Abstract
Objective
Although the systematic measurement of disease activity facilitates clinical decision making in rheumatoid arthritis (RA), no recommendations currently exist on which measures should be applied in clinical practice in the US. The American College of Rheumatology (ACR) convened a Working Group (WG) to comprehensively evaluate the validity, feasibility, and acceptability of available RA disease activity measures and derive recommendations for their use in clinical practice.
Methods
The Rheumatoid Arthritis Clinical Disease Activity Measures Working Group conducted a systematic review of the literature to identify RA disease activity measures. Using exclusion criteria, input from an Expert Advisory Panel (EAP), and psychometric analysis, a list of potential measures was created. A survey was administered to rheumatologists soliciting input. The WG used these survey results in conjunction with the psychometric analyses to derive final recommendations.
Results
Systematic review of the literature resulted in identification of 63 RA disease activity measures. Application of exclusion criteria and ratings by the EAP narrowed the list to 14 measures for further evaluation. Practicing rheumatologists rated 9 of these 14 measures as most useful and feasible. From these 9 measures, the WG selected 6 with the best psychometric properties for inclusion in the final set of ACR-recommended RA disease activity measures.
Conclusion
We recommend the Clinical Disease Activity Index, Disease Activity Score with 28-joint counts (erythrocyte sedimentation rate or C-reactive protein), Patient Activity Scale (PAS), PAS-II, Routine Assessment of Patient Index Data with 3 measures, and Simplified Disease Activity Index because they are accurate reflections of disease activity; are sensitive to change; discriminate well between low, moderate, and high disease activity states; have remission criteria; and are feasible to perform in clinical settings.
Introduction
The last 2 decades have witnessed a dramatic improvement in the treatment of rheumatoid arthritis (RA), with disease remission now considered a realistic goal for many patients (1). Surrogate measures of outcome such as disease activity measures can facilitate clinical decision making to achieve these goals, and studies in RA show that treating to a target improves outcomes (2–4). As health care reform in the US accelerates efforts to improve quality, assessment of disease activity in RA has become a nationally endorsed quality measure. For example, the Physician Quality Reporting System, a pay-for-reporting program administered by the Centers for Medicare and Medicaid Services, includes a quality measure assessing whether physicians measure RA disease activity using a standardized scale or composite index and classify RA disease activity as low, moderate, or high at least yearly (5).
Despite the push from various stakeholders in the health care system to standardized disease activity assessment in RA and growing evidence that treating to target is effective, most US rheumatologists do not routinely use standardized measures in clinical practice. The myriad available instruments and lack of formal recommendations on which measures are best suited for different practice settings likely contribute to this lack of implementation. As a first and important step in addressing this issue, the American College of Rheumatology (ACR) commissioned a Working Group (WG) to use a validated multistep process to derive recommendations for the use of RA disease activity measures in clinical practice.
The goal of this effort was to determine which disease activity measures reliably distinguish between different levels of disease activity in RA and are feasible to implement in clinical practice. In this article, we review the methods for arriving at our recommendations, summarize the important components of the selected measures, and also discuss some of the practical issues that rheumatologists should consider in deciding which measure to implement in their practice setting.
Materials and Methods
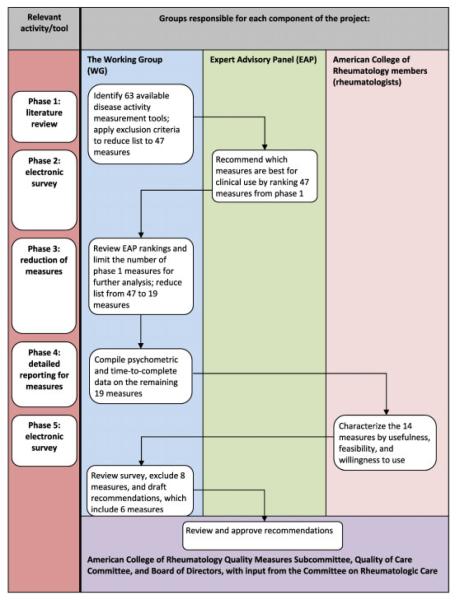
The methods used to derive our recommendations are summarized very briefly here, but are outlined in significantly greater detail in Supplementary Appendix A (available in the online version of this article at http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)2151-4658). Figure 1 shows an overview of the methodology used, which included a combination of a systematic literature review, psychometric analyses, validated group methods, and physician surveys.
Figure 1.
Flow diagram of methods.
First, the WG conducted a systematic literature review to identify available RA disease activity measurement tools and applied initial exclusion criteria (see Supplementary Figure 1 and Supplementary Table 1, available in the online version of this article at http://onlinelibrary. wiley.com/journal/10.1002/(ISSN)2151-4658). Next, an RA disease activity measurement Expert Advisory Panel (EAP), consisting of rheumatologists with published expertise in RA disease activity measurement, was consulted to review measures meeting criteria for inclusion. The EAP ranked the recommended measures by using the Outcome Measures in Rheumatology filter on truth, discrimination, and feasibility (6). The WG then further condensed the number of measures based on a detailed review of the psychometric properties of each measure. The abbreviated measure set was then incorporated into a survey administered to practicing rheumatologists to gather information regarding usefulness, feasibility, and willingness to use in daily clinical practice. Collation of the information gathered in these steps formed the basis for the final WG recommendations.
Results
Systematic literature review and EAP ratings
Sixty-three tools to measure RA disease activity were initially identified, 16 of which met prespecified exclusion criteria (see Supplementary Figure 1 and Supplementary Table 1, available in the online version of this article at http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)2151-4658). From the remaining 47 measures, 4 were combined into 2 measures. Forty-five measures were then presented to members of the EAP, and ratings of familiarity with the measure, personal experience using the measure, and a final recommendation regarding use in clinical practice resulted in selection of 31 measures (see Supplementary Table 2, available in the online version of this article at http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)2151-4658).
Qualitative feedback from the EAP was then used to further abbreviate this list of measures, including recommendations to exclude instruments that primarily measure quality of life (n = 10), do not indicate the current state of disease activity (i.e., require the calculation of change scores [n = 2]), are confined to a single body region (n = 1), or are antiquated and no longer in current use (n = 1).
Psychometric analyses
The remaining 19 measures were then subjected to a detailed review of psychometric properties, including evaluation of their reliability, validity, and responsiveness. Five measures were excluded in this phase based on the lack of defined remission and/or disease activity criteria. The psychometric analyses have been published previously in Arthritis Care & Research (7). Table 1 summarizes the psychometric properties of the 14 measures resulting from the steps above.
Table 1.
Summary of RA disease activity measures recommended for point-of-care clinical use*
| Measure/scale | Measure outputs | No. of items |
Response format | Method of administration |
Time for administration |
Validated in RA |
Psychometric properties† |
||
|---|---|---|---|---|---|---|---|---|---|
| Reliability | Validity | Responsiveness | |||||||
| Patient-driven composite tools |
|||||||||
| PAS | A single score on a continuous scale (0–10) |
3 | HAQ: 0–3 Pain VAS: 0–10 Pt Global VAS: 0–10 |
Patient questionnaire |
Patient: <3.5 minutes Provider: <1 minute Lab: N/A |
Yes | Acceptable; test–retest reliability for composite has not been evaluated |
Acceptable | Acceptable for individual components |
| PAS-II | A single score on a continuous scale (0–10) |
3 | HAQ-II: 0–3 Pain VAS: 0–10 Pt Global VAS: 0–10 |
Patient questionnaire |
Patient: <1.5 minutes Provider: <30 seconds Lab: N/A |
Yes | Acceptable; test–retest reliability for composite has not been evaluated |
Acceptable | Acceptable for individual components |
| RAPID-3 | A single score on a continuous scale (0–10) |
3 | MDHAQ: 0–3 Pain VAS: 0–10 Pt Global VAS: 0–10 |
Patient questionnaire |
Patient: ≃1.5 minutes Provider: <30 seconds Lab: N/A |
Yes | Acceptable; test–retest reliability for composite has not been evaluated |
Good | Acceptable for individual components |
| Patient and provider composite tool |
|||||||||
| CDAI | A single score on a continuous scale (0–76) |
4 | 28TJC: 0–28 28SJC: 0–28 Pt Global VAS: 0–10 Pr Global VAS: 0–10 |
Provider item; patient item |
Patient: ≃10 seconds Provider: <2 minutes Lab: N/A |
Yes | Good; test–retest reliability for composite has not been evaluated |
Excellent | Excellent |
| Patient, provider, and laboratory composite tools |
|||||||||
| DAS28 (ESR or CRP) |
A single score on a continuous scale (0–9.4) |
4, or 3 when general health is omitted |
28TJC: 0–28 28SJC: 0–28 ESR: 0–100 or CRP with lower detection level of 1.0 mg/liter Pt Global VAS: 0–100 |
Provider assessment; patient item; lab |
Patient: ≃10 seconds Provider: 3–5 minutes Lab: 1 hour waiting time for ESR |
Yes | Excellent | Excellent | Excellent |
| SDAI | A single score on a continuous scale (0–86) |
5 | 28TJC: 0–28 28SJC: 0–28 Pt Global VAS: 0–10 CRP: 0–10 |
Provider item; patient item; lab |
Patient: ≃10 seconds Provider: ≃2 minutes Lab: waiting time for CRP varies by lab |
Yes | Good; test–retest reliability for composite has not been evaluated |
Excellent | Excellent |
Measures are not shown in order of preference. RA = rheumatoid arthritis; PAS = Patient Activity Scale; HAQ = Health Assessment Questionnaire; VAS = visual analog scale; Pt Global VAS = patient global assessment of disease activity VAS; Lab = laboratory value required; N/A = not applicable; RAPID-3 = Routine Assessment of Patient Index Data with 3 measures; MDHAQ = Multidimensional HAQ; CDAI = Clinical Disease Activity Index; 28TJC = 28 tender joint count; 28SJC = 28 swollen joint count; Pr Global VAS = provider global assessment of disease activity VAS; DAS28 = Disease Activity Score with 28-joint counts; ESR = erythrocyte sedimentation rate; CRP = C-reactive protein; SDAI = Simplified Disease Activity Index.
Psychometric properties were subjectively ranked using an ordered category scale (excellent, good, acceptable, poor, and unacceptable) based on currently published literature.
Membership survey
The 14 measures were then grouped (Disease Activity Score [DAS]/DAS with 28-joint counts [DAS28], Simplified Disease Activity Index [SDAI]/Clinical Disease Activity Index [CDAI], Patient Activity Scale [PAS] scores, Routine Assessment of Patient Index Data [RAPID] scores, Rheumatoid Arthritis Disease Activity Index 5, Chronic Arthritis Systemic Index, and patient global assessment of disease activity [PtGA]/provider global assessment of disease activity [PrGA]) and incorporated into a survey sent to practicing rheumatologists (n = 4,368); 335 recipients (7.7%) completed the survey. Most respondents (76.8%) were currently using a measurement tool in their clinical practice (see Supplementary Table 3, available in the online version of this article at http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)2151-4658). We weighted the survey results for region, sex, and age to adjust for the low response rate (see Supplementary Table 4, available in the online version of this article at http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)2151-4658).
Derivation of final recommendations
Based on the information gathered in the preceding phases, including psychometric analyses and physician ratings, the WG selected 6 measures for point-of-care RA disease activity measurement: the CDAI, DAS28 (erythrocyte sedimentation rate [ESR] or C-reactive protein [CRP]), PAS, PAS-II, RAPID-3, and SDAI (Table 2).
Table 2.
Required components of rheumatoid arthritis disease activity measures recommended for point-of-care clinical use*
| Physician joint count |
Patient global VAS |
Provider global VAS |
HAQ version |
Pain | Defined remission criteria |
|
|---|---|---|---|---|---|---|
| Patient-driven composite tools | ||||||
| PAS | • | HAQ | • | • | ||
| PAS-II | • | HAQ-II | • | • | ||
| RAPID-3 | • | MDHAQ | • | • | ||
| Patient and provider composite tool | ||||||
| CDAI | • | • | • | N/A | • | |
| Patient, provider, and laboratory composite tools |
||||||
| DAS28 (ESR or CRP) | • | • | N/A | • | ||
| SDAI | • | • | • | N/A | • |
Measures are not shown in order of preference. VAS = visual analog scale; HAQ = Health Assessment Questionnaire; PAS = Patient Activity Scale; RAPID-3 = Routine Assessment of Patient Index Data with 3 measures; MDHAQ = Multidimensional HAQ; CDAI = Clinical Disease Activity Index; N/A = not applicable; DAS28 = Disease Activity Score with 28-joint counts; ESR = erythrocyte sedimentation rate; CRP = C-reactive protein; SDAI = Simplified Disease Activity Index
Discussion
Since the 1950s, when the first composite disease activity measurement tool for use in RA was developed (8), many attempts have been made to improve RA disease activity monitoring. Of the 63 currently available RA disease activity measurement tools, we used a multistep process to progressively filter down to 6 recommended measures: the CDAI, DAS28 (ESR or CRP), PAS, PAS-II, RAPID-3, and SDAI. All 6 produce a single continuous index and have defined ranges for indicating low, moderate, or high disease activity or clinical remission (Table 3). By applying these tools systematically in clinical practice, physicians will be able to “treat to target” and effectively implement the ACR recommendations for the treatment of RA (9).
Table 3.
Disease activity cutoffs for each American College of Rheumatology–recommended disease activity measure*
| Disease activity measure | Scale | Remission | Low/minimal | Moderate | High/severe |
|---|---|---|---|---|---|
| Patient-driven composite tools | |||||
| PAS | 0–10 | 0.00–0.25 | 0.26–3.70 | 3.71 to <8.0 | 8.00–10.00 |
| PAS-II | 0–10 | 0.00–0.25 | 0.26–3.70 | 3.71 to <8.0 | 8.00–10.00 |
| RAPID-3 | 0–10 | 0–1.0 | >1.0 to 2.0 | >2.0 to 4.0 | >4.0 to 10 |
| Patient and provider composite tool | |||||
| CDAI | 0–76 | ≤2.8 | >2.8 to 10.0 | >10.0 to 22.0 | >22.0 |
| Patient, provider, and laboratory composite tools | |||||
| DAS28 (ESR or CRP) | 0–9.4 | <2.6 | ≥2.6 to <3.2 | ≥3.2 to ≃5.1 | >5.1 |
| SDAI | 0–86 | ≤3.3 | >3.3 to ≤11.0 | >11.0 to ≤26 | >26 |
PAS = Patient Activity Scale; RAPID-3 = Routine Assessment of Patient Index Data with 3 measures; CDAI = Clinical Disease Activity Index; DAS28 = Disease Activity Score with 28-joint counts; ESR = erythrocyte sedimentation rate; CRP = C-reactive protein; SDAI = Simplified Disease Activity Index.
Realizing the heterogeneity of settings in which health care is delivered to patients with RA in the US, we selected measures that offer a full range of data collection options. Some are purely patient reported (PAS, PAS-II, and RAPID-3), some add provider assessment (CDAI), and some add provider assessment and laboratory acute-phase reactants (SDAI and DAS28). Below we summarize the key features of each measure to allow those caring for patients with RA to select the one best suited to their clinical practice.
Patient-driven composite tools (PAS [10], PAS-II [10], and RAPID-3 [11]) have the advantage of being relatively easy to use in clinical practice because they do not require provider assessments such as formal joint counts (tender or swollen). Patients can often complete these measures on standardized paper or electronic forms in the waiting room, making them very practical to use.
There are 3 core components to each of the patient-driven measures: visual analog scale patient pain score (12), PtGA (12), and a measure of functional assessment. Since 2 of 3 components are similar across these measures, the distinguishing feature is the functional assessment tool (PAS includes the Health Assessment Questionnaire [HAQ] [13], PAS-II includes the HAQ-II [10,14], and RAPID-3 uses the Multidimensional HAQ [MDHAQ] [15]). In choosing a measure, providers may want to consider the length (the MDHAQ in RAPID-3 and the HAQ-II in PAS-II are 10 items each, while the original HAQ in PAS is 41 items) and psychometric properties of each functional assessment tool, discussed briefly below. For a more detailed review of the psychometric properties of the 3 patient-driven disease activity measures, please refer to the recent assessment by Anderson et al (7).
All 3 measures take less than 3 minutes for patients to complete and have simple mathematical scoring. However, the RAPID-3 has been tested in many rheumatic diseases, not just RA, and therefore may be applied to patients more broadly in clinic practice. Beyond these practical issues, there are other issues related to the reliability and validity of these measures that should be considered. For example, although components of each of the 3 measures have been found to be reliable, valid, and sensitive to change, information is not available about how the composite score for each measure performs. Additionally, long-term outcomes regarding joint damage with these 3 measures have not been studied directly. Therefore, it is theoretically possible that the composite score may be less reliable, valid, or sensitive to change than its individual components.
Finally, patient-driven tools lack a formal joint assessment, something that is thought to lend face validity to a measure of RA disease activity (2). However, it is important to realize that the inclusion of provider joint counts is highly examiner dependent, and while fairly reproducible by a single assessor, may be unreliable if the assessor changes and may not correlate completely with sono-graphic evidence of synovitis (16,17). While patient-reported measures have important limitations, such as reflecting a patient’s perception of their disease, which may be influenced by a variety of factors (e.g., cultural beliefs, self-efficacy, mood), they do produce a less random and more reliable measurement of change over time. In addition, some studies suggest that patient-reported measures predict long-term outcomes better than provider joint counts and acute-phase reactants (18–21). The formulas used to calculate each measurement tool are shown in Table 4.
Table 4.
Formulas for measurement tools*
| Measurement tool | Formula | Online source |
|---|---|---|
| CDAI | 28SJC + 28TJC + PrGA + PtGA | http://www.rheumatology.org/CDAI |
| DAS28 | ||
| DAS28-ESR | 0.56 × √(28TJC) + 0.28 × √(28SJC) + 0.70 × ln(ESR) + 0.014 × PtGA |
http://www.das-score.nl/ |
| DAS28-CRP | 0.56 × √(28TJC) + 0.28 × √(28SJC) + 0.36 × ln(CRP + 1) + 0.014 × PtGA + 0.96 |
http://www.das-score.nl/ |
| PAS | (HAQ × 3.33 + pain VAS + PtGA VAS)/3 | http://www.arthritis-research.org/research/pas |
| PAS-II | (HAQ-II × 3.33 + pain VAS + PtGA VAS)/3 | http://www.arthritis-research.org/research/pas |
| RAPID-3 | (MDHAQ × 3.33 + pain VAS + PtGA VAS)/3 | http://mdhaq.org/Public/Questionnaires.aspx |
| SDAI | 28SJC + 28TJC + PrGA + PtGA + CRP | http://www.rheumatology.org/SDAI |
Numerical rating scales may be substituted for visual analog scales (VAS) in all measures. CDAI = Clinical Disease Activity Index; 28SJC = 28 swollen joint count; 28TJC = 28 tender joint count; PrGA = provider global assessment of disease activity; PtGA = patient global assessment of disease activity; DAS28 = Disease Activity Score with 28-joint counts; ESR = erythrocyte sedimentation rate; CRP = C-reactive protein; PAS = Patient Activity Scale; HAQ = Health Assessment Questionnaire; RAPID-3 = Routine Assessment of Patient Index Data with 3 measures; MDHAQ = Multidimensional HAQ; SDAI = Simplified Disease Activity Index.
The CDAI, a patient and provider composite tool (22), adds 3 components of the provider assessment (PrGA, 28 swollen joint count, and 28 tender joint count) to the PtGA. Designed as a simple numerical addition of the component scores, it avoids point-of-care issues such as needing a calculator to enter differentially weighted components. Further, it does not require an acute-phase reactant, enhancing its feasibility in some clinical settings since results are available in real time. The measure does require providers to perform detailed joint counts reliably and consistently.
Available studies show excellent psychometric properties, including validity and sensitivity to change. Although the components are reliable, it should be noted that the reliability of the composite measure is unknown.
The patient, provider, and laboratory composite tools (SDAI [23] and DAS28 [24]) have several components in common: provider tender and swollen joint counts, PtGA, and an acute-phase reactant. The main distinctions between these 2 measures are that the DAS28 does not include a PrGA and it requires a more complex calculation to arrive at a score based on differential weighting of individual indices (e.g., the DAS28 weighs tender joint count more heavily than swollen joint count).
The SDAI (like the CDAI) is a simpler numerical addition of individual components that are weighted evenly. Generally, the DAS28 uses the ESR (although the DAS28-CRP is also available) and the SDAI uses the CRP level. The SDAI is the most sensitive and specific for prediction of clinical decisions to change disease-modifying antirheumatic drugs, and an SDAI score ≤3.3 is one of two recommended definitions of remission by the ACR and the European League Against Rheumatism to be used in RA clinical trials (1). Although remission criteria for the DAS28 are less conservative than several other measures, including both the SDAI and CDAI, treating to a DAS28 target level of ≤2.4 has been shown to improve RA outcomes (3,25).
It is important to note that the inclusion of acute-phase reactants in these measures introduces a new set of variables, and therefore adds some complexity. The ESR contributes a sizeable portion (15%) of the information in the DAS28-ESR. Therefore, remission may be underestimated in high ESR states with few active joints. Similarly, in low ESR states, remission criteria may be met but patients may still have a significant number of swollen joints (26,27). Like the DAS28-ESR, it has been shown that DAS28-CRP scores may also incorrectly estimate remission as they are generally lower than DAS28-ESR scores (28). Another consideration is that newer biologic agents that target specific inflammatory cytokines are differentially reflected in the ESR and CRP and may therefore disproportionately deflate the composite score.
In addition, inclusion of acute-phase reactants in the DAS28 and SDAI complicates the logistics and timing of using these measures in point-of-care clinical decision making. Although these measures have traditionally been used in clinical trials, academic medical centers, and large multispecialty clinics, logistical barriers have likely delayed their widespread adoption in smaller practice settings.
ACR members responding to our survey rated the PtGA and PrGA as the most feasible tools for use in clinical practice. However, these measures are not included in the final set of selected measures. Limitations of these measures include the lack of standardization in structure (e.g., horizontal and vertical visual analog scale, anchored versus unanchored lines, boxes or circles) and the paucity of well-defined disease activity categories, which are important in creating thresholds for clinical decision making and for quality reporting.
The WG recognizes that there is currently no ideal measure of disease activity, and acknowledges that some measures excluded in our review may later be found to possess adequate, or even superior, psychometric properties to the 6 recommended measures. In the absence of head-to-head comparisons of the different disease activity tools, we had to rely on a process that involved a combination of expert opinion and literature review to select measures. Nevertheless, based on available evidence and expert opinion, we believe we have identified measures that are currently the most reliable, valid, feasible, and acceptable measures of disease activity in RA.
It is important to note that we made no attempt to perform de novo psychometric analyses on measures and restricted our work to reviewing published literature. In addition, our survey of the ACR membership had a low response rate. This may signal that few clinicians currently measure RA disease activity systematically, or that most feel unqualified to rate the utility of different measurement tools. To partially address the low response rate, we weighted the characteristics of the responders versus those of nonresponders for region, sex, and age. These weighted responses suggest that the responders were generally representative of the ACR membership on the examined characteristics.
Finally, although remission is the ultimate therapeutic goal in RA, remission and even attainment of low disease activity may not be appropriate targets for all patients due to comorbid conditions, treatment toxicity, and patient preference. In addition, some measure components are influenced by comorbid conditions that elevate acute-phase reactants or result in tender joints (e.g., fibromyalgia) (2). Despite these complexities, standardized assessment of disease activity in RA facilitates treating to target, which has been shown to improve disease outcomes (2).
The CDAI, DAS28 (ESR or CRP), PAS, PAS-II, RAPID-3, and SDAI all accurately reflect disease activity; are sensitive to change; discriminate between low, moderate, and high disease activity states; are feasible to perform at the point of care; and are acceptable to most practicing rheumatologists. Above we have outlined our methods for arriving at these recommended measures and summarized some of the key features that clinicians should consider when choosing a measure to adopt in their clinical practice. Incorporation of these validated RA disease activity measures into a practice’s workflow will facilitate adherence to the ACR guidelines for the treatment of RA and provide the necessary tools for treating to target. Moreover, routine use of these measures will allow clinicians to demonstrate that they are providing high-quality care for RA, as measured by nationally endorsed quality measures.
Supplementary Material
Significance & Innovations.
This study systematically examines published measures of disease activity in rheumatoid arthritis (RA), identifying those measures that are valid and feasible to perform in clinical settings.
Incorporation of these validated RA disease activity measures into a practice’s workflow will facilitate adherence to the American College of Rheumatology guidelines for the treatment of RA and provide the necessary tools for treating to target.
Routine use of these measures will allow clinicians to demonstrate that they are providing high-quality care for RA, as measured by nationally endorsed quality measures.
Guidelines and recommendations developed and/or endorsed by the American College of Rheumatology (ACR) are intended to provide guidance for particular patterns of practice and not to dictate the care of a particular patient. The ACR considers adherence to these guidelines and recommendations to be voluntary, with the ultimate determination regarding their application to be made by the physician in light of each patient’s individual circumstances. Guidelines and recommendations are intended to promote beneficial or desirable outcomes but cannot guarantee any specific outcome. Guidelines and recommendations developed or endorsed by the ACR are subject to periodic revision as warranted by the evolution of medical knowledge, technology, and practice.
The American College of Rheumatology is an independent, professional, medical and scientific society which does not guarantee, warrant, or endorse any commercial product or service.
ACKNOWLEDGMENTS
We thank Harlan Sayles, University of Nebraska Medical Center, Omaha, Nebraska, for statistical analysis of the member survey, and all those who participated in the surveys performed as part of this project. We also thank Amy Miller, American College of Rheumatology, Atlanta, Georgia, for editorial assistance.
Footnotes
AUTHOR CONTRIBUTIONS All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Kazi had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Anderson, Caplan, Neogi, Michaud, Saag, O’Dell, Kazi.
Acquisition of data. Anderson, Kazi.
Analysis and interpretation of data. Anderson, Caplan, Yazdany, Robbins, Neogi, Michaud, O’Dell, Kazi.
ADDITIONAL DISCLOSURE Dr. Anderson is currently employed by Abbott Laboratories but was not at the time of the study. Abbott Laboratories had no financial interest in this project and had no input in the design, content, data collection, or analysis, and had no role in the writing or approval of this article, with all opinions and conclusions expressed herein those of the authors.
REFERENCES
- 1.Felson DT, Smolen JS, Wells G, Zhang B, van Tuyl LH, Funovits J, et al. American College of Rheumatology/European League Against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Arthritis Rheum. 2011;63:573–86. doi: 10.1002/art.30129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smolen JS, Aletaha D, Bijlsma JW, Breedveld FC, Boumpas D, Burmester G, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69:631–7. doi: 10.1136/ard.2009.123919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grigor C, Capell H, Stirling A, McMahon AD, Lock P, Vallance R, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet. 2004;364:263–9. doi: 10.1016/S0140-6736(04)16676-2. [DOI] [PubMed] [Google Scholar]
- 4.Verstappen SM, Jacobs JW, van der Veen MJ, Heurkens AH, Schenk Y, ter Borg EJ, et al. Intensive treatment with methotrexate in early rheumatoid arthritis: aiming for remission. Computer Assisted Management in Early Rheumatoid Arthritis (CAMERA, an open-label strategy trial) Ann Rheum Dis. 2007;66:1443–9. doi: 10.1136/ard.2007.071092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Committee for Quality Assurance (NCQA) Physician Consortium for Performance Improvement (PCPI) American College of Rheumatology (ACR) Rheumatoid arthritis physician performance measurement set. 2008 URL: http://www.ama-assn.org/ama1/pub/upload/mm/pcpi/rheumatoid-arthritis.pdf.
- 6.Boers M, Brooks P, Strand CV, Tugwell P. The OMERACT filter for Outcome Measures in Rheumatology [editorial] J Rheumatol. 1998;25:198–9. [PubMed] [Google Scholar]
- 7.Anderson JK, Zimmerman L, Caplan L, Michaud K. Measures of rheumatoid arthritis disease activity: patient (PtGA) and provider (PrGA) global assessment of disease activity, Disease Activity Score (DAS) and Disease Activity Score with 28-joint counts (DAS28), Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI), Patient Activity Score (PAS) and Patient Activity Score-II (PASII), Routine Assessment of Patient Index Data (RAPID), Rheumatoid Arthritis Disease Activity Index (RADAI) and Rheumatoid Arthritis Disease Activity Index-5 (RADAI-5), Chronic Arthritis Systemic Index (CASI), patient-based Disease Activity Score with ESR (PDAS1) and patient-based Disease Activity Score without ESR (PDAS2), and Mean Overall Index for Rheumatoid Arthritis (MOI-RA) Arthritis Care Res (Hoboken) 2011;63(Suppl):S14–36. doi: 10.1002/acr.20621. [DOI] [PubMed] [Google Scholar]
- 8.Lansbury J. Quantitation of the activity of rheumatoid arthritis. 5. A method for summation of the systemic indices of rheumatoid activity. Am J Med Sci. 1956;232:300–10. [PubMed] [Google Scholar]
- 9.Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:625–39. doi: 10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolfe F, Michaud K, Pincus T. A composite disease activity scale for clinical practice, observational studies, and clinical trials: the Patient Activity Scale (PAS/PAS-II) J Rheumatol. 2005;32:2410–5. [PubMed] [Google Scholar]
- 11.Pincus T, Swearingen CJ, Bergman M, Yazici Y. RAPID3 (Routine Assessment of Patient Index Data 3), a rheumatoid arthritis index without formal joint counts for routine care: proposed severity categories compared to Disease Activity Score and Clinical Disease Activity Index categories. J Rheumatol. 2008;35:2136–47. doi: 10.3899/jrheum.080182. [DOI] [PubMed] [Google Scholar]
- 12.Felson DT, Anderson JJ, Boers M, Bombardier C, Chernoff M, Fried B, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. Arthritis Rheum. 1993;36:729–40. doi: 10.1002/art.1780360601. [DOI] [PubMed] [Google Scholar]
- 13.Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23:137–45. doi: 10.1002/art.1780230202. [DOI] [PubMed] [Google Scholar]
- 14.Wolfe F, Michaud K, Pincus T. Development and validation of the Health Assessment Questionnaire II: a revised version of the Health Assessment Questionnaire. Arthritis Rheum. 2004;50:3296–305. doi: 10.1002/art.20549. [DOI] [PubMed] [Google Scholar]
- 15.Pincus T, Yazici Y, Bergman M. Development of a Multidimensional Health Assessment Questionnaire (MDHAQ) for the infrastructure of standard clinical care. Clin Exp Rheumatol. 2005;23(Suppl):S19–28. [PubMed] [Google Scholar]
- 16.Lassere MN, van der Heijde D, Johnson KR, Boers M, Edmonds J. Reliability of measures of disease activity and disease damage in rheumatoid arthritis: implications for smallest detectable difference, minimal clinically important difference, and analysis of treatment effects in randomized controlled trials. J Rheumatol. 2001;28:892–903. [PubMed] [Google Scholar]
- 17.Marhadour T, Jousse-Joulin S, Chales G, Grange L, Hacquard C, Loeuille D, et al. Reproducibility of joint swelling assessments in long-lasting rheumatoid arthritis: influence on Disease Activity Score-28 values (SEA-Repro study part I) J Rheumatol. 2010;37:932–7. doi: 10.3899/jrheum.090879. [DOI] [PubMed] [Google Scholar]
- 18.Michaud K, Messer J, Choi HK, Wolfe F. Direct medical costs and their predictors in patients with rheumatoid arthritis: a three-year study of 7,527 patients. Arthritis Rheum. 2003;48:2750–62. doi: 10.1002/art.11439. [DOI] [PubMed] [Google Scholar]
- 19.Wolfe F, Michaud K, Gefeller O, Choi HK. Predicting mortality in patients with rheumatoid arthritis. Arthritis Rheum. 2003;48:1530–42. doi: 10.1002/art.11024. [DOI] [PubMed] [Google Scholar]
- 20.Wolfe F, Zwillich SH. The long-term outcomes of rheumatoid arthritis: a 23-year prospective, longitudinal study of total joint replacement and its predictors in 1,600 patients with rheumatoid arthritis. Arthritis Rheum. 1998;41:1072–82. doi: 10.1002/1529-0131(199806)41:6<1072::AID-ART14>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 21.Wolfe F, Hawley DJ. The longterm outcomes of rheumatoid arthritis. Work disability: a prospective 18 year study of 823 patients. J Rheumatol. 1998;25:2108–17. [PubMed] [Google Scholar]
- 22.Aletaha D, Nell VP, Stamm T, Uffmann M, Pflugbeil S, Machold K, et al. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther. 2005;7:R796–806. doi: 10.1186/ar1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smolen JS, Breedveld FC, Schiff MH, Kalden JR, Emery P, Eberl G, et al. A Simplified Disease Activity Index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxford) 2003;42:244–57. doi: 10.1093/rheumatology/keg072. [DOI] [PubMed] [Google Scholar]
- 24.Prevoo ML, van ’t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight–joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–8. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 25.Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Kerstens PJ, Nielen MM, Vos K, van Schaardenburg D, et al. DAS-driven therapy versus routine care in patients with recent-onset active rheumatoid arthritis. Ann Rheum Dis. 2010;69:65–9. doi: 10.1136/ard.2008.097683. [DOI] [PubMed] [Google Scholar]
- 26.Fransen J, van Riel PL. DAS remission cut points. Clin Exp Rheumatol. 2006;24(Suppl):S29–32. [PubMed] [Google Scholar]
- 27.Smolen JS, Aletaha D. Interleukin-6 receptor inhibition with tocilizumab and attainment of disease remission in rheumatoid arthritis: the role of acute-phase reactants. Arthritis Rheum. 2011;63:43–52. doi: 10.1002/art.27740. [DOI] [PubMed] [Google Scholar]
- 28.Hensor EM, Emery P, Bingham SJ, Conaghan PG. Discrepancies in categorizing rheumatoid arthritis patients by DAS-28(ESR) and DAS-28(CRP): can they be reduced? Rheumatology (Oxford) 2010;49:1521–9. doi: 10.1093/rheumatology/keq117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.