the unadjusted Cox model, and therefore only the Cox model findings are reported. The statistical software used for the analyses was SAS version 9.20 (SAS Institute Inc, Cary, NC). A 2-sided 0.05 significance level was used for hypothesis testing.
Results
The clinical characteristics of the study patients by gender are shown in Table 1. Baseline QTc was somewhat higher among women, however, the frequency of patients with prolonged QTc (≥500 msec) was similar in men and women. In addition, the frequency of patients with pore-loop mutations was the same in the 2 groups. During follow-up, there was no statistically significant difference between men and women in the frequency of medical therapy with beta-blockers, whereas the frequency of device therapy (including pacemakers and ICDs) was significantly higher among women. The frequency of both nonfatal syncopal episodes and life-threatening cardiac events during follow-up was significantly higher among women as compared with men (Table 1).
Table 1.
Baseline and follow-up characteristics of the study population by gender
| Characteristics | Male | Female | P-value |
|---|---|---|---|
|
| |||
| N=490 | N=676 | ||
|
| |||
| QTc (msec) | |||
| Continuous | 478 ± 57 | 484 ± 52 | 0.02 |
| ≥500, % | 32 | 34 | 0.44 |
|
| |||
| RR (sec) | 860 ± 250 | 856 ±216 | 0.91 |
|
| |||
| Location of mutation | |||
| Pore-loop, % | 28 | 28 | 0.93 |
| Nonpore-loop: | |||
| TM, % | 4 | 4 | 0.98 |
| N-term/C-term, % | 35 | 34 | 0.98 |
|
| |||
| Type of mutation | |||
| Missense, % | 65 | 68 | 0.33 |
| Non-missense, % | 35 | 32 | |
|
| |||
| LQTS-Therapies | |||
| β-blockers, % | 52 | 55 | 0.22 |
| Pacemaker, % | 3 | 6 | 0.02 |
| LSCD, % | 0.6 | 2 | 0.12 |
| ICD, % | 8 | 16 | <0.001 |
|
| |||
| Cardiac events during follow-up | |||
| Syncope, % | 24 | 46 | <0.001 |
| ACA, % | 3 | 9 | <0.001 |
| SCD, % | 8 | 12 | 0.02 |
| Appropriate ICD shocks, % | 1.5 | 1.9 | 0.58 |
|
| |||
| First SCD or ACA, %* | 10 | 19 | <0.001 |
Plus – minus values are means ± SD.
ACA = aborted cardiac arrest; ICD = implantable cardioverter defibrillator; LCSD = left cervical sympathetic denervation; SCD = sudden cardiac death; TM = transmembrane
Only the first event for each patient was considered.
Risk factors for ACA or SCD in the total LQT2 population
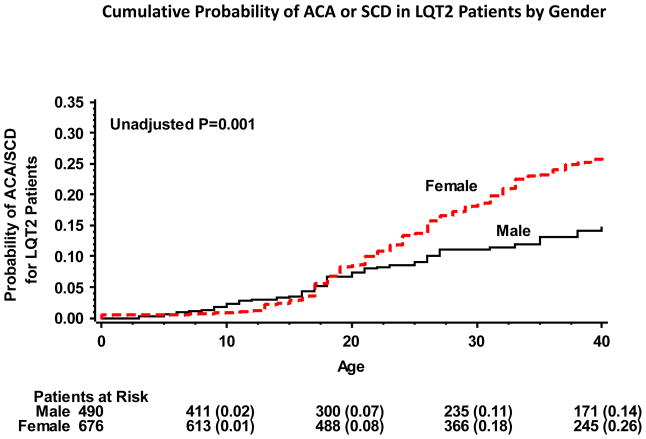
During follow-up, 179 (15%) study patients experienced the primary end point of a first ACA or SCD. Event rates were similar between men and women during childhood, whereas after onset of adolescence and during adulthood LQT2 women experienced a significantly higher rate of ACA or SCD as compared with LQT2 men (Fig. 2). Accordingly, the cumulative probability of a first ACA or SCD from birth through age 40 years was significantly higher in women (26%) as compared with men (14%; p<0.001 [Fig. 2]).
Figure 2.
Kaplan-Meier estimates of the cumulative probability of aborted cardiac arrest or sudden cardiac death in LQT2 patients by gender
ACA = aborted cardiac arrest; LQT2 = long QT syndrome type 2; SCD = sudden cardiac death.
Multivariate analysis in the total study population (Table 2) showed that during childhood (age 0–13 years) the risk of ACA or SCD was similar between women and men (HR=1.53; p=0.33), whereas after the onset of adolescence (age >13 years) women exhibited a significantly higher risk for ACA or SCD as compared with men (HR=2.23; p<0.001]). Mutations located in the pore-loop region of the KCNH2 channel were shown to be associated with a significant 39% (p=0.04) increase in the risk for ACA or SCD as compared with other ion channel mutations (Table 2). Results were similar when the secondary end point of a first ACA, SCD, or appropriate ICD shock was assessed.
Table 2.
Multivariate analysis: Risk factors for ACA/SCD among all LQT2 patients*
| Risk Factor | Relative Risk | ||
|---|---|---|---|
| Hazard Ratio | 95% Confidence Interval | P=Value | |
| Gender: Female vs. Male | |||
| Age group: 0–13 years | 1.53 | 0.72 – 3.26 | 0.33 |
| Age group: 14–40 years | 2.23 | 1.55 – 3.21 | <0.001 |
| Mutation-location | |||
| Pore-loop vs. nonpore-loop | 1.39 | 1.02 – 1.91 | 0.04 |
| Pore-loop vs. C/N-term | 1.44 | 1.06 – 1.97 | 0.02 |
| TM (nonpore) vs. C/N-term | 0.91 | 0.45 – 1.87 | 0.80 |
| Mutation Type | |||
| Missense vs. non-missense | 0.87 | 0.62–1.23 | 0.43 |
| QTc duration (msec) | |||
| ≥500 vs. <500 | 3.24 | 2.05 – 5.12 | <0.001 |
| Time-Dependent Syncope | |||
| Syncope vs. no syncope | 3.15 | 2.26 – 4.38 | <0.001 |
Models were further adjusted for missing QTc values, time-dependent β-blocker therapy, and the occurrence of syncope prior to the end point (assessed as a time-dependent covariate).
ACA = aborted cardiac arrest; LQT2 = long QT syndrome type 2; SCD = sudden cardiac death; TM = transmembrane.
Gender-specific risk factors for life-threatening cardiac events in LQT2 patients
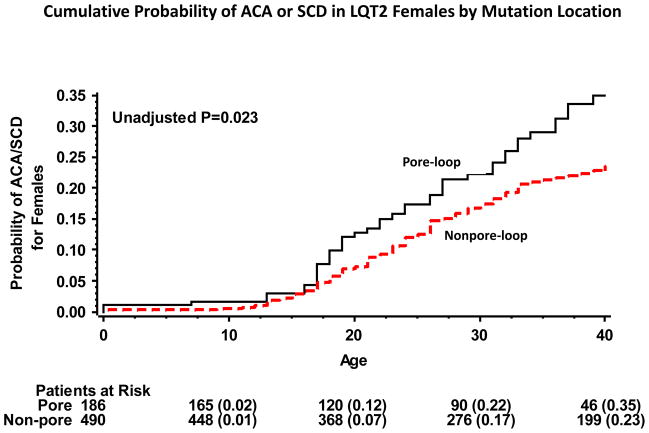
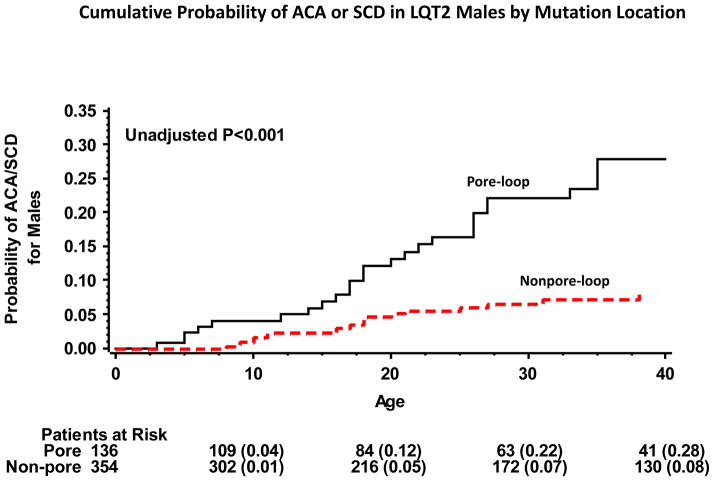
Kaplan-Meier survival analysis showed that the cumulative probablity of ACA or SCD by age 40 years was high in women with or without pore-loop mutations (35% and 23%, respectively; p=0.02 [Fig. 3]). In contrast, in men the rate of ACA or SCD was high among those with pore-loop mutations (28%) and relatively low among nonpore-loop mutations carriers (8%; p<0.001 [Fig. 4]). Consistent with these findings, gender-specific multivariate analysis (Table 3) showed that the risk for ACA or SCD was not significantly different among women with or without pore-loop mutations (HR=1.20; p=0.33), whereas men with pore-loop mutations exhibited a significantly higher risk for ACA or SCD as compared to men without pore-loop mutations (HR=2.18; P=0.01). Results for both men and women were consistent when the reference group of nonpore-loop mutations was further subcategorized into the transmembrane (nonpore-loop) and C/N-terminus regions (Table 3).
Figure 3.
Kaplan-Meier estimates of the cumulative probability of aborted cardiac arrest or sudden cardiac death in LQT2 women by mutation-location
ACA = aborted cardiac arrest; LQT2 = long QT 2 syndrome; SCD = sudden cardiac death.
Figure 4.
Kaplan-Meier estimates of the cumulative probability of a first aborted cardiac arrest or sudden cardiac death in LQT2 men by mutation-location
ACA = aborted cardiac arrest; LQT2 = long QT 2 syndrome; SCD = sudden cardiac death.
Table 3.
| LQT2 Males | LQT2 Females | |||
|---|---|---|---|---|
| HR (95% CI) | P-Value | HR (95% CI) | P-Value | |
| Mutation-Location | ||||
| Pore-loop vs. nonpore-loop | 2.18 (1.28 – 3. 72) | 0.01 | 1.20 (0.83 – 1.74) | 0.33 |
| Pore-loop vs. C/N-term | 2.04 (1.15 – 3.61) | 0.01 | 1.18 (0.81 – 1.70) | 0.39 |
| TM (nonpore) vs. C/N-term | NA‡ | 1.25 (0.60 – 2.58) | 0.56 | |
| Mutation-Type | ||||
| Missense vs. non-missense | 0.56 (0.29 – 1.06) | 0.08 | 1.29 (0.82 – 1.74) | 0.25 |
| QTc duration (msec) | ||||
| ≥500 vs. <500 | 2.16 (1.08 – 5.06) | 0.03 | 4.05 (2.33 – 7.04) | <0.001 |
| Time-Dependent Syncope | ||||
| Syncope vs. no syncope | 2.83 (1.36 – 5.58) | 0.01 | 3.32 (2.19 – 4.87) | <0.001 |
Findings were further adjusted for missing QTc values, time-dependent β-blocker therapy, and the occurrence of syncope prior to the end point (assessed as a time-dependent covariate)
Models were carried out in the total population using interaction-term analysis, with interactions tested one at a time; all interaction p-values were >0.05.
Hazard ratio was not computed due to a low event rate in male patients with TM mutations.
ACA = aborted cardiac arrest; LQT2 = long QT syndrome type 2; SCD = sudden cardiac death; TM = transmembrane.
QTc ≥ 500 msec was associated with >2-fold and >4-fold risk increase in men and women, respectively, whereas the mutation-type was not associated with a statistically significant risk increase (Table 3). Similarly, the occurrence of syncope during follow-up was associated with nearly a 3-fold increase in the risk of subsequent ACA or SCD in men, with a >3-fold risk increase in women (Table 3).
Time-dependent medical therapy with beta-blockers was associated with a significant 61% reduction in the risk fo ACA or SCD in the total study population (HR=0.39 [95% CI 0.20 – 0.74]). The benefit of treatment with beta-blockers was not significantly different between men and women (p-value for beta-blocker-by-gender interaction =0.23).
Risk stratification for ACA or SCD in LQT2 patients
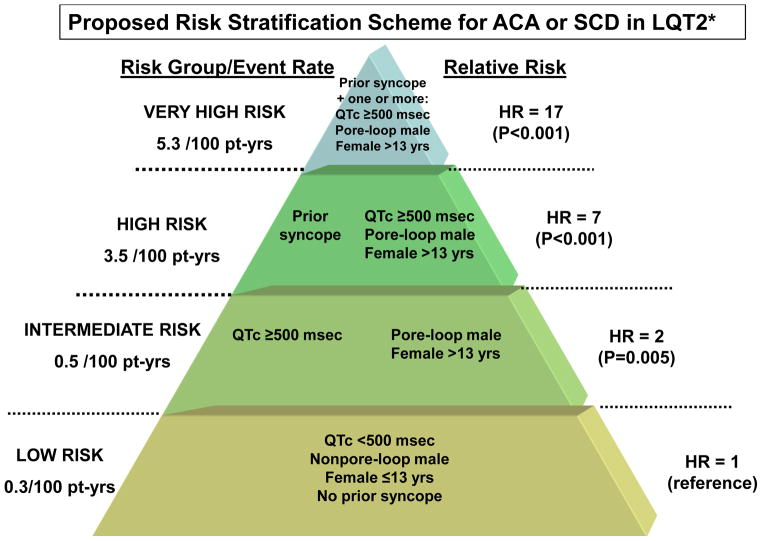
Using interaction terms among risk factors related to gender, mutation-location, QTc duration, and time-dependent syncope in the time-dependent Cox models we identified 4 risk-groups with significantly different risk for the endpoint of ACA or SCD (Fig. 5): 1) a low-risk group, comprising LQT2 patients with no risk factors (i.e. QTc <500 msec, no prior syncope, males without pore-loop mutations or females ≤ 13 years of age); 2) an intermediate-risk group (HR vs. low-risk group = 2.14; p=0.005), including a) males with pore-loop mutations or women >13 years of age [regardless of mutation location] and no additional risk factors; and b) patients with QTc ≥ 500 msec and no additional risk factors; 3) a high--risk group (HR vs. low-risk group = 7.22; p<0.001), including a) patients with prior syncope and no additional risk factors; and b) males with pore-loop mutations or females >13 years of age with QTc ≥ 500 msec, but without prior syncope; and 4) a very high-risk group (HR vs. low-risk group = 17.01; p<0.001), comprising patients who experienced prior syncope and also had one or more additional risk factor (i.e. QTc ≥ 500 msec, male with a pore-loop mutation or female >13 years of age).
Figure 5.
Proposed scheme for risk stratification for the end point of ACA or SCD in LQT2 patients by gender, mutation location, QTc, and a history of prior syncope.
*Hazard ratios and score estimates were obtained from a multivariate Cox model that included interactions among the identified risk factors (categorized by QTc duration, time-dependent syncope, gender and mutation-location) ; decimals points in HRs are rounded to the nearest whole number.; event rates per 100 person-years were calculated by dividing the number of life-threatening cardiac events (comprising ACA or SCD) in each risk category by the total follow-up time in the category (with follow-up censored after the occurrence of a ACA) and multiplying the result by 100.
ACA = aborted cardiac arrest; SCD = sudden cardiac death.
The nature of time-dependent covariates precludes assessment of cumulative event rates based only on the covariate pattern at the time origin. Therefore, to obtain an estimate of event rates we adjusted the number of events for the follow-up time in each risk-group. Thus, among very high-risk patients the rate of ACA or SCD was 5.3 per 100 patient-years; high-risk patients experienced 3.5 life-threatening cardiac events per 100 patient-years; intermediate risk patients had an event rate of 0.5 per 100 patient-years, whereas among low-risk patients the rate of ACA or SCD was only 0.3 per 100 patient years (Fig. 5).
Discussion
The present study is the first to assess gender differences in the risk of life-threatening cardiac events in LQT2, and to relate gender-specific risk in this population to the location of the disease-causing mutation. We have shown that among patients with LQT2: 1) both men and women have a relatively low rate of ACA or SCD during childhood, whereas after the onset of adolescence and throughout adulthood women exhibit a significantly higher rate of life-threatening events as compared with men; 2) the risk of ACA or SCD in women is high regardless of the location of the disease-causing mutation in the KCNH2 channel, whereas pore-loop mutations identify increased risk for ACA or SCD in men; and 3) combined assessment of clinical and mutation-specific risk factors can be used for improved risk stratification for life-threatening cardiac events in patients with type-2 long QT syndrome.
In a prior study Zareba et al. assessed age-dependent gender differences in the risk of cardiac events (comprising mostly nonfatal syncopal episodes) among 533genotyped patients from the International LQTS Registry.7 The study included 209 LQT2 patients, and showed that in this population no significant gender-related difference in the risk of cardiac events were present duirng childhood, whereas in the age-range of 16 through 40 years LQT2 women had >3-fold higher risk of cardiac events as compared with men.7 Possibly due to sample size limitations, the study did not identify a significant gender-related risk difference when the more severe end point of a first life-threatening cardiac event was assessed. The present study comprises the largest LQT2 population reported to date of 1,166 patients. We have shown that after the onset of adolescence there is a pronounced increase in the risk of ACA or SCD among LQT2 women (resulting in a cumulative event rate of 26% by age 40 years), whereas the risk of ACA or SCD among LQT2 men remains significantly lower throughout follow-up (resulting in a cumulative event rate of 14% by age 40 years). These age-gender risk differences in the clinical course of LQT2 patients may be mediated by the opposing effects of male and female sex hormones on the potassium channel. Testosterone was found to shorten the action potential duration and the QT interval through enhancement of the IKr current,12,13 and thus may be associated with QT shortening in males after childhood. In contrast, estrogen was shown to exhibit both acute and genomic effects on IKr, including reduction in channel function and prolongation of ventricular repolarization.14,15 Thus, LQT2 women who harbor mutations impairing potassium channel activity may be specifically sensitive to estrogen activity that may result in an increase in the risk for arrhythmic events after the onset of adolesence.
Recent data from the International LQTS Registry show that the location of the mutation in the ion channel is an important determinant of arrhythmic risk in LQTS patients. In a study of 201 LQT2 subjects with a total of 44 different KCNH2 mutations, Moss et al showed that subjects harboring pore mutations exhibited a more severe clinical course and experienced a higher frequency of cardiac events, occurring at an earlier age, than did subjects with nonpore mutations.5 Consistent with these findings, in a more recent study, Shimizu et al. showed that mutations in the pore region were associated with a greater risk of cardiac events as compared with mutations located in other regions in the KCNH2 channel.6 The pore region forms the potassium conductance pathway, and most mutations present in this region have a dominant-negative effects on IKr,10 suggesting that the pore region is critical for channel function. The findings of the present study are consistent with the previous link of high cardiac risk to pore-domain mutations, and show that the presence of pore-loop mutations was independently associated with a significant 39% increase in the risk of ACA or SCD in the total LQT2 population. Our findings, however, extend prior data and show a differential effect of mutation-related risk between LQT2 men and women. Thus, among men the presence of pore-loop mutations was associated with >2-fold (p=0.01) increase in the risk of ACA or SCD, whereas women with pore-loop mutations did not display a significant increase in risk as compared with those with nonpore-loop mutations. Accordingly, by age 40 years the rate of life-threatening cardiac events among men with pore-loop mutations was >3-fold higher as compared with those with other mutations (28% vs. 8%, respectively), whereas the corresponding event rates among women were high regardless of mutation-location (35% and 23%, respectively). Possible mechanisms that may explain the observed gender-related differences include the fact that estrogen increases IKr independently of mutation-location, thereby increasing arrhythymic risk even among women who carry lower-risk (nonpore) mutations in the KCNH2channel. In contrast, the protective effects of testosterone on IKr and ventricular repolarization in post-adolescent males result in a reduction in the risk of arrhytmic events among carriers of low-risk mutations, with a possible remaining residual risk in men who harbor higher risk mutations in the functionally more important pore-loop region.
In a prior study, Priori et al. proposed a risk stratification scheme for LQTS patients that is based on the LQTS genotype, QTc, and gender.16 This study, however, assessed a composite end point of cardiac events of any type, comprising mostly nonfatal symcopal episodes,16 whereas the large sample size of genotyped LQT2 patients in the present study facilitated for the first time the development of a risk stratification scheme for the end point of life-threatening cardiac events within the LQT2 population. We show that combined assessed of clinical and genetic data, related to mutation-location, can be used to identify risk groups of LQT2 patients with a significantly different risk of ACA or SCD and with a pronounced difference in the rate of ACA or SCD during follow-up. These findings suggest that risk stratification in long QT syndrome should combine clinical and mutation-related risk factors that are specific for each of the 3 main LQTS genotypes.
Prior data suggest that LQT2 patients expereince a relatively high rate of cardiac events during beta-blocker therapy.17 In the present study medical therapy with beta-blockers was associated with pronounced 61% reduction in the risk of ACA or SCD in the total LQT2 population. However, the present findings also suggest that careful follow-up, with consideration of ICD therapy for primary prevention, is warranted in high- and very-risk LQT2 patients. These patient subsets were shown to experience 3.5 to 5.3 events per 100 patient years (which correspond to a high rate of 1.5 to 2.1 life-threatening cardiac events per patient from birth through age 40 years) despite frequent usage of beta-blocker therapy (>80%).
Study limitations
We did not carry out expression studies to assess the effects of estrogen and testosterone on ion channel mutations by their location. Therefore, further studies are necessary to evaluate the mechanism related to the observed gender-specific risk related to mutation-location.
Due to sample size limitations we did not carry out comprehensive analysis of the relation between all function regions of the KCNH2 channel (including the PAS, CNBD, other C-terminus and N-terminus domains) and gender-specific risk. However, the results from the secondary analysis in which nonpore-loop mutations were further subcategorized into mutations in the transmembrane and C/N-terminus regions support the consistency of our findings.
Conclusions and Clinical Implications
The present study shows a distinct association between mutation characteristics and time-dependent differences in the clinical course of LQT2 patients. We have shown that after the onset of adolescence women with and without high risk mutations exhibit increased risk for life-threatening cardiac events, whereas the risk of ACA or SCD in men is increased only among carriers of the higher-risk pore-loop mutations. Thus, a comprehensive approach, that combines clinical and genetic data, should be employed for risk assessment and management of LQTS patients.
Supplementary Material
List of KCNH2 mutations among study patients by location and type
Acknowledgments
Funding/Support: This work was supported by research grants HL-33843 and HL-51618 from the National Institutes of Health, Bethesda, MD and by a research grant from GeneDx to the Heart Research Follow-Up Program in support of the LQTS Registry.
Abbreviations
- ACA
aborted cardiac arrest
- ECG
electrocardiogram
- ICD
implantable cardioverter defibrillator
- LQTS
long QT syndrome
- LQT2
long QT syndrome type 2
- QTc
corrected QT
- SCD
sudden cardiac death
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moss AJ, Kass RS. Long QT Syndrome: from channels to cardiac arrhythmias. J Clin Invest. 2005;115:2018–2024. doi: 10.1172/JCI25537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldenberg I, Zareba W, Moss AJ. Long QT syndrome. Curr Probl Cardiol. 2008;33:629–694. doi: 10.1016/j.cpcardiol.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Sanguinetti MC, Tristan-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature. 2006;440:463–469. doi: 10.1038/nature04710. [DOI] [PubMed] [Google Scholar]
- 4.Sanguinetti MC. HERG1 channelopathies. Eur J Physiol. 2010;460:265–276. doi: 10.1007/s00424-009-0758-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moss AJ, Zareba W, Kaufman ES, et al. Increased risk of arrhythmic events in long-QT syndrome with mutations in the pore region of the human ether-a-go-go-related gene potassium channel. Circulation. 2002;105:794–799. doi: 10.1161/hc0702.105124. [DOI] [PubMed] [Google Scholar]
- 6.Shimizu W, Moss AJ, Wilde AAM, et al. Genotype-phenotype aspects of type 2 long QT syndrome. J Am Coll Cardiol. 2009;54:2052–2062. doi: 10.1016/j.jacc.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zareba W, Moss AJ, Locati EH, et al. Modulating effects of age and gender on the clinical course of cong QT syndrome by genotype. J Am Coll Cardiol. 2003;42:103–109. doi: 10.1016/s0735-1097(03)00554-0. [DOI] [PubMed] [Google Scholar]
- 8.Bazett HC. An analysis of the time relations of electrocardiograms. Heart. 1920;7:353–67. [Google Scholar]
- 9.Splawski I, Shen J, Timothy KW, et al. Spectrum of mutations in long-QT syndrome genes: KVLQT1, HERG, SCN5A, KCNE1, and KCNE2. Circulation. 2000;102:1178–1185. doi: 10.1161/01.cir.102.10.1178. [DOI] [PubMed] [Google Scholar]
- 10.January CT, Gong Q, Zhou Z. Long-QT syndrome: cellular basis and arrhythmia mechanism in LQT2. J Cardiovasc Electrophysiol. 2000;11:1413–1418. doi: 10.1046/j.1540-8167.2000.01413.x. [DOI] [PubMed] [Google Scholar]
- 11.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York, NY: Springer-Verlag; 2000. [Google Scholar]
- 12.Bidoggia H, Maciel JP, Capalozza N, et al. Sex differences on the electrocardiographic pattern of cardiac repolarization: possible role of testosterone. Am Heart J. 2000;140:678–683. doi: 10.1067/mhj.2000.109918. [DOI] [PubMed] [Google Scholar]
- 13.Ridley JM, Shuba YM, James AF, Hancox JC. Modulation by testosterone of an endogenous hERG potassium channel current. J Physiol Pharmacol. 2008;59:395–407. [PubMed] [Google Scholar]
- 14.Hara M, Danilo P, Jr, Rosen MR. Effects of gonadal steroids on ventricular repolarization and on the response to E4031. J Pharmacol Exp Ther. 1998;285:1068–1072. [PubMed] [Google Scholar]
- 15.Song M, Helguera G, Eghbali M, et al. Remodeling of Kv4. 3 potassium channel gene expression under the control of sex hormones. J Biol Chem. 2001;276:31883–31890. doi: 10.1074/jbc.M101058200. [DOI] [PubMed] [Google Scholar]
- 16.Priori SG, Schwartz PJ, Napolitano C, et al. Risk stratification in the long-QT syndrome. N Engl J Med. 2003;348:1866–1874. doi: 10.1056/NEJMoa022147. [DOI] [PubMed] [Google Scholar]
- 17.Priori SG, Napolitano C, Schwartz PJ, et al. Association of long QT syndrome loci and cardiac events among patients treated with beta-blockers. JAMA. 2004;292:1341–1344. doi: 10.1001/jama.292.11.1341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of KCNH2 mutations among study patients by location and type