Abstract
Recent investigations indicate that innate immune “danger-signaling” pathways mediate metal implant debris induced-inflammatory responses, e.g. NALP3 inflammasome. How the physical characteristics of particles, (size, shape and chemical composition) affect this inflammatory reactivity remains controversial. We examined the role of Cobalt-Chromium-Molybdenum (CoCrMo) alloy particle shape and size on human macrophage phagocytosis, lysosomal destabilization, and inflammasome activation. Round/smooth vs. irregularly shaped/rough CoCrMo-alloy particles of ~1µm and 6 to 7µm diameter were investigated for differential lysosomal damage and inflammasome activation in human monocytes/macrophages. While spherical/smooth 1µm CoCrMo-alloy particles did not measurably affect macrophage IL-1β production, irregular 1µm CoCrMo-alloy particles induced significant IL-1β increases over controls. Both round/smooth particles and irregular CoCrMo-alloy particles that were 6 to 7µ min size induced >10-fold increases in IL-1β production compared to similarly shaped smaller particles (p<0.05). Larger irregular particles induced a greater degree of intracellular lysosomal damage and a >3-fold increase in IL-1β vs. similarly sized round/smooth particles (at an equal dose, particles/cell). CoCrMo-alloy particle-size-induced IL-1β production was dependent on the lysosomal protease Cathepsin B, further supporting lysosomal destabilization as causative in inflammation. Phagocytosable larger/irregular shaped particles (6µm) demonstrated the greatest lysosomal destabilization (observed immunofluorescently) and inflammatory reactivity when compared on an equal dose basis (particles/cell) to smaller/spherical 1µm particles in vitro.
Keywords: Inflammasome, Monocytes/macrophages, Lysosomal destabilization, Cathepsin B, Metal particles
INTRODUCTION
It is well established that soluble and particulate metal debris from orthopedic implants induce a local inflammatory response mediated by resident and recruited immune cells (i.e., monocytes, macrophages, and T lymphocytes)(1–5). Key inflammatory cytokines in this monocyte/macrophage response to particulate and soluble metal debris are PGE-2, IL-1β, IL-6, and TNFα (3; 6–8). The Nalp3 inflammasome “danger signaling” pathway was previously identified as an initial and central mechanism by which monocytes/macrophages react to soluble and particulate implant debris-induced cellular damage (8), initiating an inflammatory cascade of events that begin with the secretion of the potent pro-inflammatory cytokine IL-1β. While the Nalp3 inflammasome pathway is a well established mechanism of immune recognition of cellular damage by several non-pathogenic agents (i.e., vaccine adjuvants, silica, asbestos, uric acid, and cholesterol crystals), the precise intracellular danger signal or combination of signals that trigger Nalp3 oligomerization, and thus inflammasome assembly and secretion of IL-1β, remains unknown. Some reports indicate potassium efflux from distressed cells as a possible trigger of Nalp3 oligomerization and pathway activation, while others showed that reactive oxygen species (ROS) produced by endo-lysosomes and/or mitochondria are essential for Nalp3 activation (9–11). Recent studies reported that monocyte/macrophage phagocytosis of particulate agents can activate the Nalp3 inflammasome pathway due to lysosomal rupture and leakage of the protease cathepsin B into the cytoplasm (12–14), suggesting that cathepsin B could be an important lysosomal danger signal detected by Nalp3 in the cytosol of cells under mechanical stress.
Previous studies showed that biomaterial debris type, size, and shape influence subsequent tissue bio-reactivity, suggesting that different debris types induce differential inflammatory responses (15–18). While all types of debris induce some degree of biologic reactivity, particulate debris <10 µm is of greater concern because it can be readily phagocytosed by single cells and lead to a stronger inflammatory reaction (2; 8). However, debate continues on exactly what size particle produces the greatest inflammatory response for different materials and shapes and under what conditions (19–22) Thus, it remains contentious as to which specific size(s) and/or dose of particles (particles/cell or particles/tissue volume) are maximally inflammatory (7; 23; 24). Though heavily digested tissues and processed simulator fluids show debris from metal-on-metal implants as small as ~5 nm, the particle sizes in both implant simulator fluids and in peri-implant tissues range from 5nm to 100’s of microns in diameter with the typically observed in the 0.02–5 micron range (25–28). Although the inflammasome “danger signaling” pathway is involved in debris reactivity(8; 29), it remains unknown to what extent size and shape (surface texture) can induce inflammasome activation and if this occurs via lysosomal rupture and destabilization.
We hypothesized that increasing size, dose, and surface irregularity would induce more inflammasome mediated inflammation (IL-1β production) by inducing a greater degree of lysosomal damage (cellular mechanical strain) after phagocytosis. We tested this hypothesis by challenging a human macrophage cell line (THP-1) and primary human monocytes/macrophages with increasing dose, increasing size, and increasing surface irregularity of CoCrMo-alloy particles, and measured lysosomal destabilization-mediated IL-1β secretion using Nalp3 inflammasome pathway specific target blockers and confocal microscopy of lysosome destabilization.
MATERIALS AND METHODS
Media and Challenge Agents
Growth media for the human primary monocytes or cell lines (THP-1 (ATCC) was RPMI 1640 supplemented with L-Glutamine, Penicillin, Streptomycin, 25mM Hepes (Lonza, Walkersville, MD), and 10% heat inactivated fetal bovine serum (FBS; Hyclone Laboratories, Logan, UT) or 10% heat inactivated autologous serum.
The particles used in this study were comprised of clinically available CoCrMo alloy commonly used in hip and knee implants. Four types of particles were used: 1.1 µm (ECD) Irregularly shaped CoCrMo-alloy particles (ASTM F75) ; 6.3 µm (ECD) Irregularly shaped CoCrMo-alloy particles (ASTM F75) ; 1.4 µm (ECD) Smooth shaped CoCrMo-alloy particles (ASTM F75); and 7.3 µm (ECD) Smooth shaped CoCrMo-alloy particles (ASTM F75).
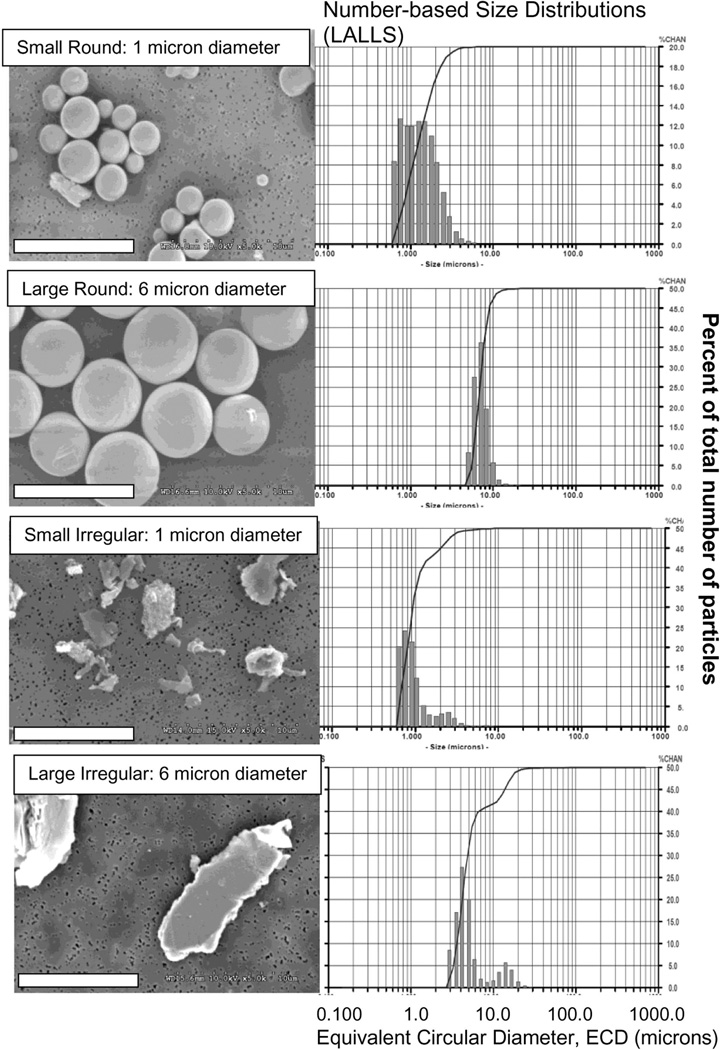
All particle powders were analyzed by Low Angle Laser Light Scattering (LALLS, Microtrac X-100), which measures millions to billions of particles (Fig. 1). Irregularly shaped alloy particles were produced by gas atomization (prior to implant casting) and high-speed cyromilled under proprietary methods (BioEngineering Solutions Inc., Oak Park, IL) using commercially available and packaged total hip replacement femoral heads (Zimmer Inc, Warsaw IN). While simulator fluids and peri-implant tissue-digests have shown metal particles as small as 5–10 nm (30; 31), currently nanoparticles have not been identified in virgin tissue sections (due to the practical difficulty associated with detecting particles in non-digested tissue sections under high power SEM or TEM). In contrast, individual particles and particle agglomerates in the 0.1, 1, 10, and 100 micron ranges are well established and identified in tissue sections (and tissues digests) of failed implants, particularly in those of failed metal-on-metal implants (32–39). Thus the midranges of these well documented sizes (i.e. µm) was selected due to its commercial availability, the ability to efficaciously use similar sized particles of different surface topology (validated by SEM and LALLS), and the long history of histologically identifiable particles within retrieved tissues. All CoCrMo-alloy particles were endotoxin cleaned and tested (<0.01eU, Kinetic QCL) and sterilized by steam sterilization. Controls were Alum (a particle/adjuvant Nalp3 inflammasome positive control), zVAD (Selective blocker of caspase-1 activity), and CA-074-Me (selective Cathepsin B inhibitor), (Sigma-Aldrich).
Figure 1.
(Left Panels) Scanning electron micrographs show the 2 sizes (~1 µm and 6 µm) of round and irregularly shaped CoCrMo-alloy particles used to challenge cells (Note: Bars=10 µm). (Right Panels) Associated Low Angle Laser Light Scattering generated number distributions show the range of sizes for each particle sizes and shape challenge.
Cell Purification
Blood samples were obtained intravenously from healthy volunteers with IRB approved informed consent (Rush University). Peripheral blood mononuclear cells (PBMCs) were isolated by Ficol gradient separation and collected for further purification. Peripheral blood CD14+ monocytes were isolated from PBMCs by negative selection with a specific magnetic bead antibody cocktail to CD3, CD7, CD16, CD19, CD56, CD123, and Glycophorin A (Miltenyi Biotec). Isolated human primary monocytes were assessed for >90% purity using FACS.
Cell Culture and Blocking Experiments
THP-1 monocytes were differentiated into macrophages by culturing 2.0×105 monocytes in 48 well plates with a phorbol ester (TPA) for 18–24 hrs (determined previously to yield maximal inflammasome macrophage/monocyte responses) (8). Newly differentiated macrophages were challenged with: irregularly shaped CoCrMo-alloy particles of 6µm diam (large) and 1um (small) (ASTM F-75) at a 0.25:1, 0.5:1, and 1:1(particles:cell) ratio; and round CoCrMo-alloy particles of 6µm diam (large) and 1 µm (small) (ASTM F-75) at a .25:1, .5:1, and 1:1 ratios for 24 hrs. Supernatants were analyzed for mature IL-1β production subsequently. Caspase-1 inhibitor zVAD (20uM) or Cathepsin B inhibitor were added with THP-1 cells and human primary monocytes during metal challenge cultures to block caspase-1 cleavage of pro-IL-1β or to block the effects of cytosolic lysosomal Cathepsin B on Nalp3 activation respectively.
ELISA
Sandwich ELISAs for IL-1β (R&D systems) were used to detect metal-induced THP-1 and human primary monocyte/macrophage mature IL-1β secretion using manufacturer’s instructions.
Luminex Assays
Metal-induced IL-1β by THP-1 monocytes and human primary monocytes were analyzed with Luminex suspension multiplex array technology. Supernatants from metal-challenged monocytes were collected 24 hrs after initial metal challenge and frozen at –80 C°.
Monocyte/macrophage supernatants were then thawed and analyzed for IL-1β concentrations with a pro-inflammatory-bead based cytokine array kit (Invitrogen). All Luminex assays were analyzed in triplicates with manufacturer’s provided buffers and protocols.
Stealth RNA Interference (RNAi)
To determine if down regulating the inflammasome pathway in macrophages/monocytes diminished the inflammasome responses by all challenge agents (and that other mechanisms were not responsible for cytokine production), RNAi was transfected into a THP-1 human macrophage cell line to knock down inflammasome proteins (Nalp3, ASC). THP-1 cells (2.5×105) were transfected with 10 nM RNAi using Lipofectamine RNAimax transfection reagent following manufacturer’s protocols (Invitrogen). THP-1 cells were transfected with RNAi for 48 hrs before fresh media and metal challenge agents were introduced into the culture system. Non-treated, Mock RNAi-treated, Nalp3 RNAi-treated and ASC RNAi-treated THP-1 were challenged with: irregularly shaped particles of 6µm and 1µm diam (ASTM F-75) at a 0.25:1, 0.5:1, and 1:1particles:cell ratio for 24 hrs.
Confocal Microscopy
THP-1 monocytes (ATCC) were cultured in RPMI-1640 10% fetal bovine serum (FBS) (Hyclone Laboratories, Inc) at 37°C and 0.5% Co2 and differentiated into macrophages by culturing 2.5 × 105 in a four well glass chamber slide with phorbol ester (TPA) for 18–24 hrs. Differentiated THP-1 macrophages challenged with particles and incubated at 37°C for 3 hrs with 15µg/ml DQ Ovalbumin (Invitrogen) were fixed with histochoice for 30 mim, washed 4x (PBS), mounted (vectasheild) and imaged (Zeiss LSM 510, 488nm).
Statistical Analysis
A two-tailed t-test was used to determine significance in cytokine production in THP-1 cells and human primary monocytes/macrophages. Paired t-test was used to determine significance in cytokine secretion between metal-treated and non-treated human primary monocytes/macrophages as a group (n=5).
RESULTS
CoCrMo-alloy particle-induced monocyte/macrophage IL-1β secretion is caspase-1 dependent
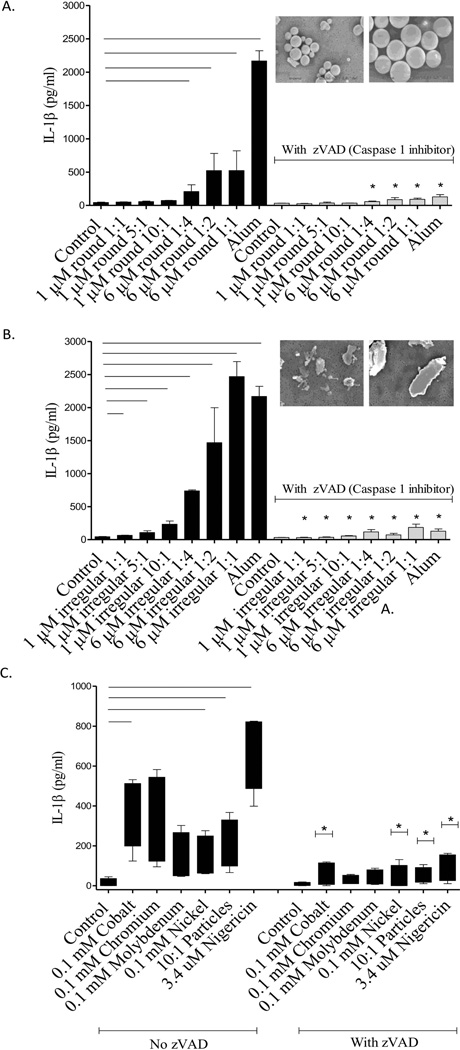
IL-1β secretion increased with increasing concentrations of 6 um round CoCrMo-alloy particles (positive dose responses) and induced a modest production of IL-1β (up to 523 pg/ml) compared to untreated controls and smaller 1um round particles at any dose (p<0.05) (Fig. 2A). Smaller (1um) round particles did not induce a significant increase in IL-1β production even at the highest concentration tested (10:1 particles:cell) (Fig. 2A). In contrast to small 1um round particles, small 1um irregular particles induced a significant increase in IL-1β production (up to 234 pg/mL) with increasing concentrations of particles vs. untreated controls (p < 0.05) (Fig. 2B). Large 6um irregular particles also induced a concentration dependent effect on IL-1β production (up to 2468 pg/mL), which was significantly higher than untreated controls and higher than the effect induced by their large round particle counterpart (523 pg/mL) (Fig. 1B).
Figure 2.
THP-1 cells were treated with increasing concentrations of 1µM or 6 µM smooth (A) or rough (B) particles in the presence or absence of caspase-1 inhibitor Z-VAD C) Human primary monocytes/macrophages (n=4) were treated with 0.1 mM Cobalt (CoCl2), Chromium (CrCl3), Molybdenum (MoCl5), Nickel (NiCl2) and 6 µM Co-Cr-Mo alloy particles (10:1 Particles:cell) for 16 hrs. * denotes significance between ZVAD treated cells vs. no ZVAD treatment for THP-1 and for human primary monocytes/macrophages. Bars indicate significance at p < 0.05 between metal particle/ion treated cells vs. non-treated controls.
These phenomena depended on inflammasome activation as determined by concomitant treatment with ZVAD, an inhibitor of Caspase-1 (which cleaves pro- IL-1β into IL-1β). Addition of the caspase-1 inhibitor abolished secretion of IL-1β in response to all CoCrMo-alloy particle challenges (Fig. 2A-B). These data establish that the differences in particle size, shape, and dose that induce differential IL-1β secretion are Caspase-1 dependent (inflammasome activated). To confirm the reduction in IL-1β in THP-1 macrophages in the presence of capase-1 inhibitor ZVAD, we tested whether soluble and particulate metal-induced IL-1β secretion in primary human monocytes/macrophages (n=3) was also abolished in the presence of caspase-1 inhibitor (Fig. 2C). Co and Cr ions induced significantly more IL-1β secretion than Ni in human primary monocytes/macrophages. These data imply that metal particle-induced IL-1β production in human THP-1 and human primary monocytes are in part to released metal ions and is fully or partially dependent on the active form of Caspase-1.
Round and irregular CoCrMo-alloy particle-induced IL-1β secretion in THP-1 cells is Nalp3 dependent
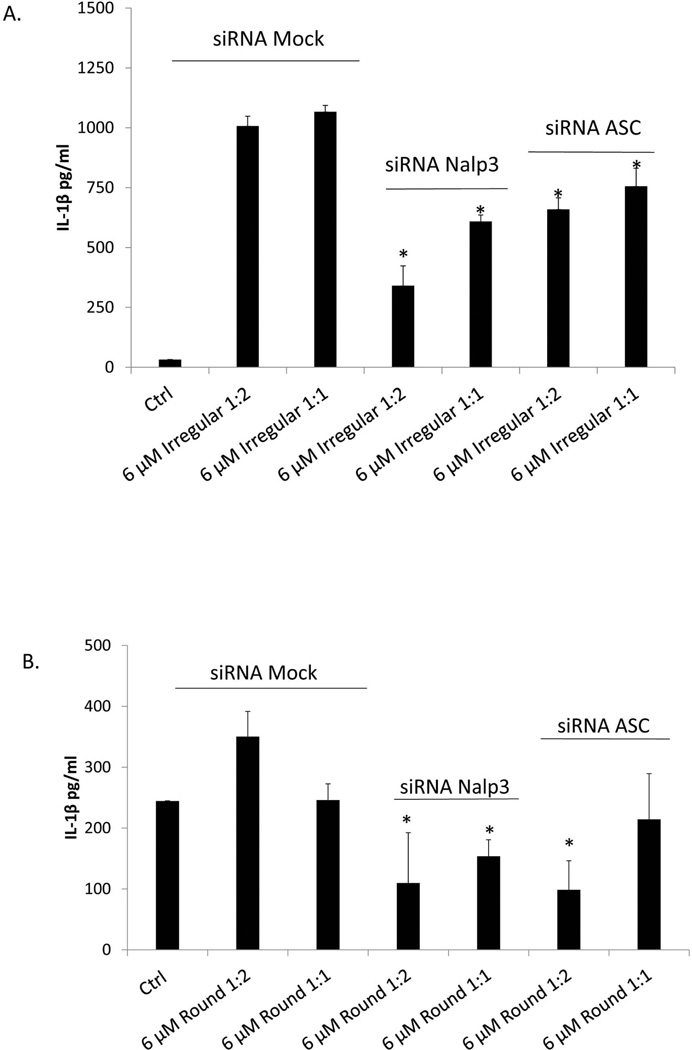
Consistent with previous caspase-1 inhibition results, THP-1 cells that were knocked down for Nalp3 expression had a significant (p<0.05) decrease in IL-1β production compared to their Mock siRNA-treated control cells (Fig 3A,B). ASC knockdown induced a more modest, but significant decrease in IL-1β secretion for irregular particles and a non-significant decrease for round particles.
Figure 3.
THP-1 cells were not-transfected (Mock) or transfected with RNAi constructs specific for Nalp3 and ASC for 48 hrs to allow for Nalp3 and ASC knock down. Nalp3 RNAi, ASC RNAi or Mock-transfected THP-1 cells were subsequently challenged with or without (negative control) 6 µM irregular (A) or round (B) particles, for 16hrs (Alum = positive control). * denotes significance at p < 0.05.
Round, but not irregular CoCrMo-alloy particle-induced IL-1β production is dependent on macrophage phagocytosis
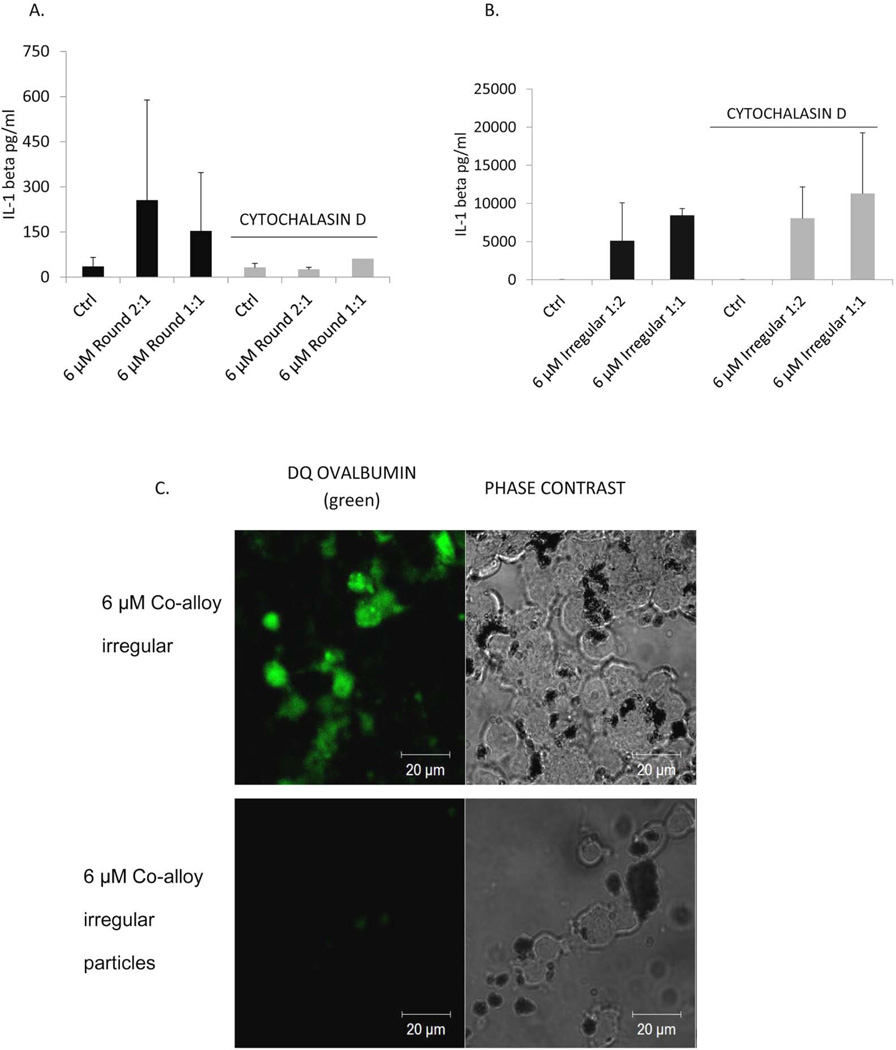
While cytochalasin D inhibited macrophage phagocytosis of both round and irregular particles as illustrated by lack DQ Ova-Albumin uptake by THP-1 cells (Fig.4 C), it only inhibited the production of IL-1β in round particle-treated macrophages (Fig. 4A). Treatment with cytochalasin D inhibited phagocytosis, but did not inhibit the production of IL-1β in irregular particle treated macrophages (Fig. 4B). Similar results were obtained from human primary monocytes, where cytochalasin D treated monocytes/macrophages had a decrease in IL-1β production in response to round particles, but not to irregular particles (data not shown). IL-1β production despite blockage of phagocytosis of irregularly shaped particles implicates differential ion release from different types of CoCrMo-alloy particles (irregular vs. round) (40).
Figure 4.
THP-1 cells were pre-treated with Cytochalasin D for 4 hrs and challenged with 6 µM round (A) or irregular (B) particles . THP-1 cell secretion of IL-1β was analyzed 24 hrs after culture. (C) THP-1 phagocytosis inhibition was confirmed by confocal microscopy 24 hrs after culture. * denotes significance at p < 0.05.
Round and irregular CoCrMo-alloy particle-induced lysosomal destabilization and leakage
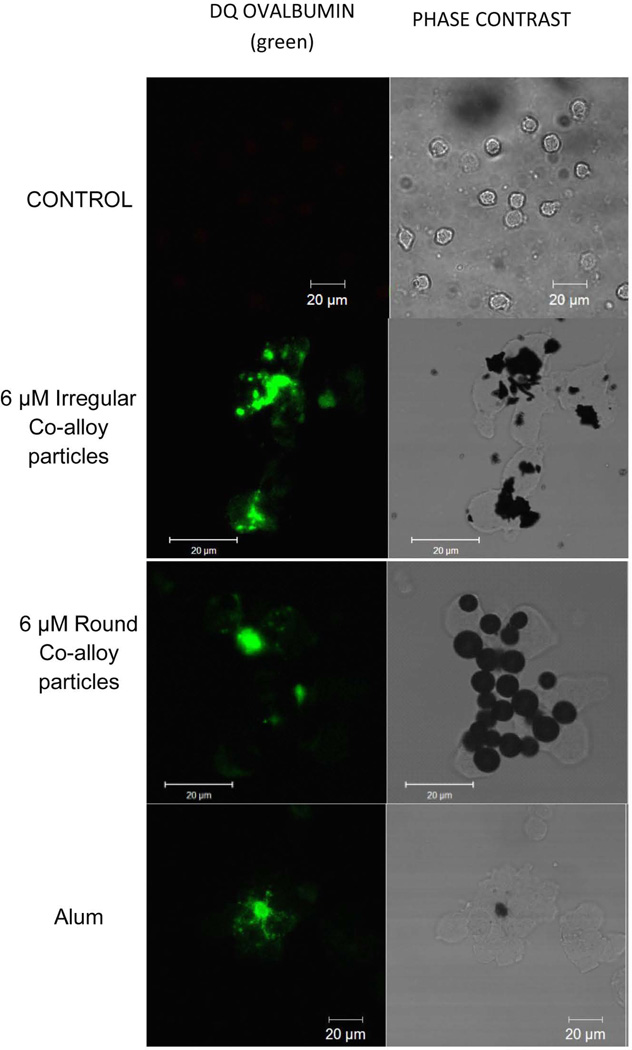
Particulate challenge agents phagocytosed by macrophages can lead to destabilization, and the extent of lysosomal damage can be assessed using confocal microscopy. DQ ovalbumin is internalized by cells simultaneously with particles into the phagolysosomal compartment and fluoresces in the lysosome acidic environment. Large fluorescent pools of DQ ovalbumin in the cytosol of cells are indicative of compromised lysosomal compartments that have leaked the fluorescent protein (Fig. 5). While THP-1 cells not treated with particles did not exhibit significant fluorescence pools in the cytosol (no lysosomal damage), round and irregular particles showed increased lysosomal damage as indicated by large fluorescent areas in particle-treated cells. These results show that both round and irregular particles can induce lysosomal destabilization in THP-1 macrophages.
Figure 5.
THP-1 macrophages were challenged with and without 6µM irregular or round particles and incubated with 15µg/ml DQ ovalbumin simultaneously for 4 hrs. THP-1 macrophages were fixed and evaluated for the presence or absence of large pools of DQ ovalbumin fluorescence indicative of lysosomal destabilization. White arrows indicate lysosomal destabilization.
CoCrMo-alloy particle-induced IL-1β in human macrophages is cathepsin B dependent
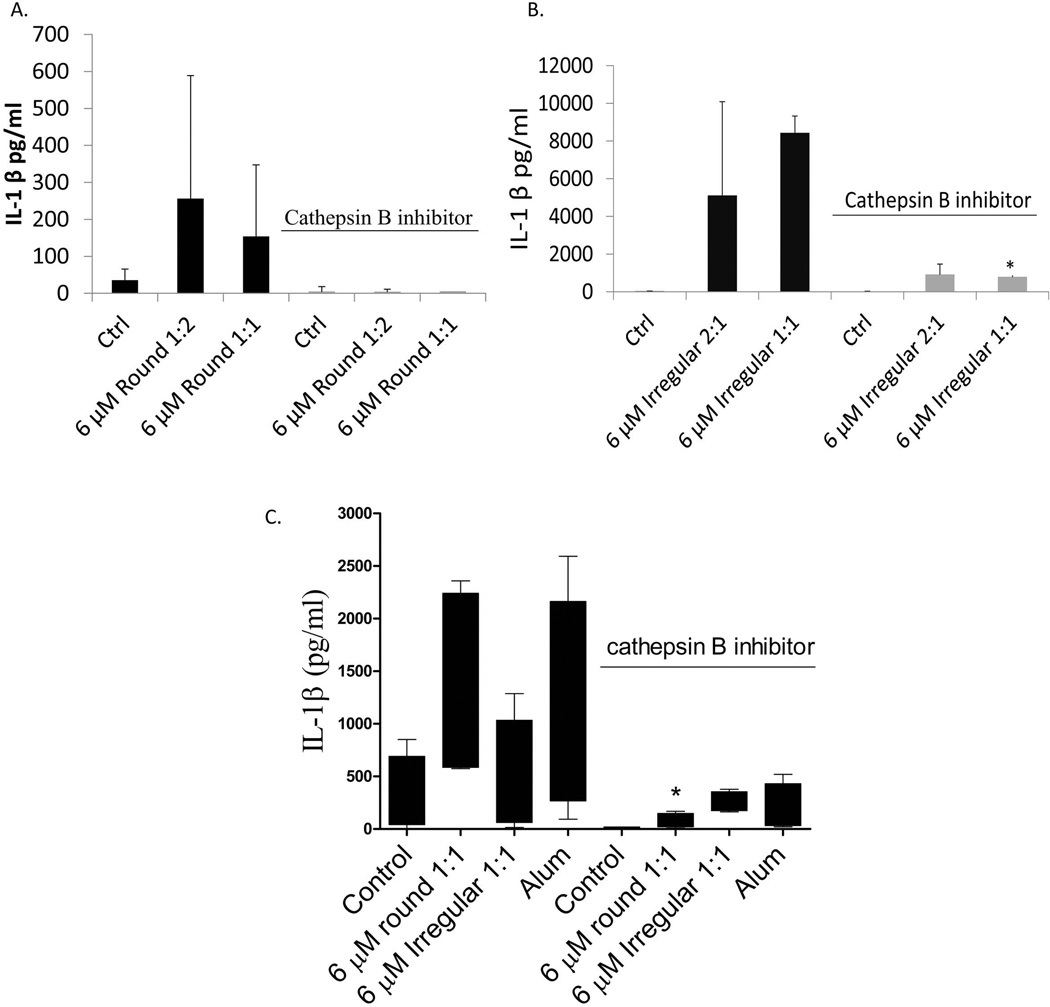
While THP-1 cells showed a more robust IL-1β response to irregular particles, the cathepsin B inhibitor reduced IL-1β to almost control/background levels (p<0.05) (Fig. 6). Human primary monocytes (n=4) exhibited a similar response where round particles increased IL-1β production that was completely abolished in the presence of cathepsin B inhibitor. Irregular particles induced higher production of IL-1β compared to untreated controls and the presence of Cathepsin B inhibitor also abolished IL-1β production (Fig. 6C). These data suggest that Cathepsin B is a key activator of the IL-1β inflammasome response to both round and irregular particles.
FIGURE 6.
THP-1 cells were treated with increasing 6 µM smooth (A) or rough (B) particles at 2 concentrations in the presence or absence of cathepsin B inhibitor; IL-1β was measured after 16 hrs of challenge. C) Freshly isolated human primary monocytes/macrophages (n=4) were treated with 6 µM Cobalt-alloy smooth (A) or rough (B) particles for 24 hrs. Alum was used as positive control for cathepsin B- induced IL-1β production.
DISCUSSION
We found that CoCrMo-alloy particle size, shape, and number induces differential macrophage IL-1β production dependent on several inflammasome “danger signaling” pathway components. The data support our hypothesis that irregularly shaped particles and larger sized particles (at equal dose) will induce more IL-1β than spherical/smooth or smaller particles due to a greater degree of lysosomal damage and inflammasome activation after phagocytosis. Particle number and shape influenced the degree of inflammatory response at the molecular level related to the seeming propensity to disrupt lysosome integrity (i.e cathepsin B leakage into the cytosol, and Caspase 1 activity). This provides insight into how different types of debris generated from the degradation of metal implants can induce different inflammatory responses.
It was previously established that the phagocytosis of non-biological particulate challenge agents, such as the vaccine adjuvant alum, induce lysosomal destabilization that leads to cathepsin B leakage in the cytosol and subsequent activation of the Nalp3 inflammasome that results in IL-1β secretion (13). Given that metal implant particles induce the inflammasome/IL-1β (6; 8; 41), we tested the degree to which factors such as particle number, size, and shape dictate inflammasome mediated IL-1β production in human macrophages. Larger round CoCrMo-alloy particles (6µM diam), induced a significant increase in IL-1β at low particle concentrations. In contrast smaller round particles (1 µM diam) did not induce IL-1β in THP-1 cells at any particle concentration tested (up to 10 particles per cell). This difference was also true of irregular (granular) smaller (1µM) and larger (6 µM) particles where larger particles induced significantly higher IL-1β (p<0.05) at an equal dose basis in THP-1 cells. It was not surprising that increased size resulted in an increased inflammatory response on an equal dose basis for both smooth and irregular geometries given that these sizes represent a 5to 25% increase in proportion compared to the 20µm diam of a non-spread monocyte/macrophage. However, the extreme non-proportional increase in inflammatory response over this size range suggests both non-linearity of response and the existence of a “sensitivity to size” threshold in the range of 1 to 10 µm.
Caspase-1 inhibitor zVAD diminished macrophage production of IL-1β to background control levels in response to both round and irregular particles. However, IL-1β production when challenged with highest concentration of large irregular particles was not reduced in the presence of Cytochalasin D (phagocytosis inhibitor). This suggests that increasing particle burden (particularly by larger particles) can potentially reach a threshold where alternate inflammatory or toxic pathways may be mediated by increased metal ion exposure and not by phagocytosis of the particle itself. This phenomenon could be explained by the fact that irregularly shaped particles have a greater surface area compared to the smooth surface of round particles and thus a greater propensity to release metal ions. Initial testing shows evidence that irregularly shaped particles release a higher concentration of metal ions into the surrounding environment compared to round particles (data not shown). The complex relationship between metal ion release and particle topology needs further characterization. We also showed that the presence of high ionic concentrations in addition to the particulate challenge agent can induce different mechanisms of inflammation and/or toxicity (soluble vs. particulate challenge agents) that can potentially overwhelm the cellular inflammatory response (6; 8).
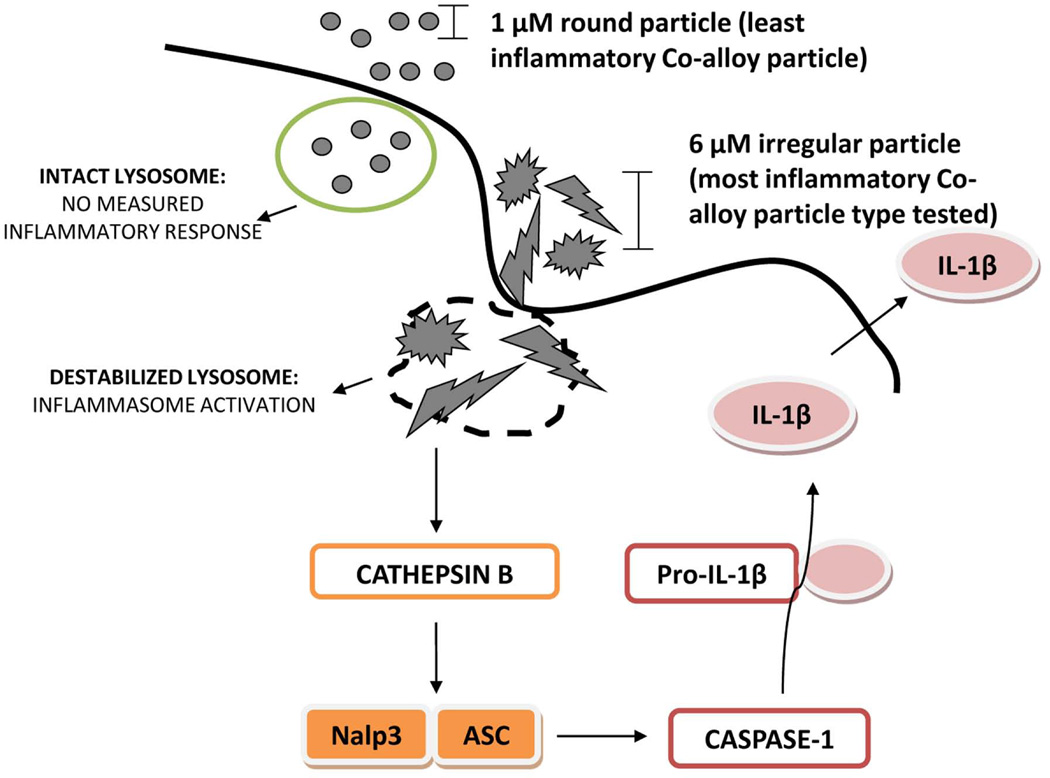
Danger signaling is a central immune mechanism that helps form an effective immune response to pathogen and non-pathogen derived cell damage, where lysosomal destabilization occurs from phagocytosis of particulate non-pathogenic challenge agents leading to Nalp3 inflammasome IL-1β production (13; 42; 42; 43). Here we have extended this type of reactivity to include implant debris and more importantly to vary in accordance with the surface geometries more able to destabilize intracellular lysosomes, i.e. increasing particle size and roughness (Fig. 7).
Figure 7.
Small 1 µM round or large 6 µM irregular particles are internalized by macrophages through active phagocytosis. While small 1 µM round particles do not induce a measurable response, large 6 µM irregular particles induce lysosomal destabilization leading to the release of Cathepsin B from the compromised lysosome. Cathepsin B acts as a danger signal to activate the Nalp3 –ASC inflammasome complex of proteins leading to the activation of Caspase-1 and cleavage of pro- IL-1β into active IL-1 β, which is subsequently released from the cell to exert its pro-inflammatory functions.
Lysosomal destabilization and leakage of proteases like Cathepsin B result in Nalp3 inflammasome activation and subsequent IL-1β production. Shape-dependent macrophage lysosomal destabilization to CoCrMo-alloy particles was further demonstrated by blocking this key lysosomal protease and observing abolished production of IL-1β. However, when increasing size, dose, and irregularity stop producing a commiserate increase in cell remains unknown and, given our results, is likely material and environment specific. Because it remains largely unknown which implant designs produce different shaped metal debris, the clinical utility of our results cannot be used (as yet) to predict the reactivity of one design over another. However, more generally, our results suggest that nano-debris (or other small sized particles) that are less likely to destabilize intracellular lysosomal compartments are also less likely to generate IL-1β mediated inflammatory responses. Thus, as metal particles decrease in size the greater the contribution of metal ions to the overall induction of IL-1β mediated responses and less important is that of particulate-specific effects. Further efforts are required to confirm this relationship, and testing is underway using nano-metallic particles and ions.
While the scope of this investigation is limited to particulate debris associated with CoCrMo-alloy implants, the application of our data to other implant metals, ceramics, and polymers is supported by our and others previous work (8; 44; 45). Understanding the danger signal components triggered by particulate debris that activate the Nalp3 inflammasome is of paramount importance given the millions of people with total joint replacements and the one million people in the U.S. with a metal-on-metal implant. Understanding specific cellular triggers of inflammasome involvement in particle-induced inflammation, such as lysosome destabilization, is key to designing better implants (e.g., evaluating the relative ability of CoCrMo-alloy vs. Ti-alloy particles to induce this response) and incorporating this pathway into therapeutic strategies to pharmacologically treat debris-induced inflammation/hypersensitivity by interrupting the initiation of inflammation (e.g., blocking inflammasome activation by blocking Cathepsin-B activation in the cytosol or IL-1β potentiation).
ACKNOWLEDGEMENTS
This research was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number AR060782. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Reference List
- 1.Greenfield EM, Bechtold J. What other biologic and mechanical factors might contribute to osteolysis? J Am Acad Orthop Surg. 2008;16(Suppl 1):S56–S62. doi: 10.5435/00124635-200800001-00012. [DOI] [PubMed] [Google Scholar]
- 2.Hallab NJ, Jacobs JJ. Biologic effects of implant debris. Bull NYU Hosp Jt Dis. 2009;67(2):182–188. [PubMed] [Google Scholar]
- 3.Schwarz EM, Lu AP, Goater JJ, Benz EB, Kollias G, Rosier RN, et al. Tumor necrosis factor-alpha/nuclear transcription factor-kappaB signaling in periprosthetic osteolysis. J Orthop Res. 2000 May;18(3):472–480. doi: 10.1002/jor.1100180321. [DOI] [PubMed] [Google Scholar]
- 4.Smith RA, Hallab NJ. In vitro macrophage response to polyethylene and polycarbonate-urethane particles. J Biomed Mater Res A. 2010 Apr;93(1):347–355. doi: 10.1002/jbm.a.32529. [DOI] [PubMed] [Google Scholar]
- 5.Hallab NJ, Anderson S, Stafford T, Glant T, Jacobs JJ. Lymphocyte responses in patients with total hip arthroplasty. J Orthop Res. 2005 Mar;23(2):384–391. doi: 10.1016/j.orthres.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Caicedo MS, Pennekamp PH, McAllister K, Jacobs JJ, Hallab NJ. Soluble ions more than particulate cobalt-alloy implant debris induce monocyte costimulatory molecule expression and release of proinflammatory cytokines critical to metal-induced lymphocyte reactivity. J Biomed Mater Res A. 2010 Jun 15;93(4):1312–1321. doi: 10.1002/jbm.a.32627. [DOI] [PubMed] [Google Scholar]
- 7.Shanbhag AS, Jacobs JJ, Black J, Galante JO, Glant TT. Human monocyte response to particulate biomaterials generated in vivo and in vitro. J Orthop Res. 1995 Sep;13(5):792–801. doi: 10.1002/jor.1100130520. [DOI] [PubMed] [Google Scholar]
- 8.Caicedo MS, Desai R, McAllister K, Reddy A, Jacobs JJ, Hallab NJ. Soluble and particulate Co-Cr-Mo alloy implant metals activate the inflammasome danger signaling pathway in human macrophages: a novel mechanism for implant debris reactivity. J Orthop Res. 2009 Jul;27(7):847–854. doi: 10.1002/jor.20826. [DOI] [PubMed] [Google Scholar]
- 9.Schorn C, Frey B, Lauber K, Janko C, Strysio M, Keppeler H, et al. Sodium overload and water influx activate the NALP3 inflammasome. J Biol Chem. 2011 Jan 7;286(1):35–41. doi: 10.1074/jbc.M110.139048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, et al. The Nalp3 inflammasome is essential for the development of silicosis. Proc Natl Acad Sci U S A. 2008 Jul 1;105(26):9035–9040. doi: 10.1073/pnas.0803933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011 Mar;12(3):222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meunier E, Coste A, Olagnier D, Authier H, Lefevre L, Dardenne C, et al. Double-walled carbon nanotubes trigger IL-1beta release in human monocytes through Nlrp3 inflammasome activation. Nanomedicine. 2011 Nov;:16. doi: 10.1016/j.nano.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008 Aug;9(8):847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Werneburg NW, Guicciardi ME, Bronk SF, Gores GJ. Tumor necrosis factor-alpha-associated lysosomal permeabilization is cathepsin B dependent. Am J Physiol Gastrointest Liver Physiol. 2002 Oct;283(4):G947–G956. doi: 10.1152/ajpgi.00151.2002. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs JJ, Shanbhag A, Glant TT, Black J, Galante JO. Wear Debris in Total Joint Replacements. J Am Acad Orthop Surg. 1994 Jul;2(4):212–220. doi: 10.5435/00124635-199407000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Shanbhag AS, Jacobs JJ, Glant TT, Gilbert JL, Black J, Galante JO. Composition and morphology of wear debris in failed uncemented total hip replacement. J Bone Joint Surg Br. 1994 Jan;76(1):60–67. [PubMed] [Google Scholar]
- 17.Shanbhag AS, Jacobs JJ, Black J, Galante JO, Glant TT. Macrophage/particle interactions: effect of size, composition and surface area. J Biomed Mater Res. 1994 Jan;28(1):81–90. doi: 10.1002/jbm.820280111. [DOI] [PubMed] [Google Scholar]
- 18.Glant TT, Jacobs JJ, Molnar G, Shanbhag AS, Valyon M, Galante JO. Bone resorption activity of particulate-stimulated macrophages. J Bone Miner Res. 1993 Sep;8(9):1071–1079. doi: 10.1002/jbmr.5650080907. [DOI] [PubMed] [Google Scholar]
- 19.Green TR, Fisher J, Matthews JB, Stone MH, Ingham E. Effect of size and dose on bone resorption activity of macrophages by in vitro clinically relevant ultra high molecular weight polyethylene particles. J Biomed Mater Res. 2000 Sep;53(5):490–497. doi: 10.1002/1097-4636(200009)53:5<490::aid-jbm7>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 20.Green TR, Fisher J, Stone M, Wroblewski BM, Ingham E. Polyethylene particles of a 'critical size' are necessary for the induction of cytokines by macrophages in vitro. Biomaterials. 1998 Dec;19(24):2297–2302. doi: 10.1016/s0142-9612(98)00140-9. [DOI] [PubMed] [Google Scholar]
- 21.Matthews JB, Green TR, Stone MH, Wroblewski BM, Fisher J, Ingham E. Comparison of the response of primary human peripheral blood mononuclear phagocytes from different donors to challenge with model polyethylene particles of known size and dose. Biomaterials. 2000 Oct;21(20):2033–2044. doi: 10.1016/s0142-9612(00)00089-2. [DOI] [PubMed] [Google Scholar]
- 22.Matthews JB, Besong AA, Green TR, Stone MH, Wroblewski BM, Fisher J, et al. Evaluation of the response of primary human peripheral blood mononuclear phagocytes to challenge with in vitro generated clinically relevant UHMWPE particles of known size and dose. J Biomed Mater Res. 2000 Nov;52(2):296–307. doi: 10.1002/1097-4636(200011)52:2<296::aid-jbm8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 23.Ingram J, Matthews JB, Tipper J, Stone M, Fisher J, Ingham E. Comparison of the biological activity of grade GUR 1120 and GUR 415HP UHMWPE wear debris. Biomed Mater Eng. 2002;12(2):177–188. [PubMed] [Google Scholar]
- 24.Matthews JB, Green TR, Stone MH, Wroblewski BM, Fisher J, Ingham E. Comparison of the response of primary murine peritoneal macrophages and the U937 human histiocytic cell line to challenge with in vitro generated clinically relevant UHMWPE particles. Biomed Mater Eng. 2000;10(3–4):229–240. [PubMed] [Google Scholar]
- 25.Catelas I, Campbell PA, Bobyn JD, Medley JB, Huk OL. Wear particles from metal-on-metal total hip replacements: effects of implant design and implantation time. Proc Inst Mech Eng H. 2006 Feb;220(2):195–208. doi: 10.1243/09544119JEIM112. [DOI] [PubMed] [Google Scholar]
- 26.Savio JA, III, Overcamp LM, Black J. Size and shape of biomaterial wear debris. Clin Mater. 1994;15(2):101–147. doi: 10.1016/0267-6605(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 27.Lee JM, Salvati EA, Betts F, DiCarlo EF, Doty SB, Bullough PG. Size of metallic and polyethylene debris particles in failed cemented total hip replacements. J Bone Joint Surg Br. 1992 May;74(3):380–384. doi: 10.1302/0301-620X.74B3.1587882. [DOI] [PubMed] [Google Scholar]
- 28.Doorn PF, Campbell PA, Worrall J, Benya PD, McKellop HA, Amstutz HC. Metal wear particle characterization from metal on metal total hip replacements: transmission electron microscopy study of periprosthetic tissues and isolated particles. J Biomed Mater Res. 1998 Oct;42(1):103–111. doi: 10.1002/(sici)1097-4636(199810)42:1<103::aid-jbm13>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 29.Winter M, Beer HD, Hornung V, Kramer U, Schins RP, Forster I. Activation of the inflammasome by amorphous silica and TiO2 nanoparticles in murine dendritic cells. Nanotoxicology. 2011 Sep;5(3):326–340. doi: 10.3109/17435390.2010.506957. [DOI] [PubMed] [Google Scholar]
- 30.Catelas I, Medley JB, Campbell PA, Huk OL, Bobyn JD. Comparison of in vitro with in vivo characteristics of wear particles from metal-metal hip implants. J Biomed Mater Res B Appl Biomater. 2004 Aug 15;70(2):167–178. doi: 10.1002/jbm.b.20036. [DOI] [PubMed] [Google Scholar]
- 31.Brown C, Williams S, Tipper JL, Fisher J, Ingham E. Characterisation of wear particles produced by metal on metal and ceramic on metal hip prostheses under standard and microseparation simulation. J Mater Sci Mater Med. 2007 May;18(5):819–827. doi: 10.1007/s10856-006-0015-z. [DOI] [PubMed] [Google Scholar]
- 32.Natu S, Sidaginamale RP, Gandhi J, Langton DJ, Nargol AV. Adverse reactions to metal debris: histopathological features of periprosthetic soft tissue reactions seen in association with failed metal on metal hip arthroplasties. J Clin Pathol. 2012 May;65(5):409–418. doi: 10.1136/jclinpath-2011-200398. [DOI] [PubMed] [Google Scholar]
- 33.Ollivere B, Darrah C, Barker T, Nolan J, Porteous MJ. Early clinical failure of the Birmingham metal-on-metal hip resurfacing is associated with metallosis and soft-tissue necrosis. J Bone Joint Surg Br. 2009 Aug;91(8):1025–1030. doi: 10.1302/0301-620X.91B8.21701. [DOI] [PubMed] [Google Scholar]
- 34.Engh CA, Jr, Moore KD, Vinh TN, Engh GA. Titanium prosthetic wear debris in remote bone marrow. A report of two cases. J Bone Joint Surg Am. 1997 Nov;79(11):1721–1725. doi: 10.2106/00004623-199711000-00014. [DOI] [PubMed] [Google Scholar]
- 35.Urban RM, Jacobs JJ, Gilbert JL, Galante JO. Migration of corrosion products from modular hip prostheses. Particle microanalysis and histopathological findings. J Bone Joint Surg Am. 1994 Sep;76(9):1345–1359. doi: 10.2106/00004623-199409000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Maloney WJ, Smith RL, Schmalzried TP, Chiba J, Huene D, Rubash H. Isolation and characterization of wear particles generated in patients who have had failure of a hip arthroplasty without cement. J Bone Joint Surg Am. 1995 Sep;77(9):1301–1310. doi: 10.2106/00004623-199509000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Shahgaldi BF, Heatley FW, Dewar A, Corrin B. In vivo corrosion of cobalt-chromium and titanium wear particles. J Bone Joint Surg Br. 1995 Nov;77(6):962–966. [PubMed] [Google Scholar]
- 38.Urban RM, Jacobs JJ, Tomlinson MJ, Gavrilovic J, Black J, Peoc'h M. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J Bone Joint Surg Am. 2000 Apr;82(4):457–476. doi: 10.2106/00004623-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Guyer RD, Shellock J, MacLennan B, Hanscom D, Knight RQ, McCombe P, et al. Early failure of metal-on-metal artificial disc prostheses associated with lymphocytic reaction: diagnosis and treatment experience in four cases. Spine(Phila Pa 1976) 2011 Apr 1;36(7):E492–E497. doi: 10.1097/BRS.0b013e31820ea9a2. [DOI] [PubMed] [Google Scholar]
- 40.Bearinger JP, Orme CA, Gilbert JL. In situ imaging and impedance measurements of titanium surfaces using AFM and SPIS. Biomaterials. 2003 May;24(11):1837–1852. doi: 10.1016/s0142-9612(02)00547-1. [DOI] [PubMed] [Google Scholar]
- 41.Kanaji A, Caicedo MS, Virdi AS, Sumner DR, Hallab NJ, Sena K. Co-Cr-Mo alloy particles induce tumor necrosis factor alpha production in MLO-Y4 osteocytes: a role for osteocytes in particle-induced inflammation. Bone. 2009 Sep;45(3):528–533. doi: 10.1016/j.bone.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joy B, Sivadasan R, Abraham TE, John M, Sobhan PK, Seervi M, et al. Lysosomal destabilization and cathepsin B contributes for cytochrome c release and caspase activation in embelin-induced apoptosis. Mol Carcinog. 2010 Apr;49(4):324–336. doi: 10.1002/mc.20599. [DOI] [PubMed] [Google Scholar]
- 43.Rajamaki K, Lappalainen J, Oorni K, Valimaki E, Matikainen S, Kovanen PT, et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One. 2010;5(7):e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maitra R, Clement CC, Scharf B, Crisi GM, Chitta S, Paget D, et al. Endosomal damage and TLR2 mediated inflammasome activation by alkane particles in the generation of aseptic osteolysis. Mol Immunol. 2009 Dec;47(2–3):175–184. doi: 10.1016/j.molimm.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.St Pierre CA, Chan M, Iwakura Y, Ayers DC, Kurt-Jones EA, Finberg RW. Periprosthetic osteolysis: characterizing the innate immune response to titanium wear-particles. J Orthop Res. 2010 Nov;28(11):1418–1424. doi: 10.1002/jor.21149. [DOI] [PMC free article] [PubMed] [Google Scholar]