Abstract
We conducted a study to determine the prevalence of extraarticular manifestations (ExRA) in a cohort of predominantly Hispanic and Asian patients with rheumatoid arthritis (RA), to identify factors associated with the development of ExRA, and to compare the prevalence of ExRA between Hispanic and Asian patients.
Patients with RA followed in the outpatient rheumatology clinics of a public hospital were included if they were aged ≥18 years and met the 1987 American College of Rheumatology criteria for the diagnosis of RA. We performed a cross-sectional analysis in which patients with ExRA were identified based on predefined criteria. We compared sociodemographic and clinical characteristics in patients with and without ExRA. Multivariate logistic regression was used to examine the association between sociodemographic variables, clinical characteristics, and the presence of ExRA.
The prevalence of ExRA was 21.5%, and the most common manifestations were subcutaneous nodules (17.2%) and interstitial lung disease (3.6%). Hispanic patients were significantly more likely to develop ExRA than Asian patients (odds ratio, 2.53; 95% confidence interval, 1.26–5.09). The development of ExRA was also associated with disease duration, male sex, and seropositivity for serum rheumatoid factor.
INTRODUCTION
Although articular inflammation and damage usually dominate the clinical course of rheumatoid arthritis (RA), extraarticular manifestations (ExRA) develop in 17.8%–50.1% of patients with RA and are associated with poor outcomes.2,6–8,13,20,26,30,35–38,41,43 Patients with ExRA have higher mortality, poorer functional status, and significantly greater comorbidity than patients without ExRA.26,35–38,41
RA occurs in all racial and ethnic groups, but little is known regarding possible ethnic or racial differences in the development of ExRA. Most studies of ExRA have examined homogenous patient populations.2,6–9,12,13,20,26,37 Comparisons between studies of ExRA can often be misleading because referral bias, secular trends in the incidence of ExRA, differences in defining criteria for ExRA, and differential exposure to medications can contribute to differences in the reported prevalence of ExRA.2,4–8,13,20,26,32,37
In the current study, we examined a multiethnic cohort of predominantly Hispanic and Asian patients with RA, 2 rapidly growing demographic groups in the United States. The goals of the study were to determine prevalence and manifestations of ExRA, to compare the prevalence of ExRA between different ethnic groups, and to identify those factors that are associated with the development of ExRA.
METHODS
Study Population
We performed a cross-sectional analysis of all patients with RA who were enrolled in the University of California, San Francisco (UCSF) RA Cohort between August 23, 2006 and September 29, 2010 and followed at San Francisco General Hospital. All patients were ≥18 years of age at the time of enrollment and met the 1987 American College of Rheumatology (ACR) criteria for the diagnosis of RA.3 The UCSF Committee on Human Research approved this study. Written informed consent was obtained from all patients enrolled in the RA cohort.
Assessment of ExRA
We systematically reviewed outpatient rheumatology records to identify the presence or history of ExRA using the criteria of Turesson et al37 with the following exceptions. First, for interstitial lung disease (ILD), we included patients who had all of the following clinical and diagnostic criteria: 1) dyspneic by history or crackles on auscultation of the lungs, 2) radiographic changes on chest computed tomography consistent with ILD, and 3) reduced diffusion capacity on pulmonary function testing. Second, we also included panniculitis and large granular lymphocyte (LGL) syndrome as manifestations of ExRA. The diagnosis of panniculitis, including pyoderma gangrenosum, was made by reviewing outpatient rheumatology records. Panniculitis was confirmed by reviewing dermatology records and pathologic reports. LGL syndrome was diagnosed based on the laboratory findings of neutropenia (absolute neutrophil count <1500) and flow cytometry consistent with LGL. Alternative diagnoses, including malignancy, infection, and adverse drug reactions, were excluded. The date at which a patient first met the inclusion criteria for a diagnosis of ExRA was defined as the ExRA index date.
Secondary Sjögren syndrome and sicca symptoms were excluded from our definition of ExRA because objective testing for Sjögren syndrome was not routinely performed and sicca symptomatology was not systemically ascertained. Our definition of ExRA was limited to nonarticular forms of ExRA; therefore, patients with cervical myelopathy were not included in the final analysis. Pulmonary nodules, which are nonspecific and often associated with alternative etiologies, were excluded from our definition of ExRA. All uncertain cases of ExRA were adjudicated by a second investigator (JBI).
Sociodemographic and Clinical Measures
Sociodemographic variables, including the age at which a patient was diagnosed with RA, disease duration before enrollment into our cohort, self-reported ethnicity, sex, and history of tobacco use (recorded as current or former), were compared in patients with and without ExRA. Clinical characteristics, including the presence of serum rheumatoid factor (RF) positivity (RF >20 IU/mL or ≥1:40), and anti-cyclic citrullinated peptide (CCP) antibody positivity (anti-CCP antibodies >20 units) at any point in the follow-up period were compared among patients with and without ExRA. Data for antinuclear antibody (ANA) positivity (ANA ≥1:40) were also recorded.
Medication use (recorded as present or not present), including biologic agents (etanercept, adalimumab, infliximab, rituximab, certolizumab, and abatacept) and nonbiologic disease-modifying antirheumatic drugs (DMARDs; methotrexate, leflunomide, sulfasalazine, hydroxychloroquine), was recorded in patients with ExRA at the ExRA index date. If a patient was not taking either a biologic medication or a DMARD, we recorded whether the patient reported noncompliance (as documented by the treating physician in the medical record), had been recently diagnosed with RA, and/or had contraindications to the use of these medications.
Statistical Analysis
We performed chi-square tests to compare the sociodemographic and serologic profiles of patients with and without ExRA. We used the Student t test to compare the age at RA diagnosis and disease duration before cohort enrollment in patients with and without ExRA. We used univariate logistic regression to determine the association of sociodemographic variables (age at RA diagnosis, disease duration, ethnicity, sex, and history of tobacco use) and serologic profiles (serum RF and anti-CCP antibodies) with the presence of ExRA. All sociodemographic and serologic profiles used in the univariate model, regardless of statistical significance, were used in the multivariate model in an effort to determine which variables were independently associated with the development of ExRA while controlling for other covariates. Data analysis was performed using STATA software v. 11.1 (College Station, TX).
RESULTS
Patient Characteristics
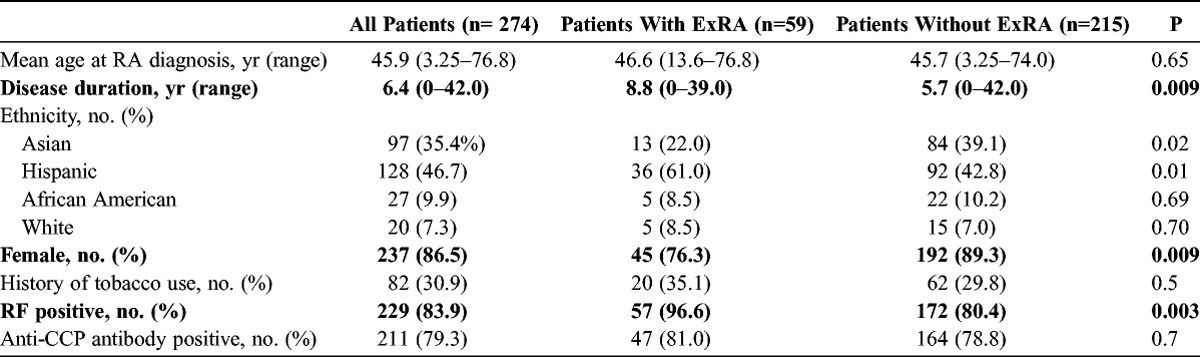
Two hundred seventy-four patients with RA were included in this study. Sociodemographic and clinical characteristics are shown in Table 1. The mean age at the time of RA diagnosis was 45.9 years, and the mean disease duration before the observation period was 6.4 years. Two hundred thirty-seven patients (86.5%) were female; 97 patients (35.4%) were Asian, 128 patients (46.7%) were Hispanic, 27 patients (9.9%) were African American, and 20 patients (7.3%) were white. Of the 97 Asian patients in the study, 81 were immigrants, all of whom were born in either East Asia or Southeast Asia; none came from the Indian subcontinent. Of the 128 Hispanic patients, 110 immigrated from Mexico, Central America, or South America. Eighty-two patients (30.9%) reported a history of tobacco use. Two hundred twenty-nine (83.6%) patients were seropositive for RF, and 211 (79.3%) patients had anti-CCP antibodies. After enrollment in the cohort, patients were followed for a mean of 2.16 years, during which time almost all patients (97.5%) received a nonbiologic DMARD and 41.1% received a biologic agent (data not shown).
TABLE 1.
Sociodemographic and Clinical Characteristics of a Multiethnic Cohort of Patients With RA
Clinical Characteristics of Patients With and Without ExRA
Fifty-nine patients (21.5%) either had ExRA before enrollment in the cohort or developed ExRA during the period of observation (see Table 1). Patients with ExRA were significantly more likely to be male, (23.7% vs. 10.7%, p = 0.009), to be Hispanic (p = 0.01), and to have a longer disease duration (8.8 yr vs. 5.7 yr, p = 0. 009). They were less likely to be Asian (p = 0.02). The prevalence of ExRA among Hispanic patients was significantly greater than the prevalence among Asian patients (28.1% vs. 13.4%, p < 0.001). This difference was largely due to the differential prevalence in subcutaneous nodules, which was significantly greater in Hispanic patients compared to Asian patients (23.4% vs. 10.3%, p = 0.001). There was no statistically significant difference in the use of methotrexate and hydroxychloroquine between Hispanic and Asian patients (data not shown), or with regard to tobacco use in patients with and without ExRA.
Patients with ExRA were more likely to be positive for serum RF (96.6% vs. 80.4%, p = 0.003) but not for anti-CCP antibodies. There was no statistically significant difference in ANA positivity between these 2 groups, but these data were available for only 232 patients (data not shown).
Manifestations of ExRA
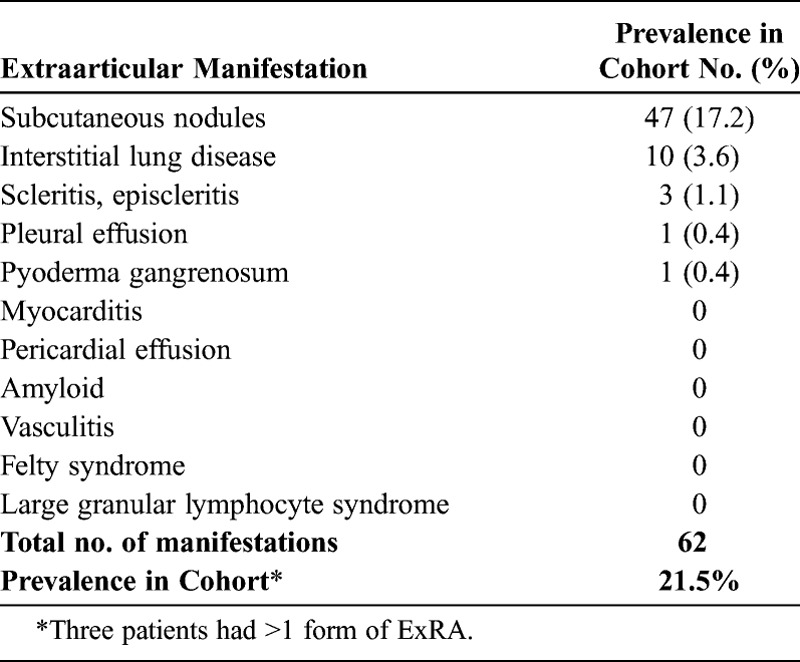
There were 62 manifestations of ExRA in 59 patients (Table 2). Subcutaneous nodules were the most common form of ExRA (n = 47, 17.2%), followed by ILD (n = 10, 3.6%). Scleritis, episcleritis, pleural effusions, and pyoderma gangrenosum were seen in ≤1% of patients.
TABLE 2.
Prevalence of ExRA in a Multiethnic Cohort of Patients With RA
Thirty-seven manifestations of ExRA (37/62, 60%) occurred in treated patients: 26 cases (26/62, 42% of total manifestations) were diagnosed in patients on monotherapy with a nonbiologic DMARD and 11 cases (11/62, 18%) occurred in patients using a biologic agent, either as monotherapy or in combination with a nonbiologic DMARD (data not shown). Twenty-five manifestations of ExRA (25/62, 40%) were diagnosed in patients who were not using biologic agents or nonbiologic DMARDs. In 17 cases, ExRA either preceded the diagnosis of RA or appeared early in the course of RA. In the remaining cases, ExRA developed in patients who were noncompliant with medical management or who had contraindications to DMARD therapy (for example, malignancy or contemplating pregnancy).
Sociodemographic and Clinical Characteristics Associated With the Development of ExRA
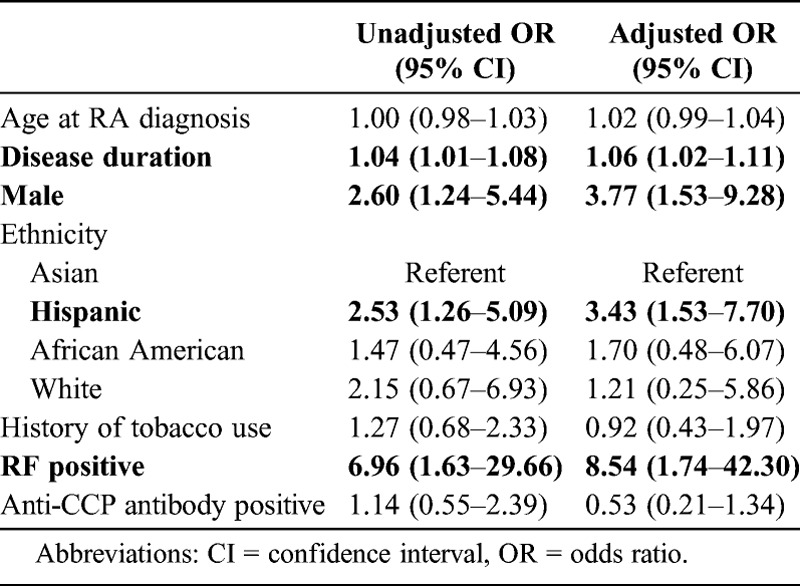
Table 3 reports the unadjusted and adjusted regression models examining the sociodemographic and clinical characteristics associated with the development of ExRA. In the unadjusted model, longer disease and male sex were associated with the development of ExRA. The odds of Hispanic patients developing ExRA were 2.53 times those of Asian patients. RF positivity, but not anti-CCP antibody positivity, was associated with the development of ExRA. There were no associations of ExRA with the age of diagnosis or tobacco use. These associations remained significant in the adjusted analyses, with longer disease duration, ethnicity, male sex, and serum RF independently associated with the development of ExRA.
TABLE 3.
Unadjusted and Adjusted Models of Clinical Associations of ExRA
DISCUSSION
We studied ExRA in a multiethnic cohort composed predominantly of Hispanic and Asian patients with RA. The prevalence of ExRA was 21.5%, and the most common manifestations were subcutaneous nodules and ILD (prevalence of 17.2% and 3.6%, respectively). The development of ExRA was associated with Hispanic ethnicity, longer disease duration, male sex, and seropositivity for serum RF but not for anti-CCP antibodies.
The prevalence of ExRA in Hispanic patients was significantly greater than the prevalence of ExRA in Asian patients (28.1% vs. 13.4%), due largely due to the differential prevalence of subcutaneous nodules between these 2 groups. Subcutaneous nodules are known to cluster with more severe forms of ExRA, such as ILD, scleritis, vasculitis, and Felty syndrome, and are associated with erosive disease and radiographic progression.29,34
Others have shown that ethnic groups with RA can differ in disease severity.11,18 Compared to white patients, Hispanic patients have higher disease activity, and Asian patients have less joint damage.11,18 The differential prevalence of ExRA we observed between Hispanic and Asian patients suggests a similar pattern: that Hispanic patients have a higher propensity to develop ExRA, while Asian patients are at lower risk. Genetic differences are plausible contributors to the differential development of ExRA in Hispanic and Asian patients.39,40 These 2 ethnic groups differ in the HLADRB1 allelic variants that contribute to genetic risk of developing RA, and in the prevalence of the PTPN22 C1858T polymorphism, which confers risk for development of RA in white patients.10,11,17,19,22,25 It is unlikely, however, that these particular risk alleles contribute to the differences in ExRA in our cohort. Both confer risk for anti-CCP-positive disease (rather than RF-positive disease), but ExRA is strongly associated with RF positivity. Moreover, in white patients at least, HLADRB1 risk variants are not associated with the development of rheumatoid nodules.16
The presence of serum RF, but not anti-CCP antibodies, was strongly associated with the development of ExRA. Serum RF is well known to be associated with ExRA; for example, rheumatoid nodules develop almost exclusively in patients who are positive for serum RF.15,33,42,44 There are conflicting data as to whether or not anti-CCP antibodies are associated with the development of ExRA.1,24,31,33 The results of the current study agree with those of Korkmaz et al24 and Rycke et al,31 who found no association between the presence of anti-CCP antibodies and the presence of ExRA. Anti-CCP antibodies are more sensitive and specific for the diagnosis of RA than serum RF and are associated with more erosive disease and greater radiologic progression.14,27 The pathophysiology underlying the differential association of serum RF and anti-CCP antibodies with the development of ExRA remains unclear.
Although we did not find an association between ExRA and smoking, several other studies have linked tobacco use with the development of ExRA, particularly nodules.20,28,37 The prevalence of tobacco use in our cohort (30.9%) is substantially lower than the prevalence in studies reporting an association, and, therefore, may have limited our ability to find an association. It is noteworthy that a number of epidemiologic studies indicate that tobacco use is an environmental risk factor for the development of anti-CCP-positive RA, but only in individuals who carry HLADRB1 risk alleles.21 As noted above, HLADRB1 risk alleles are not associated with nodules, at least in white patients; therefore, a pathway linking smoking to the development of nodules would appear to be distinct from the genetic pathways linking tobacco use to the development of RA itself.16
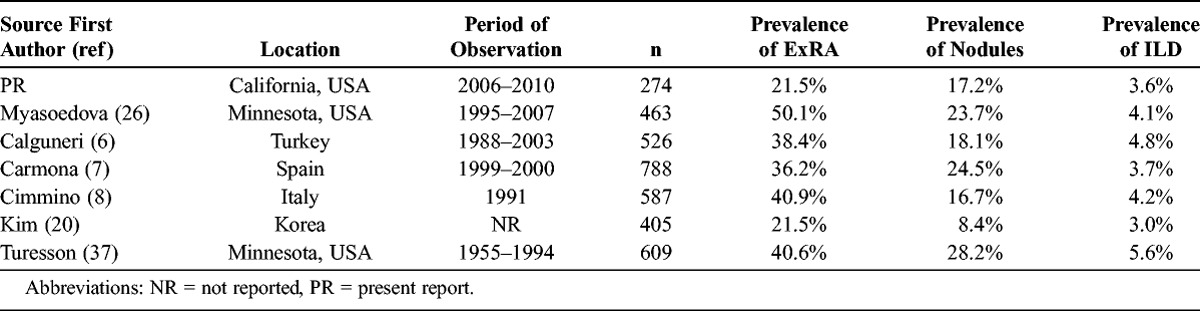
The overall prevalence of ExRA in our cohort (21.5%) is within the broad range of the reported incidence and prevalence of ExRA in cohorts from Italy, Spain, Greece, Turkey, Korea, Saudi Arabia, and Minnesota (17.8%–50.1%)2,6–8,13,20,26,37 (Table 4). The prevalence of subcutaneous nodules (17.2%) in our study is similar to that in cohorts from Italy and Spain (24.5% and 16.7%, respectively), but less than the 30-year cumulative incidence in a cohort from Minnesota (34.0%)7,8,37 (see Table 4). The prevalence of ILD in our cohort (3.6%) is strikingly similar to the prevalence of RA-associated ILD in an English cohort (3.56%) that used similar criteria to identify ILD.23 Other forms of ExRA were either rare or not seen—a finding in agreement with those of Myasoedova et al26 who reported very low 10-year cumulative incidences of scleritis, episcleritis, pleural effusions, vasculitis, and Felty syndrome (0, 0.7%, 1.9%, 0.6%, and 0.5%, respectively). In fact, studies have shown that secular trends of certain forms of ExRA, such as vasculitis, are declining.4,5,26
TABLE 4.
Prevalence of Subcutaneous Nodules and ILD in RA Cohorts, Present and Previous Reports
There are several limitations to the current study. First, the study design limited detection of ExRA to cases that were reported in the medical record. This method of data collection, however, was unlikely to miss more severe forms of ExRA, such as clinically apparent ILD, vasculitis, and scleritis, which are unlikely to escape medical attention. Second, the mean disease duration in our study was 6.4 years, and certain forms of ExRA, such as amyloidosis and Felty syndrome, develop late in the disease course. Third, differences in the criteria used to define ExRA make comparisons between cohorts difficult. For example, secondary Sjögren syndrome, sicca symptoms, and cervical myelopathy were not included in our definition of ExRA. Nonetheless, the overall prevalence of ExRA reported in our cohort is within the broad range of the reported incidence and prevalence of ExRA in other cohorts. Fourth, the population studied had few white patients and, therefore, did not permit comparison of the prevalence of ExRA in white patients with that in Asian or Hispanic patients. Finally, we did not have data on ANA positivity in 15% of patients, and such missing data can mask potentially important clinical associations.
We observed ethnic differences in the prevalence of ExRA. Future studies should directly compare the prevalence of ExRA in Hispanic and Asian patients to that in white patients. Determining which patients may be at risk for the development of ExRA becomes clinically useful when considering the poor outcomes that are associated with ExRA. The above findings show that patients who are Hispanic, male, with long-standing disease, and who are seropositive for serum RF are at risk for the development of ExRA. Our study shows that certain forms of ExRA, including subcutaneous nodules and ILD, remain prevalent, while other forms of ExRA are rarely seen.
Abbreviations
- ANA
antinuclear antibody
- CCP
cyclic citrullinated peptide
- DMARD
disease-modifying antirheumatic drug
- ExRA
extraarticular manifestations of rheumatoid arthritis
- ILD
interstitial lung disease
- LGL
large granular lymphocyte
- RA
rheumatoid arthritis
- RF
rheumatoid factor
Footnotes
Financial support and conflicts of interest: Supported by grants from the Rosalind Russell Medical Research Center for Arthritis and the National Institutes of Health (JY is supported by K23AR060259; NCR is supported by T32 AR007304). The authors have no conflicts of interest to disclose.
REFERENCES
- 1. Alexiou I, Germenis A, Koutroumpas A, Kontogianni A, Theodoridou K, Sakkas LI. Anti-cyclic citrullinated peptide-2 (CCP2) autoantibodies and extra-articular manifestations in Greek patients with rheumatoid arthritis. Clin Rheumatol. 2008; 27: 511– 513. [DOI] [PubMed] [Google Scholar]
- 2. Al-Ghamdi A, Attar SM. Extra-articular manifestations of rheumatoid arthritis: a hospital-based study. Ann Saudi Med. 2009; 29: 189– 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arnett FC, Edworthy SM, Bloch DA, Mcshane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, Medsger TA, Jr, Mitchell DM, Neustadt DH, Pinals RS, Schaller JG, Sharp JT, Wilder RL, Hunder GG. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988; 31: 315– 324. [DOI] [PubMed] [Google Scholar]
- 4. Bartels C, Bell C, Shinki K, Rosenthal A, Bridges AJ. Changing trends in serious extra-articular manifestations of rheumatoid arthritis among United States veterans over 20 years. Rheumatology. 2010; 49: 1670– 1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bartels C, Bell C, Rosenthal A, Shinki K, Bridges A. Decline in rheumatoid vasculitis prevalence among US veterans: a retrospective cross sectional study. Arthritis Rheum. 2009; 60: 2553– 2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Calguneri M, Ureten K, Akif Ozturk M, Onat A, Ertenli I, Kiraz S, Akdogan A. Extra-articular manifestations of rheumatoid arthritis: results of a university hospital of 526 patients in Turkey. Clin Exp Rheumatol. 2006; 24: 305– 308. [PubMed] [Google Scholar]
- 7. Carmona L, Gonzalez-Alvaro I, Balsa A, Angel Belmonte M, Tena X, Sanmarti R. Rheumatoid arthritis in Spain: occurrence of extra-articular manifestations and estimates of disease severity. Ann Rheum Dis. 2003; 62: 897– 900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cimmino MA, Salvarani C, Macchioni P, Montecucco C, Fossaluzza V, Mascia MT, Punzi L, Davoli C, Filippini D, Numo R. Extra-articular manifestations in 587 Italian patients with rheumatoid arthritis. Rheumatol Int. 2000; 19: 213– 217. [DOI] [PubMed] [Google Scholar]
- 9. Cohen MG, Li EK, Ng PY, Chan KL. Extra-articular manifestations are uncommon in southern Chinese with rheumatoid arthritis. Br J Rheumatol. 1993; 32: 209– 211. [DOI] [PubMed] [Google Scholar]
- 10. Delgado-Vega AM, Anaya JM. Meta-analysis of HLA-DRB1 polymorphism in Latin American patients with rheumatoid arthritis. Autoimmun Rev. 2007; 6: 402– 408. [DOI] [PubMed] [Google Scholar]
- 11. Del Rincon I, Battafarano DF, Arroyo RA, Murphy FT, Fischbach M, Escalante A. Ethnic variation in the clinical manifestations of rheumatoid arthritis: role of HLA-DRB1 alleles. Arthritis Rheum. 2003; 49: 200– 208. [DOI] [PubMed] [Google Scholar]
- 12. Drosos AA, Lanchbury JS, Panayi GS, Moutsopoulos HM. Rheumatoid arthritis in Greek and British patients. A comparative clinical, radiologic, and serologic study. Arthritis Rheum. 1992; 35: 745– 748. [DOI] [PubMed] [Google Scholar]
- 13. Eser F, Garip Y, Bodur H. Extraarticular manifestations in Turkish patients with rheumatoid arthritis: impact of EAMs on the health-related quality of life in terms of disease activity, functional status, severity of pain, and social and emotional functioning. Rheumatol Int. 2012; 32: 1521– 1525. [DOI] [PubMed] [Google Scholar]
- 14. Forslind K, Ahlmen M, Eberhardt K, Hafstrom I, Svensson B. Prediction of radiological outcome in early rheumatoid arthritis in clinical practice: role of antibodies to citrullinated peptides (anti-CCP). Ann Rheum Dis. 2004; 63: 1090– 1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gordon DA, Stein JL, Broder I. The extra-articular features of rheumatoid arthritis: a systematic analysis of 127 cases. Am J Med. 1973; 54: 445– 452. [DOI] [PubMed] [Google Scholar]
- 16. Gorman JD, David-Vaudey E, Pai M, Lum RF, Criswell LA. Lack of association of the HLA-DRB1 shared epitope with rheumatoid nodules: an individual patient data meta-analysis of 3,272 Caucasian patients with rheumatoid arthritis. Arthritis Rheum. 2004; 50: 753– 762. [DOI] [PubMed] [Google Scholar]
- 17. Gourh P, Tan FK, Assassi S, Ahn CW, McNearney TA, Fischbach M, Arnett FC, Mayes MD. Association of the PTPN22 R620W polymorphism with anti-topoisomerase I- and anticentromere antibody-positive systemic sclerosis. Arthritis Rheum. 2006; 54: 3945– 3953. [DOI] [PubMed] [Google Scholar]
- 18. Griffiths B, Situnayake RD, Clark B, Tennant A, Salmon M, Emery P. Racial origin and its effect on disease expression and HLA-DRB1 types in patients with rheumatoid arthritis: a matched cross-sectional study. Rheumatology. 2000; 39: 857– 864. [DOI] [PubMed] [Google Scholar]
- 19. Jun KR, Choi SE, Cha CH, Oh HB, Heo YS, Ahn HY, Lee KJ. Meta-analysis of the association between HLA-DRB1 allele and rheumatoid arthritis susceptibility in Asian populations. J Korean Med Sci. 2007; 22: 973– 980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim S, Park S, Shin IH, Choe JY. Anti-cyclic citrullinated peptide antibody, smoking, alcohol consumption, and disease duration as risk factors for extraarticular manifestations in Korean patients with rheumatoid arthritis. J Rheumatol. 2008; 35: 995– 1001. [PubMed] [Google Scholar]
- 21. Klareskog L, Ronnelid J, Lundberg K, Padyukov L, Alfredsson L. Immunity to citrullinated proteins in rheumatoid arthritis. Annu Rev Immunol. 2008; 26: 651– 675. [DOI] [PubMed] [Google Scholar]
- 22. Kochi Y, Suzuki A, Yamada R, Yamamoto K. Genetics of rheumatoid arthritis: underlying evidence of ethnic differences. J Autoimmun. 2009; 32Z: 158– 162. [DOI] [PubMed] [Google Scholar]
- 23. Koduri G, Norton S, Young A, Cox N, Davies P, Devlin J, Dixey J, Gough A, Prouse P, Winfield J, Williams P. Interstitial lung disease has a poor prognosis in rheumatoid arthritis: results from an inception cohort. Rheumatology. 2010; 49: 1483– 1489. [DOI] [PubMed] [Google Scholar]
- 24. Korkmaz C, Us T, Kasifoglu T, Akgun Y. Anti-cyclic citrullinated peptide (CCP) antibodies in patients with long-standing rheumatoid arthritis and their relationship with extra-articular manifestations. Clin Biochem. 2006; 39: 961– 965. [DOI] [PubMed] [Google Scholar]
- 25. Lin L, Chen Y, Xiao Z, Huang S, Yang Z. The association of HLA-DRB1 alleles with rheumatoid arthritis in the Chinese Shantou population: a follow-up study. Biochem Cell Biol. 2007; 85: 227– 238. [DOI] [PubMed] [Google Scholar]
- 26. Myasoedova E, Crowson CS, Turesson C, Gabriel SE, Matteson EL. Incidence of extraarticular rheumatoid arthritis in Olmsted County, Minnesota, in 1995–2007 versus 1985–1994: a population-based study. J Rheumatol. 2011; 38: 983– 989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nishimura K, Sugiyama D, Kogata Y, Tsuji G, Nakazawa T, Kawano S, Saigo K, Morinobu A, Koshiba M, Kuntz KM, Kamae I, Kumagai S. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med. 2008; 146: 797– 808. [DOI] [PubMed] [Google Scholar]
- 28. Nyhall-Wahlin BM, Jacobsson LT, Petersson IF, Turesson C. Smoking is a strong risk factor for rheumatoid nodules in early rheumatoid arthritis. Ann Rheum Dis. 2006; 65: 601– 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nyhall-Wahlin BM, Turesson C, Jacobsson L, Nilsson J-A, Forslind K, Albertsson K, Ronnelid J, Petersson IF. The presence of rheumatoid nodules at early rheumatoid arthritis diagnosis is a sign of extra-articular disease and predicts radiographic progression of joint destruction over 5 years. Scand J Rheumatol. 2011; 40: 81– 87. [DOI] [PubMed] [Google Scholar]
- 30. Prete M, Racanelli V, Digiglio L, Vacca A, Dammacco F, Perosa F. Extra-articular manifestations of rheumatoid arthritis: an update. Autoimmun Rev. 2011; 11: 123– 131. [DOI] [PubMed] [Google Scholar]
- 31. Rycke LD, Peene I, Hoffman IE, Kruithof E, Union A, Meheus L, Lebeer K, Wyns B, Vincent C, Mielants H, Boullart L, Serre G, Veys EM, De Keyser F. Rheumatoid factor and anticitrullinated protein antibodies in rheumatoid arthritis: diagnostic value, associations with radiological progression rate, and extra-articular manifestations. Ann Rheum Dis. 2004; 63: 1587– 1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Turesson C, Eberhardt K, Jacobsson LT, Lindqvist E. Incidence and predictors of severe extra-articular disease manifestations in an early rheumatoid arthritis inception cohort. Ann Rheum Dis. 2007; 66: 1543– 1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Turesson C, Jacobsson LTH, Sturfelt G, Matteson EL, Mathsson L, Ronnelid J. Rheumatoid factor and antibodies to cyclic citrullinated peptides are associated with severe extra-articular manifestations in rheumatoid arthritis. Ann Rheum Dis. 2007; 66: 59– 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Turesson C, McClelland RL, Christianson T, Matteson E. Clustering of extraarticular manifestations in patients with rheumatoid arthritis. J Rheumatol. 2008; 35: 179– 180. [PubMed] [Google Scholar]
- 35. Turesson C, McClelland RL, Christianson TJ, Matteson EL. Severe extra-articular disease manifestations are associated with an increased risk of first ever cardiovascular events in patients with rheumatoid arthritis. Ann Rheum Dis. 2006; 66: 70– 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Turesson C, McClelland RL, Christianson TJ, Matteson EL. Multiple extra-articular manifestations are associated with poor survival in patients with rheumatoid arthritis. Ann Rheum Dis. 2006; 65: 1533– 1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Turesson C, O’Fallon W, Crowson CS, Gabriel SE, Matteson EL. Extra-articular disease manifestations in rheumatoid arthritis: incidence trends and risk factors over 46 years. Ann Rheum Dis. 2003; 62: 722– 727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Turesson C, O’Fallon WM, Crowson CS, Gabriel SE, Matteson EL. Occurrence of extraarticular disease manifestations is associated with excess mortality in a community based cohort of patients with rheumatoid arthritis. J Rheumatol. 2002; 29: 62– 67. [PubMed] [Google Scholar]
- 39. Turesson C, Schaid DJ, Weyand C, Jacobsson LTH, Goronzy JJ, Petersson IF, Sturfelt G, Nyhall-Wahlin BM, Truedsson L, Dechant SA, Matteson EL. The impact of HLA-DRB1 genes of extra-articular disease manifestations in rheumatoid arthritis. Arthritis Res Ther. 2005; 7: R1386– 1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Turesson C, Weyand CM, Matteson EM. Genetics of rheumatoid arthritis: is there a pattern predicting extraarticular manifestations? Arthritis Rheum. 2004; 51: 853– 863. [DOI] [PubMed] [Google Scholar]
- 41. Turesson C, Jacobsson L, Bergstrom U. Extra-articular rheumatoid arthritis: prevalence and mortality. Rheumatology. 1999; 38: 668– 674. [DOI] [PubMed] [Google Scholar]
- 42. Turesson C, Jacobsson L, Bergstrom U, Truedsson L, Sturfelt G. Predictors of extra-articular manifestations in rheumatoid arthritis. Scand J Rheumatol. 2000; 29: 358– 364. [DOI] [PubMed] [Google Scholar]
- 43. Young A, Koduri G. Extra-articular manifestations and complications of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2007; 21: 907– 927. [DOI] [PubMed] [Google Scholar]
- 44. Ziff M. The rheumatoid nodules. Arthritis Rheum. 1980; 33: 761– 767. [DOI] [PubMed] [Google Scholar]


