Abstract
Purpose of review
This review presents novel findings regarding the renal angiotensin-converting enzyme (ACE) and its role in blood pressure (BP) control.
Recent findings
The textbook flow diagram of the renin–angiotensin system (RAS) shows the pulmonary endothelium as the main source of the ACE that converts angiotensin I to angiotensin II. However, ACE is made in large quantities by the kidneys, which raises the important question of what precisely is the function of renal ACE? Recent studies in gene-targeted mice indicates that renal ACE plays a dominant role in regulating the response of the kidney to experimental hypertension. In particular, renal ACE and locally generated angiotensin II affect the activity of several key sodium transporters and the induction of sodium and water retention resulting in the elevation of BP.
Summary
New experimental data link the renal ACE/angiotensin II pathway and the local regulation of sodium transport as key elements in the development of hypertension.
Keywords: angiotensin-converting enzyme, angiotensin II, blood pressure, kidney, sodium transport
INTRODUCTION
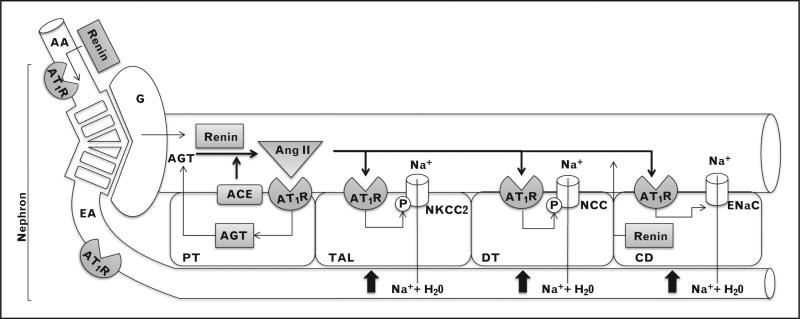
If forced to explain the control of blood pressure (BP) in six words or less, we would respond ‘the kidney and the renin–angiotensin system (RAS)’. An enormous quantity of experimental and theoretical observations, ranging from the studies of Arthur Guyton to the analysis of Mendelian forms of hypertension, have clearly established the kidney as the organ chiefly responsible for maintaining normal BP [1–3]. Many experimental studies, as well as clinical experience with angiotensin-converting enzyme (ACE) inhibitors and AT1 receptor antagonists, have demonstrated the central role of the RAS in BP regulation and, perhaps not surprisingly, the kidney and the RAS are intimately linked. Renin is made in the kidney. ACE is found in the kidney and angiotensin II has many effects on the kidney. It is therefore natural to consider what the relationship is between the RAS located within the kidney and the overall control of BP (Fig. 1). Such a discussion inevitably touches upon the subject of the local vs. the systemic production of angiotensin II. Although the lung is a major source of ACE and most angiotensin I is converted to angiotensin II by a single pass through this organ [4], there is still substantial data indicating a functional role of locally generated angiotensin II, predominantly in the brain, the heart and the kidney [5,6]. Rat kidney contains relatively little ACE, but human renal tissues make large amounts, even, by some measures, more than is found in human lung [7,8]. Thus, this review will focus on the role of ACE and the RAS locally within the kidney.
FIGURE 1.
Elements of the renal renin–angiotensin system (RAS). Depicted in grey boxes are RAS components made locally. AA, afferent arteriole; AGT, angiotensinogen; AT1R, angiotensin II type 1 receptor; CD, collecting duct; DT, distal tubule; EA, efferent arteriole; G, glomerulus; PT, proximal tubule; TAL, thick ascending limb.
Renal angiotensin-converting enzyme and kidney development
Clinical and experimental evidence supports an important role for the RAS in renal organogenesis. Human autosomal recessive renal tubular dysgenesis (RTD) was described in the 1980s and is a severe (often fatal) fetal disorder characterized by under-perfusion of the fetal kidney, persistent anuria, oligohydramnios and the underdevelopment of renal proximal tubules. Recent studies by Gribouval et al. [9■■] demonstrate that this disease is caused by mutations inactivating the fetal RAS, including mutations in angiotensinogen, renin, ACE and AT1 receptor genes. Such mutations result in renal hypoperfusion. Indeed, in humans, any condition that results in severe hypoperfusion of the developing kidney can result in the RTD phenotype. The resulting insufficiency of amniotic fluid causes fetal compression and a variety of facial, limb and lung defects, often referred to as the Potter sequence. Most fetuses with RTD die soon after birth due to anuria and pulmonary hypoplasia. A comparable situation is seen in mice lacking a functional RAS, but an important distinction is that whereas nephrogenesis in humans is completed before birth, nephrogenesis in mice continues after birth. Because of this, mice lacking a functioning RAS are born with normal renal structure. However, by as early as 16 days after birth, renal lesions have developed, characterized by renal arteriolar thickening, juxtaglomerular cell hypertrophy, and expanded renal medulla with blunted papilla and hydronephrosis [10–14].
In the mouse, gene targeting has been used to create animals in which renal ACE is nearly or completely abolished, though ACE expression by other tissues remains sufficient to maintain normal BP (Table 1) [15–19]. For example, a line of mice termed ACE 3/3 expresses ACE predominantly in hepatocytes; renal ACE expression is only 14% of wild-type levels [16]. In another mouse model termed ACE 10/10, ACE expression is under the control of the c-fms promoter. Here, ACE is only made by myelomonocytic cells [19]. In ACE 8/8 mice, the control of ACE by the a-myosin heavy chain promoter leads to expression predominant by cardiac myocytes [17]. In both the ACE 10/10 and ACE 8/8 mice, renal ACE is essentially abolished. ACE 3/3 and ACE 10/10 mice show normal basal BPs; ACE 8/8 mice have a mild reduction of basal BP, probably as a function of cardiac abnormality. However, in all three models, there is normal renal development and a normal ability to concentrate urine despite a substantial reduction in (ACE 3/3) or a total lack of renal ACE (ACE 8/8 and ACE 10/10). A very different picture is seen in a mouse termed ACE 9/9 in which the ACE gene has been genetically modified such that ACE is expressed only by renal tubular cells [18] (Table 1). In this model, the concentration of renal ACE is equivalent to that of control mice, but very little ACE is found in plasma or in other organs. The result is an animal with a marked reduction in BP that pheno-typically has the renal changes associated with hypoperfusion, similar to that observed in an ACE null (knockout) mouse.
Table 1.
ACE gene-targeted mice
| Strain name | ACE promoter | Target tissues | BP | Phenotype |
|---|---|---|---|---|
| ACE 1/1 (ACE null) [15] | Normal ACE promoter but gene inactivated by intragenic neo cassette | No tissues express ACE | Very low | Renal medullary expansion. Inability to concentrate urine. Male infertility. Anemia |
| ACE 3/3 [16] | Albumin promoter | Hepatocytes. Normal circulating ACE. 14% normal renal ACE | Normal | None |
| ACE 8/8 [17] | α-Myosin heavy chain promoter | Cardiac myocytes. Low circulating ACE. No renal ACE | Low (nearly normal) | Early death due to cardiac arrhythmias. Normal renal phenotype |
| ACE 9/9 [18] | Kidney specific cadherin promoter | Renal tubular epithelium | Low | Renal phenotype was similar but milder than ACE null mouse |
| ACE 10/10 [19] | c-fms promoter | Myelomonocytic cells. Normal circulating ACE. No renal ACE | Normal | Normal renal phenotype. Enhanced immune response |
ACE, angiotensin-converting enzyme; BP, blood pressure. These mice were created by targeted homologous recombination in embryonic stem cells. ACE expression is determined by the ability of individual tissues to use the promoter positioned to control tissue and temporal activity of the ACE gene.
Taken overall, these observations suggest that if renal perfusion pressure is normal, it does not appear that renal expression of ACE is necessary for normal renal development or even for basal control of BP. The opposite is also true in that the presence of renal ACE does not compensate for the absence of systemic ACE and low perfusion pressure.
Renal angiotensin-converting enzyme and the sodium excretory function
If renal ACE is not important for normal renal development or baseline function, what is the role of the large amounts of ACE normally made in the kidneys? Analyses in mice have indicated that glomerular filtration rate (GFR) and single nephron GFR are not affected by the absence of tissue-bound renal ACE [20]. In contrast, tubuloglomerular feedback responses are markedly attenuated in the absence of renal ACE [20]. Angiotensin II, generated in the renal tubular lumen, may enhance NaCl transport across macula densa cells and thus increase the basal sensitivity of tubuloglomerular feedback [21]. Indeed, studies show that angiotensin II modulates Na+/H+ exchanger and Na+/K+/2Cl− co-transporter activity in macula densa cells [22,23]. Under normal conditions, the tubuloglomerular mechanism regulates GFR by sensing the delivery of sodium chloride to macula densa cells. Under conditions of low sodium intake, tubuloglomerular feedback is critical for maintaining GFR even in the presence of decreased distal nephron sodium delivery.
In the human and mouse kidney, the greatest concentration of ACE is found in the brush border of proximal tubular epithelium [24]. The direct application of an ACE inhibitor in the proximal tubular lumen significantly reduces proximal tubular sodium reabsorption [25]. This is probably as a result of Na+/H+ exchanger 3 (NHE3) modulation, as this is the predominant mechanism for proximal tubular sodium absorption. Surprisingly, experiments with mice having no or minimal amounts of proximal tubular ACE demonstrate that proximal tubular reabsorption is not much different from that of wild-type mice [20]. One may speculate that in animals lacking proximal tubular ACE, compensatory mechanisms operate to maintain proximal reabsorption in the absence of local generation of angiotensin II.
ACE inhibitors have been applied to other nephron segments including the thick ascending limb and the collecting ducts. These inhibitors reduce sodium transport [23,26]. It is not known to what extent this is because of the effects of local ACE expression vs. ACE shed from the proximal tubular epithelium. In wild-type mice and rats, ACE expression has been reported in the distal nephron segments, including collecting duct cells [26,27]. In addition, Casarini et al. [28] report that ACE activity is found throughout the nephron and results in ACE expression in urine.
The renal angiotensin-converting enzyme/ angiotensin II pathway as master regulator of renal function during hypertension
Several independent observations suggest that local renal RAS is important in BP control, especially in experimental hypertension. A critical observation supporting this assertion is the clinical conundrum that ACE inhibitors and AT1 receptor blockers effectively reduce BP in hypertensive patients, even when there is no sign of systemic RAS activation [29,30]. Navar et al. [6] have shown that local angiotensin II content in the kidney is regulated independently from the systemic RAS. According to these investigators, angiotensin II concentrations in the total kidney are higher than plasma concentrations and reach even higher levels in many forms of experimental hypertension [6]. In addition, genetic experiments have shown that overexpressing RAS components in the nephron results in experimental hypertension [31–35]. Finally, one of the most convincing demonstrations of the importance of renal angiotensin II concentrations are the cross transplantation studies by Coffman and colleagues. By transplanting AT1a null kidneys into wild-type mice and wild-type kidneys into AT1a knockout mice, this group established that systemic AT1a receptors and AT1a receptors within the kidneys make separate and nonredundant contributions to baseline BP [36]. More importantly, this group showed that it was the presence of renal AT1a receptors that were the critical determinant of long-term sensitivity to angiotensin II-induced hypertension [37].
Although these studies emphasize the importance of angiotensin II actions locally within the kidney, they do not establish the precise role of systemic generation of angiotensin II vs. its local renal production in experimental hypertension. One experiment investigating this was performed in ACE 9/9 mice that express ACE only in renal tubules [18]. Such mice, when exposed to chronic angiotensin I infusion, showed increased levels of renal angiotensin II and urinary angiotensin II excretion. More significantly, the ACE 9/9 mice developed hypertension that was similar in magnitude (increase in systolic BP) to that observed in equally treated wild-type mice. After 3 weeks of angiotensin I infusion, ACE 9/9 mice had a BP increase of 41 mmHg over baseline vs. 36 mmHg in wild-type mice. As ACE 9/9 mice make ACE only in renal tubular epithelium, this experiment showed that renal ACE has the ability to increase the local concentration of angiotensin II and to induce hypertension even in the absence of a systemic ability to produce angiotensin II.
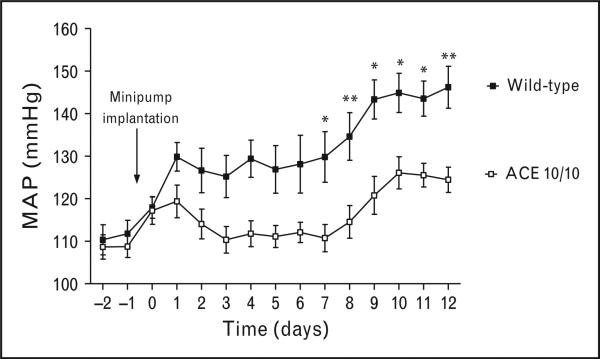
A very useful tool for investigating the exact role of renal ACE is the ACE 3/3 and ACE 10/10 mouse models previously discussed. These animals have two desirable characteristics: minimal (ACE 3/3) or no renal ACE (ACE 10/10) but ACE expression by nonrenal tissues that results in a normal BP. The hypertensive response of these mice was investigated in response to angiotensin II infusion, a high plasma angiotensin II model, and in response to nitric oxide synthesis inhibition with Nω-Nitro-L-arginine methyl ester (L-NAME), a low plasma angiotensin II model [38]. This showed that mice lacking renal ACE responded to the two very different forms of hypertension with blunted BP (Fig. 2) [38]. These experiments indicate that the absence of renal ACE protects against experimental hypertension regardless of plasma angiotensin II levels. Indeed, it was notable that during the sustained phase of angiotensin II-induced hypertension, detailed analysis showed that wild-type and mutant mice had the same high levels of plasma angiotensin II even though their BP was remarkably different.
FIGURE 2.
The absence of renal angiotensin-converting enzyme (ACE) blunts the hypertension induced by angiotensin II infusion. The mean arterial pressure of wild-type and mice lacking renal ACE (ACE 10/10 mice) was recorded by telemetry during chronic angiotensin II infusion (400 ng/kg/min via osmotic minipump). n = 7 –8 per group. * =P < 0.05, ** = P < 0.01. Values are means ± SEM. Data was originally published in [38].
The role of the local ACE in the renal responses to experimental hypertension was also investigated. For this, the angiotensin II infused model was selected for several reasons. First, previous studies have consistently demonstrated that exogenous angiotensin II infusion activates the renal RAS and induces local renal angiotensin II synthesis. For example, experiments in rats infused with Val5-angiotensin II, an isoform of angiotensin II that can be separated from endogenous angiotensin II (Ile5-angiotensin II) by high performance liquid chromatography, demonstrated that the chronic Val5-angiotensin II infusion (exogenous angiotensin II) induces renal Ile5-angiotensin II (endogenous angiotensin II) synthesis. This in turn resulted in endogenous angiotensin II accumulation and uri-nary excretion [39]. A second reason to select the angiotensin II infused model was that the renal responses to angiotensin II are well characterized [40]. Third, as mentioned before, the infusion of angiotensin II clamped plasma angiotensin II to equally high levels in wild-type and mutant mice [38].
For some researchers, the existence of a local RAS is still a point of contention, particularly proximal tubular epithelial angiotensinogen accumulation. Specifically, there is controversy as to whether proximal tubule angiotensinogen is generated locally or comes from the liver. Although Matsusaka et al. [41■] showed that, at baseline, most angiotensinogen and angiotensin II found in the kidney results from liver angiotensinogen, several studies confirm the presence of angiotensinogen mRNA in the proximal tubular epithelium [42,43]. Furthermore, in certain conditions, including angiotensin II infusion and early type 1 diabetes, increased proximal tubular angiotensinogen content and urinary angiotensinogen excretion are observed even in the absence of proteinuria [44,45]. Regardless of angiotensinogen origin, incontrovertible evidence supports the presence of renin and ACE in the tubules. We have already discussed tubular ACE. Renin from the juxtaglomerular apparatus is filtered [46]. In addition, local renin is produced by the principal cells of the collecting duct [27,47,48]. Indeed, although angiotensin II infusion induces strong plasma renin suppression, it increases collecting duct renin content [27]. As a whole, these observations suggest that angiotensin II infusion induces proximal tubular angiotensinogen accumulation that spills into the tubular fluid and then cleaved by tubular ACE and renin. The locally formed angiotensin II can bind to AT1 receptors ubiquitously expressed throughout the nephron [49]. The concept that exogenous angiotensin II can induce proximal tubule angiotensinogen, and thus endogenous angiotensin II formation, moves away from the classical RAS depiction. However, recent evidence suggests that local inflammation triggered by exogenous angiotensin II is to blame for proximal tubular angiotensinogen induction. It has been known for years that liver angiotensinogen is an acute-phase reactant that responds to inflammatory cytokines, including interleukin (IL)-1, IL-6 and TNFa [50]. Evidence from Kobori and Satou now indicates that the same is true for renal angiotensinogen [51–54]. According to the authors, angiotensin II acts synergistically with proinflammatory cytokines to stimulate angiotensinogen synthesis. Consistent with this concept of ‘exogenous angiotensin II’ stimulation of ‘endogenous local angiotensin II formation’, angiotensin II infusion increased renal angiotensin II content in wild-type but not in the ACE 10/10 mice. In other words, genetically removing ACE from renal tissues effectively prevented the local increase of angiotensin II observed in the angiotensin II-infused model [38].
The most remarkable aspect of renal ACE is the striking effect on the kidney response to experimental hypertension. Wild-type and ACE 10/10 mice were housed individually in metabolic cages to assess sodium and water handling during angiotensin II infusion. Whereas angiotensin II-infused wild-type mice showed a rapid decline in sodium excretion that returned to basal levels at the expense of hypertension, ACE 10/10 mice rapidly recovered sodium balance while maintaining normal BP levels. These very novel observations strongly support the nonintuitive conclusion that in angiotensin II-induced hypertension the shifting of the renal pressure–natriuresis relationship ultimately depends on renal ACE and the locally produced angiotensin II [38].
In searching for downstream targets, a detailed renal sodium transporter profile was performed. The analysis included NHE3, the loop of Henle Na+/K+/ 2Cl− co-transporter 2 (NKCC2), the distal tubule NaCl co-transporter (NCC), the epithelial sodium channel (ENaC), the anion exchangers pendrin and NDBCE, and the Na+/K+ ATPase. It was observed that kidney ACE, and the resultant local angiotensin II synthesis, was required to increase the abundance, phosphorylation and/or processing of NKCC2, NCC, ENaC and pendrin in the loop of Henle and the distal nephron. Indeed, most changes consistent with transporter activation (including increased in-vivo sensitivity to specific blockers) observed in angiotensin II-infused wild-type mice, were absent or significantly reduced in equally treated ACE 10/10 mice [38]. In essence, although many investigators have studied angiotensin II regulation of NKCC2, NCC [55–58], ENaC [26,59] and pendrin [60,61], a major finding is the dominant contribution of locally generated angiotensin II in the regulation of these transporters. These data support the concept that the renal ACE/angiotensin II pathway works as an important regulator of sodium transport along the nephron and that activation of this pathway plays a key role in the development of experimental hypertension.
CONCLUSION
The presence of large amounts of ACE in renal tissues is perhaps the most overlooked aspect of ACE biology. In many ways, the persistence of fundamental gaps in our understanding of renal ACE results from the success of systemic ACE inhibitors in treating hypertension and renal disease. However, emerging evidence from experiments in gene-targeted mice indicates that ACE actions within the kidney are more complex and more important than previously thought. This is especially the case for the dominant role of the renal ACE/angiotensin II pathway in regulating renal sodium excretion during experimental hypertension. We believe that a better understanding of renal ACE and its downstream targets will increase our ability to treat hypertension and renal damage.
KEY POINTS.
The presence of angiotensinogen, renin, ACE and AT1 receptors within renal tissues gives rise to a local RAS.
Mice lacking renal ACE are protected from angiotensin II-induced and L-NAME-induced hypertension.
Locally generated angiotensin II by renal ACE is a master regulator of nephron sodium transport and plays a key role in experimental hypertension.
Acknowledgements
None.
This research was supported by NIH grants R00DK083455 (RAGV), and R01HL110353 (KEB) and an AHA Beginning Grant-in-Aid 13BGIA14680069 (XZS). The authors thank Brian Taylor for his assistance in preparing this manuscript. The authors also thank Dr Sebastien Fuchs, Ellen Bernstein and Tea Janjulia for assistance.
The authors are supported by grants from NHLBI, NIDDK and AHA.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Guyton AC. Abnormal renal function and autoregulation in essential hypertension. Hypertension. 1991;18:III49–III53. doi: 10.1161/01.hyp.18.5_suppl.iii49. [DOI] [PubMed] [Google Scholar]
- 2.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hyper-tension. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 3.Coffman TM. Under pressure: the search for the essential mechanisms of hypertension. Nat Med. 2011;17:1402–1409. doi: 10.1038/nm.2541. [DOI] [PubMed] [Google Scholar]
- 4.Ng KK, Vane JR. Conversion of angiotensin I to angiotensin II. Nature. 1967;216:762–766. doi: 10.1038/216762a0. [DOI] [PubMed] [Google Scholar]
- 5.Wright JW, Harding JW. The brain renin-angiotensin system: a diversity of functions and implications for CNS diseases. Pflugers Arch. 2013;465:133–151. doi: 10.1007/s00424-012-1102-2. [DOI] [PubMed] [Google Scholar]
- 6.Navar LG, Kobori H, Prieto MC, et al. Intratubular renin-angiotensin system in hypertension. Hypertension. 2011;57:355–362. doi: 10.1161/HYPERTENSIONAHA.110.163519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cushman DW, Cheung HS. Concentrations of angiotensin-converting enzyme in tissues of the rat. Biochim Biophys Acta. 1971;250:261–265. doi: 10.1016/0005-2744(71)90142-2. [DOI] [PubMed] [Google Scholar]
- 8.Erdos EG, Skidgel RA. Structure and functions of human angiotensin I converting enzyme (kininase II). Biochem Soc Trans. 1985;13:42–44. doi: 10.1042/bst0130042. [DOI] [PubMed] [Google Scholar]
- 9■■.Gribouval O, Morinière V, Pawtowski A, et al. Spectrum of mutations in the renin–angiotensin system genes in autosomal recessive renal tubular dysgenesis. Hum Mutat. 2012;33:316–326. doi: 10.1002/humu.21661. [The study highlights the importance of genetic alterations of the RAS, and hypoperfusion, in human renal development.] [DOI] [PubMed] [Google Scholar]
- 10.Kim HS, Krege JH, Kluckman KD, et al. Genetic control of blood pressure and the angiotensinogen locus. Proc Natl Acad Sci U S A. 1995;92:2735–2739. doi: 10.1073/pnas.92.7.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanimoto K, Sugiyama F, Goto Y, et al. Angiotensinogen-deficient mice with hypotension. J Biol Chem. 1994;269:31334–31337. [PubMed] [Google Scholar]
- 12.Niimura F, Labosky PA, Kakuchi J, et al. Gene targeting in mice reveals a requirement for angiotensin in the development and maintenance of kidney morphology and growth factor regulation. J Clin Invest. 1995;96:2947–2954. doi: 10.1172/JCI118366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsuchida S, Matsusaka T, Chen X, et al. Murine double nullizygotes of the angiotensin type 1A and 1B receptor genes duplicate severe abnormal phenotypes of angiotensinogen nullizygotes. J Clin Invest. 1998;101:755–760. doi: 10.1172/JCI1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi N, Lopez ML, Cowhig JE, Jr, et al. Ren1c homozygous null mice are hypotensive and polyuric, but heterozygotes are indistinguishable from wild-type. J Am Soc Nephrol. 2005;16:125–132. doi: 10.1681/ASN.2004060490. [DOI] [PubMed] [Google Scholar]
- 15.Esther CR, Jr, Howard TE, Marino EM, et al. Mice lacking angiotensin-converting enzyme have low blood pressure, renal pathology, and reduced male fertility. Lab Invest. 1996;74:953–965. [PubMed] [Google Scholar]
- 16.Cole J, Quach DL, Sundaram K, et al. Mice lacking endothelial angiotensin-converting enzyme have a normal blood pressure. Circ Res. 2002;90:87–92. doi: 10.1161/hh0102.102360. [DOI] [PubMed] [Google Scholar]
- 17.Xiao HD, Fuchs S, Campbell DJ, et al. Mice with cardiac-restricted angiotensin-converting enzyme (ACE) have atrial enlargement, cardiac arrhythmia, and sudden death. Am J Pathol. 2004;165:1019–1032. doi: 10.1016/S0002-9440(10)63363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez-Villalobos RA, Billet S, Kim C, et al. Intrarenal angiotensin-converting enzyme induces hypertension in response to angiotensin I infusion. J Am Soc Nephrol. 2011;22:449–459. doi: 10.1681/ASN.2010060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen XZ, Li P, Weiss D, et al. Mice with enhanced macrophage angiotensin-converting enzyme are resistant to melanoma. Am J Pathol. 2007;170:2122–2134. doi: 10.2353/ajpath.2007.061205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashimoto S, Adams JW, Bernstein KE, et al. Micropuncture determination of nephron function in mice without tissue angiotensin-converting enzyme. Am J Physiol Renal Physiol. 2005;288:F445–452. doi: 10.1152/ajprenal.00297.2004. [DOI] [PubMed] [Google Scholar]
- 21.Kobori H, Nangaku M, Navar LG, et al. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 22.Peti-Peterdi J, Bell PD. Regulation of macula densa Na : H exchange by angiotensin II. Kidney Int. 1998;54:2021–2028. doi: 10.1046/j.1523-1755.1998.00200.x. [DOI] [PubMed] [Google Scholar]
- 23.Kovács G, Peti-Peterdi J, Rosivall L, et al. Angiotensin II directly stimulates macula densa Na-2Cl-K cotransport via apical AT(1) receptors. Am J Physiol Renal Physiol. 2002;282:F301–306. doi: 10.1152/ajprenal.00129.2001. [DOI] [PubMed] [Google Scholar]
- 24.Corvol P, Jeunemaitre X, Plouin PF, et al. The application of molecular genetics to the study of familial arterial hypertension. Clin Exp Hypertens A. 1989;11:1053–1073. doi: 10.3109/10641968909035391. [DOI] [PubMed] [Google Scholar]
- 25.Quan A, Baum M. Endogenous production of angiotensin II modulates rat proximal tubule transport. J Clin Invest. 1996;97:2878–2882. doi: 10.1172/JCI118745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol. 2002;13:1131–1135. doi: 10.1097/01.asn.0000013292.78621.fd. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez-Villalobos RA, Satou R, Ohashi N, et al. Intrarenal mouse reninangiotensin system during ANG II-induced hypertension and ACE inhibition. Am J Physiol Renal Physiol. 2010;298:F150–157. doi: 10.1152/ajprenal.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casarini DE, Boim MA, Stella RC, et al. Angiotensin I-converting enzyme activity in tubular fluid along the rat nephron. Am J Physiol. 1997;272:F405–409. doi: 10.1152/ajprenal.1997.272.3.F405. [DOI] [PubMed] [Google Scholar]
- 29.Atlas SA. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm. 2007;13:9–20. doi: 10.18553/jmcp.2007.13.s8-b.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alderman MH, Cohen HW, Sealey JE, et al. Plasma renin activity levels in hypertensive persons: their wide range and lack of suppression in diabetic and in most elderly patients. Am J Hypertens. 2004;17:1–7. doi: 10.1016/j.amjhyper.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 31.Kobori H, Ozawa Y, Satou R, et al. Kidney-specific enhancement of ANG II stimulates endogenous intrarenal angiotensinogen in gene-targeted mice. Am J Physiol Renal Physiol. 2007;293:F938–945. doi: 10.1152/ajprenal.00146.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lavoie JL, Lake-Bruse KD, Sigmund CD. Increased blood pressure in transgenic mice expressing both human renin and angiotensinogen in the renal proximal tubule. Am J Physiol Renal Physiol. 2004;286:F965–971. doi: 10.1152/ajprenal.00402.2003. [DOI] [PubMed] [Google Scholar]
- 33.Sachetelli S, Liu Q, Zhang SL, et al. RAS blockade decreases blood pressure and proteinuria in transgenic mice overexpressing rat angiotensinogen gene in the kidney. Kidney Int. 2006;69:1016–1023. doi: 10.1038/sj.ki.5000210. [DOI] [PubMed] [Google Scholar]
- 34.Ying J, Stuart D, Hillas E, et al. Overexpression of mouse angiotensinogen in renal proximal tubule causes salt-sensitive hypertension in mice. Am J Hypertens. 2012;25:684–689. doi: 10.1038/ajh.2012.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramkumar N, Ying J, Stuart D, et al. Overexpression of renin in the collecting duct causes elevated blood pressure. Am J Hypertens. 2013;26:965–972. doi: 10.1093/ajh/hpt071. [DOI] [PubMed] [Google Scholar]
- 36.Crowley SD, Gurley SB, Oliverio MI, et al. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest. 2005;115:1092–1099. doi: 10.1172/JCI200523378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crowley SD, Gurley SB, Herrera MJ, et al. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci U S A. 2006;103:17985–17990. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzalez-Villalobos RA, Janjoulia T, Fletcher NK, et al. The absence of intrarenal ACE protects against hypertension. J Clin Invest. 2013;123:2011–2023. doi: 10.1172/JCI65460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shao W, Seth DM, Navar LG. Augmenation of endogenous Angiotensin II levels in Val5-Ang II infused rats. JIM. 2008;56:344–492. doi: 10.1152/ajprenal.90596.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shao W, Seth DM, Navar LG. Angiotensin II type 1 receptor-mediated augmentation of urinary excretion of endogenous angiotensin II in Val5-angiotensin II-infused rats. Hypertension. 2010;56:378–383. doi: 10.1161/HYPERTENSIONAHA.110.153106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41■.Matsusaka T, Niimura F, Shimizu A, et al. Liver angiotensinogen is the primary source of renal angiotensin II. J Am Soc Nephrol. 2012;23:1181–1189. doi: 10.1681/ASN.2011121159. [The authors of this reference demonstrated, via genetic manipulation in mice, that most angiotensinogen found in the kidneys at baseline comes from the liver.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol. 2001;12:431–439. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rohrwasser A, Morgan T, Dillon HF, et al. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension. 1999;34:1265–1274. doi: 10.1161/01.hyp.34.6.1265. [DOI] [PubMed] [Google Scholar]
- 44.Saito T, Urushihara M, Kotani Y, et al. Increased urinary angiotensinogen is precedent to increased urinary albumin in patients with type 1 diabetes. Am J Med Sci. 2009;338:478–480. doi: 10.1097/MAJ.0b013e3181b90c25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gonzalez-Villalobos RA, Seth DM, Satou R, et al. Intrarenal angiotensin II and angiotensinogen augmentation in chronic angiotensin II-infused mice. Am J Physiol Renal Physiol. 2008;295:F772–779. doi: 10.1152/ajprenal.00019.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leyssac PP. Changes in single nephron renin release are mediated by tubular fluid flow rate. Kidney Int. 1986;30:332–339. doi: 10.1038/ki.1986.189. [DOI] [PubMed] [Google Scholar]
- 47.Prieto-Carrasquero MC, Botros FT, Kobori H, et al. Collecting duct renin: a major player in angiotensin II-dependent hypertension. J Am Soc Hypertens. 2009;3:96–104. doi: 10.1016/j.jash.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prieto-Carrasquero MC, Botros FT, Pagan J, et al. Collecting duct renin is upregulated in both kidneys of 2-kidney, 1-clip Goldblatt hypertensive rats. Hypertension. 2008;51:1590–1596. doi: 10.1161/HYPERTENSIONAHA.108.110916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paxton WG, Runge M, Horaist C, et al. Immunohistochemical localization of rat angiotensin II AT1 receptor. Am J Physiol. 1993;264:F989–995. doi: 10.1152/ajprenal.1993.264.6.F989. [DOI] [PubMed] [Google Scholar]
- 50.Brasier AR, Li J. Mechanisms for inducible control of angiotensinogen gene transcription. Hypertension. 1996;27:465–475. doi: 10.1161/01.hyp.27.3.465. [DOI] [PubMed] [Google Scholar]
- 51.Satou R, Miyata K, Gonzalez-Villalobos RA, et al. Interferon-gamma biphasically regulates angiotensinogen expression via a JAK-STAT pathway and suppressor of cytokine signaling 1 (SOCS1) in renal proximal tubular cells. Faseb J. 2012;26:1821–1830. doi: 10.1096/fj.11-195198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Acres OW, Satou R, Navar LG, et al. Contribution of a nuclear factor-kappaB binding site to human angiotensinogen promoter activity in renal proximal tubular cells. Hypertension. 2011;57:608–613. doi: 10.1161/HYPERTENSIONAHA.110.165464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Satou R, Miyata K, Katsurada A, et al. Tumor necrosis factor-{alpha} suppresses angiotensinogen expression through formation of a p50/p50 homo-dimer in human renal proximal tubular cells. Am J Physiol Cell Physiol. 2010;299:C750–759. doi: 10.1152/ajpcell.00078.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Satou R, Gonzalez-Villalobos RA, Miyata K, et al. IL-6 augments angiotensinogen in primary cultured renal proximal tubular cells. Mol Cell Endocrinol. 2009;311:24–31. doi: 10.1016/j.mce.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.San-Cristobal P, Pacheco-Alvarez D, Richardson C, et al. Angiotensin II signaling increases activity of the renal Na-Cl cotransporter through a WNK4-SPAK-dependent pathway. Proc Natl Acad Sci U S A. 2009;106:4384–4389. doi: 10.1073/pnas.0813238106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van der Lubbe N, Lim CH, Fenton RA, et al. Angiotensin II induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter independent of aldosterone. Kidney Int. 2010;79:66–76. doi: 10.1038/ki.2010.290. [DOI] [PubMed] [Google Scholar]
- 57.Gurley SB, Riquier-Brison AD, Schnermann J, et al. AT1A angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab. 2011;13:469–475. doi: 10.1016/j.cmet.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brooks HL, Allred AJ, Beutler KT, et al. Targeted proteomic profiling of renal Na(+) transporter and channel abundances in angiotensin II type 1a receptor knockout mice. Hypertension. 2002;39:470–473. doi: 10.1161/hy02t2.102959. [DOI] [PubMed] [Google Scholar]
- 59.Mamenko M, Zaika O, Ilatovskaya DV, et al. Angiotensin II increases activity of the epithelial Na+ channel (ENaC) in distal nephron additively to aldosterone. J Biol Chem. 2012;287:660–671. doi: 10.1074/jbc.M111.298919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Verlander JW, Hong S, Pech V, et al. Angiotensin II acts through the angiotensin 1a receptor to upregulate pendrin. Am J Physiol Renal Physiol. 2011;301:F1314–1325. doi: 10.1152/ajprenal.00114.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pech V, Kim YH, Weinstein AM, et al. Angiotensin II increases chloride absorption in the cortical collecting duct in mice through a pendrin-dependent mechanism. Am J Physiol Renal Physiol. 2007;292:F914–920. doi: 10.1152/ajprenal.00361.2006. [DOI] [PubMed] [Google Scholar]