Abstract
Objective
The purpose of this study is to examine the effects of zonisamide on ethanol self-administration and subjective effects in risky drinkers using a human laboratory paradigm.
Method
We conducted a double-blind, placebo-controlled study of the effects of zonisamide 100 mg on ethanol self-administration and urge to drink in risky drinkers (N = 10) (1).
Result
During the second hour of a 2-hour self-administration session ethanol consumption was 50% lower in the zonisamide group as compared to the placebo group. Urge to drink was also significantly lower under the zonisamide condition.
Conclusion
These results indicate that a single dose of zonisamide reduces urge to drink and the quantity of ethanol self-administered by risky drinkers during their second hour of access to alcohol.
Scientific Significance
Zonisamide may help individuals drinking at risky levels reduce their intake of alcohol.
Keywords: Alcohol urges, anticonvulsants, ethanol, Human laboratory study, pharmacotherapy, Sulfonamide
INTRODUCTION
Zonisamide is a sulfonamide anticonvulsant that is structurally similar to the sulfamate anticonvulsant topiramate. Both drugs decrease alcohol consumption in rats and mice in limited access models of drinking (2). Topiramate reduces ethanol intake in alcohol dependent subjects (3). Zonisamide shares some pharmacological actions with topiramate, including blockade of voltage-sensitive sodium channels (4), low potency inhibition of carbonic anhydrase (5), and possibly enhancing potassium channel conductance (6, 7). Zonisamide inhibits excitatory post-synaptic potentials in frontal cortical tissues consistent with blockade of AMPA/kainate receptors (8), an action that may contribute to its suppression of alcohol seeking behaviors (9). Topiramate most likely blocks kainate glutamate receptors, while its interaction with AMPA receptors has not been fully characterized (10). Zonisamide, but not topiramate increases striatal and hippocampal dopamine (11) and inhibits T calcium channel activity (12).
There are no published data on the effects of zonisamide on alcohol consumption in humans. The present study was conducted to assess whether the acute administration of zonisamide would alter alcohol self-administration in a laboratory setting by subjects meeting NIAAA criteria for risky drinking (1).
METHODS
Subjects were non–treatment seeking men and women ages 21 to 55, who met NIAAA criteria for risky drinking (1), defined as more than 4 drinks per day on any day or more than 14 drinks per week for men, and more than 3 drinks on any day or more than 7 drinks per week for women. Weekly alcohol consumption could not exceed 40 and 35 drinks for men and women, respectively. Individuals were excluded from the study if they met criteria for substance dependence other than nicotine or alcohol dependence as determined using the Structured Clinical Interview for the DSM-IV-TR (13) or had a urine positive for opioids, cocaine, amphetamines, benzodiazepines, or 2 positive urines for tetrahydrocannabinol. This study was approved by the Institutional Review Board of the Boston University Medical Center. Subjects provided written informed consent prior to enrollment into the study.
This study followed a double-blind, placebo-controlled crossover design. Drug and placebo were administered in counter-balanced manner to reduce possible order effects. Subjects attended 2 trials separated by at least 7 days. Trials consisted of a priming drink challenge session lasting 1 hour, followed by 2 consecutive 1-hour self-administration segments.
Subjects were asked not to drink any alcohol 24 hours prior to the trial. Urine toxicology and breathalyzers tests confirmed that subjects had not consumed alcohol or drugs of abuse prior to the trial. Testing was conducted in a quiet room with a lounge chair, television, table, and a computer. At approximately 11:30 a.m., subjects received a light lunch followed by baseline assessments. These included Clinical Institute Withdrawal Assessment for Alcohol scale-Revised (CIWA-AR) (14), the Time Line Follow Back (TLFB) (15) to evaluate the amount of alcohol used prior to the session. The Obsessive Compulsive Drinking scale (OCDS) (16) and Alcohol Urge Questionnaire (AUQ) (17) were used to determine baseline levels of alcohol craving.
After these assessments subjects received either a zonisamide 100 mg or placebo in identically appearing capsules. Immediately after receiving the study medication, subjects were asked to completely consume an alcoholic drink over a period of less than 5 minutes. The beverage was the subject’s drink of choice and contained sufficient ethanol to increase blood alcohol concentrations .03 g/dl, based on a published formula (18). The priming drink was administered to subjects to normalize drinking in the test setting (19). The following measures were obtained 10, 20, 30, and 40 minutes following administration of the priming drink: 1) AUQ, 2) VAS scales for high, liking, and sedation, 3) Biphasic Alcohol Effects scales (BAES) (20). Cognitive tests including the Digit Symbol Modalities test (DSMT) (21), the Controlled Word Association Test (COWAT) (22), and WMS-III Digit and Spatial Span tests (23) were also administered during the priming drink segment.
After completion of the 1 hour priming drink trial-segment, subjects were given access to 4 alcoholic drinks during 2 consecutive hour-long trial segments. Each drink was selected to produce an increase in blood alcohol concentration of .015 g/dl. Subjects were told that they could drink as many of the 4 drinks as they wished during the hour session or would receive $3 for each of the drinks that they did not consume. After the first session, the tray containing the first set of drinks was removed, and 4 freshly prepared drinks were made available. The following were administered every 30 minutes: 1) Breathalyzer, 2) AUQ, 3) VAS Sedation scales, 4) BAES. After completion of the self-administration sessions, the subjects’ levels of intoxication were assessed using BAC and clinical evaluation by the study psychiatrist to determine a safe return home.
DATA ANALYSIS
Because the proportion of alcohol content varied amongst the types of alcoholic beverage selected by subjects, estimated grams of ethanol consumed were used as the primary outcome measure. These values were determined by multiplying the volume of alcoholic beverage consumed by the proportion of ethanol content and the density of alcohol.
Separate analyses were conducted for the priming drink segment, the self-administration segments, and the last time-point (180 minute time point). Data for the last time point were analyzed separately because subjects had stopped drinking by that time and were anxious to leave the laboratory, raising questions about the value of the data collected at that point. Data between trials were compared using mixed models analysis with subject, and when appropriate, subject × visit as random effects (Proc Mixed, SAS 9.1.3, 2002–2004). Drug (treatment condition) and visit served as within-subject effects, and when appropriate, time was also used as a within-subject factor. The visit effect was included as a covariate in the analysis models to separate out drug administration order effects and to allow for the determination of whether these effects had a significant influence on the experimental outcomes. Differences in drug effects at each time point assessed were compared using a t test for model generated least squares means. For the primary outcome measures, i.e., least squares means for the amount of ethanol consumed, Tukey–Kramer adjusted p values were determined to correct for multiple comparisons.
RESULTS
Ten subjects, 7 females and 3 males, completed both the placebo and the zonisamide trials. Eight subjects were white, 1 was African American, and 1 subject was biracial. The mean subject age was 26 (SD ± 5) years. The mean number of years of education completed was 16 (SD ± 2). Female subjects drank a mean of 53.5% of the 90 days prior to study entry. They consumed a mean of 7.1 (SE ± .87) drinks per heavy drinking day. During the 90 day period preceding the study, male subjects drank on a mean of 40.4% of the days and consumed 6.3 (SE ± .37) drinks per heavy drinking day. The median number of heavy drinking days for subjects during the 90 day prescreening period was 14 (range 4–29) days.
Priming Drink Segment
Mean total OCDS scores obtained prior to ingestion of the priming drink were 10.9 (SE ± 2.7) and 9.5 (SE ± 1.5) for the placebo and zonisamide conditions, respectively. These scores did not differ significantly. Scores on the DSMT were significantly lower at baseline on the zonisamide challenge day (66.6 ± 3.2 vs. 78.9 ± 6.3), but they did not differ during testing. Significant differences in drug effects or drug × time interactions were not found for any of the measures obtained during the priming drink segment. These results indicate that subjects had similar initial responses to ethanol challenge on each test day, whether zonisamide or placebo were administrated. Notably this included both sedation measures of the VAS and BAES.
Alcohol Self-Administration Trial-Segments
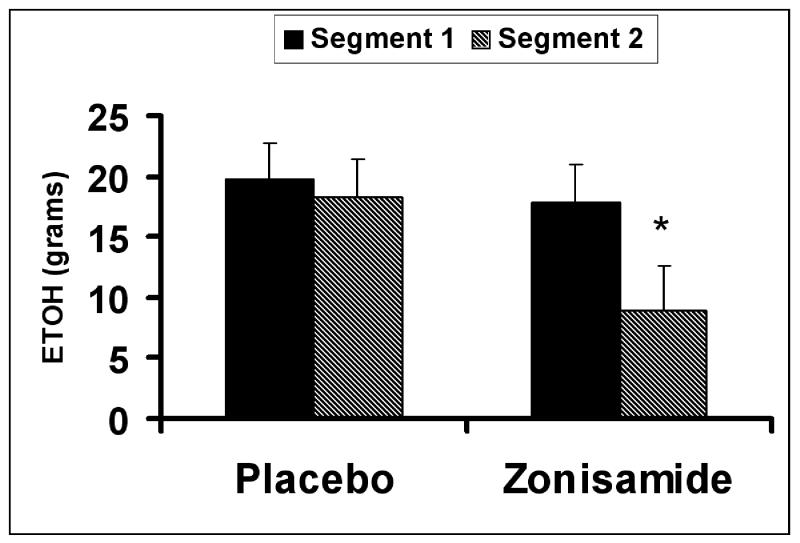
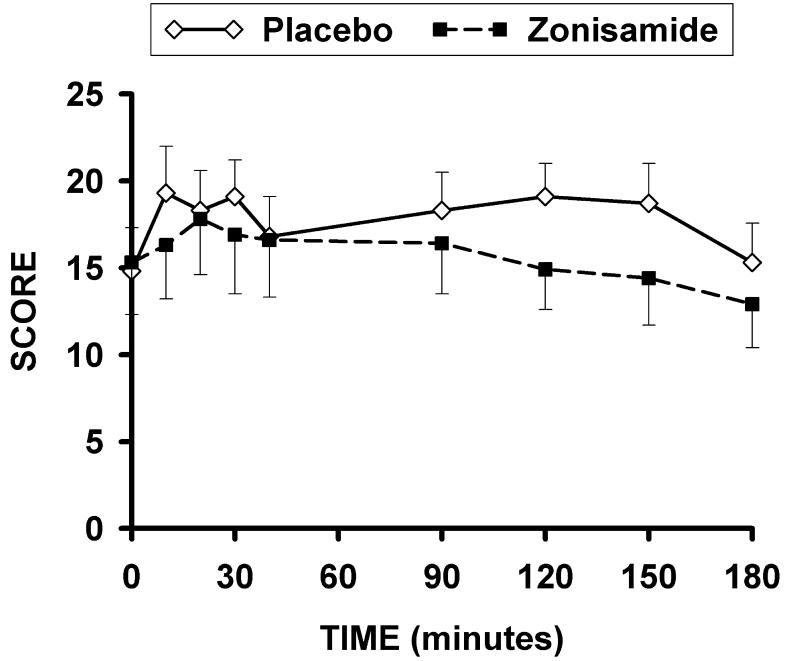
Significant order effects were not found for any measures during the self-administration trial-segments. The mean grams of ethanol ingested during the first and second self-administration trial-segments for each treatment condition are presented in Fig. 1. The drug effect was significant for the amount of ethanol consumption {F (1, 26) = 9.7; p = .005}. The drug × time interaction for this measure approached significance {F (1, 26) = 4.11; p = .053}. Post hoc testing indicated that the grams of ethanol consumed was significantly lower for the zonisamide trial than for the placebo trial during the second, but not the first self-administration trial segment. For BACs obtained during the self-administration segments, the drug effect {F(1,{ 44) = 9.4; p = .004}, but not the drug × time interaction effect was determined to be significant, reflecting lower BAC for the zonisamide as compared to the placebo treatment condition. The drug effect for BAC’s was not significant at the 180 minute time point. The drug effect was significant {F (1, 8.0) = 5.7; p .045} for AUQ scores during the self-administration segments (Fig. 2) indicating lower urge to drink with zonisamide than placebo.
FIG. 1.
Mean (+/− SE) grams of ethanol consumed during the first (Segment 1) and second (Segment 2) self-administration trial-segments for the placebo and zonisamide treatment conditions. *indicates a significant difference, p < 0.05, between the placebo and zonisamide treatment conditions for the comparable trial-segment.
FIG. 2.
Mean (+/− SE) AUQ for the priming drink (0–60 minutes) , first self-administration (90–120 minutes), and second self-administration trial-segments (150–180 minutes) obtained for both the placebo and zonisamide treatment conditions.
For the first self-administration segment mean compensation received under the placebo condition was $3.0 (SE ± .9) and $3.9 (SE ± 1.3) for the zonisamide condition. During the second segment, the mean amounts received were $5.1 (SE ± 1.3) and $6.9 (SE ± 1.4) for placebo and zonisamide treatment conditions, respectively. The drug effect and the drug × trial-segment effect were not significant for money received for either of the self-administration trial-segments.
The drug × time interaction effect {F (2, 36) = 4.0; p = .03} but not the effect was significant for the sedative scale of the BAES obtained for the self-administration segments. Mean sedative BAES scale scores for placebo increased from 6.9 (SE ± 3.8) at 90 minutes to 10.2 (SE ± 4.08) at 150 minutes. For zonisamide scores declined from 10.2 (SE ± 4.0) to 7.8 (SE ± 3.3) for the 90 and 150 minute time points, respectively. Thus, the significant drug × time interaction for sedation scores collected during the self-administration sessions reflects a moderate increase in the placebo group and a moderate decline in the zonisamide group. At no point did group differences in BAES sedation scores reach significance. When mean sedative scores from the BAES for each trial segment were used as covariates, the drug effect for volume of ethanol self-administered remained significant {F (1, 25.8) = 9.6; p = .005}, and the drug × time interaction continued to approach significance {F (1, 25.8) = 8.3; p = .054
The drug and the drug × time interaction effects were not significant on the BAES stimulation scale for the self-administration trial segments. The drug effect was not significant for either the sedative or stimulation scores acquired during the 180-minute time point.
For the VAS sedation measures obtained during the self-administration trial segments, drug effects were not significant for Sleepy, Slow, and Tired scales. For these measures the drug × time effects for the Sleepy, Slow, and Tired VAS were also not significant. The drug effects for the Sedation VAS were not in the significance range for the Sleepy, Slow, or Tired scales for the 180 minutes time point.
DISCUSSION
Zonisamide, compared to placebo, reduced the volume of ethanol consumed by risky drinkers during the second hour of the self-administration trial-segments, when plasma concentrations of zonisamide would be expected to be at, or close to, peak values (24). During the self-administration trial segments, reductions in blood alcohol concentrations paralleled reductions in urges to drink as reflected in decreased AUQ scores with zonisamide compared to placebo. These effects of zonisamide are of particular interest because they were observed after the administration of a single dose of this agent.
Subjective responses and cognitive measures obtained during the priming drink trial-segment did not differ significantly between the placebo and zonisamide conditions. Because of its comparatively slow rate of absorption, plasma levels for zonisamide would be expected to be fairly low during the priming-drink segment. BAC levels were equivalent during this segment. Thus, the lack of difference between treatment conditions in responses to ethanol suggests roughly equivalent sensitivity to the effects of alcohol on the 2 test days.
There were no significant differences in BAES sedation scores between groups, although there was a trend for sedation scores to be slightly higher during the first hour of self-administration during the zonisamide condition compared to placebo. There was also a trend for sedation to increase in the placebo group and decline in the zonisamide group. However, when BAES sedation score was used as a covariate in the comparative analysis of alcohol consumption, and ethanol intake was still significantly lower in the zonisamide group.
Despite encouraging results there are several limitations to our study. The small number of subjects tested, use of non–treatment seeking risky drinkers, and the administration of only a single dose of drug limit our findings. Chronic dosing and measurement of zonisamide plasma levels at the time of testing would be an appropriate next step. Nevertheless, this study provides evidence that a single dose of zonisamide decreases ethanol self-administration by risky drinkers 2 hours after the administration of the drug. This effect occurred in association with a decrease in the urge to drink alcohol. If our findings are replicated, zonisamide may have a therapeutic role when individuals with alcohol problems face situations that pose high risk for relapse.
ACKNOWLEDGMENTS
This work was supported by funds received from The Gennaro Acamparo Charitable Trust.
Footnotes
Declaration of Interest The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Note: Drs. Domenic A. Ciraulo, Maryam Afshar, and Ofra Sarid-Segal have received research support from Alkermes, Bristol Myers Squibb, Catalyst Pharmaceutical Partners, Drug Abuse Sciences, Jannssen, Lipha, Orhto McNeil and UCB Pharma. Dr. Domenic A. Ciraulo has received consulting fees from Alkermes, Bristol Myers Squibb, Janssen, Ortho McNeil, Organon, Trancept and Novo Nordisk. None of the other investigators have any disclosures to make.
REFERENCES
- 1.NIAAA . Rethinking Drinking, Alcohol and your Health. In: U.S.D.H.H. Services, editor. National Institute of Health. NIAAA; Washington, D.C.: 2009. [Google Scholar]
- 2.Knapp CM, Mercado M, Markley TL, Crosby S, Ciraulo DA, Kornetsky C. Zonisamide decreases ethanol intake in rats and mice. Pharmacol Biochem Behav. 2007;87(1):65–72. doi: 10.1016/j.pbb.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, Mckay A, Alt-Daoud N, Anton RF, Ciraulo DA, Kranzler HR, Mann K, Omalley, Swift RM. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298(14):1641–1651. doi: 10.1001/jama.298.14.1641. [DOI] [PubMed] [Google Scholar]
- 4.Schauf CL. Zonisamide enhances slow sodium inactivation in Myxicola. Brain Res. 1987;413(1):185–188. doi: 10.1016/0006-8993(87)90168-5. [DOI] [PubMed] [Google Scholar]
- 5.Masuda Y, Karasawa T. Inhibitory effect of zonisamide on human carbonic anhydrase in vitro. Arzneimittelforschung. 1993;43(4):416–418. [PubMed] [Google Scholar]
- 6.Herrero AI, Del Olmo N, Gonzalea-Escalada JR, Solis JM. Two new actions of topiramate: Inhibition of depolarizing GABA(A)-mediated responses and activation of a potassium conductance. Neuropharmacology. 2002;42(2):210–20. doi: 10.1016/s0028-3908(01)00171-x. [DOI] [PubMed] [Google Scholar]
- 7.Huang CW, Huang CC, Wu SN. Activation by zonisamide, a newer antiepileptic drug, of large-conductance calcium-activated potassium channel in differentiated hippocampal neuron-derived H19-7 cells. J Pharmacol Exp Ther. 2007;321(1):98–106. doi: 10.1124/jpet.106.116954. [DOI] [PubMed] [Google Scholar]
- 8.Huang CW, Ueno S, Okada M, Kaneko S. Zonisamide at clinically relevant concentrations inhibits field EPSP but not presynaptic fiber volley in rat frontal cortex. Epilepsy Res. 2005;67(1-2):51–60. doi: 10.1016/j.eplepsyres.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Backstrom P, Hyytia P. Ionotropic glutamate receptor antagonists modulate cue-induced reinstatement of ethanol-seeking behavior. Alcohol Clin Exp Res. 2004;28(4):558–565. doi: 10.1097/01.alc.0000122101.13164.21. [DOI] [PubMed] [Google Scholar]
- 10.Gryder DS, Rogawski MA. Selective antagonism of GluR5 kainate-receptor-mediated synaptic currents by topiramate in rat basolateral amygdala neurons. J Neurosci. 2003;23(18):7069–7074. doi: 10.1523/JNEUROSCI.23-18-07069.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okada M, Kaneko S, Hirano T, Mizuno K, Kondo T, Otani K, Fukushima Y. Effects of zonisamide on dopaminergic system. Epilepsy Res. 1995;22(3):193–205. doi: 10.1016/0920-1211(95)00078-x. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki S, Kawakami K, Nishimura S, Watanabe Y, Yagi K, Seino M, Miyamoto K. Zonisamide blocks T-type calcium channel in cultured neurons of rat cerebral cortex. Epilepsy Res. 1992;12(1):21–27. doi: 10.1016/0920-1211(92)90087-a. [DOI] [PubMed] [Google Scholar]
- 13.First M, Spitzer RL, Robert L, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient Edition (SCIDI/P) State Psychiatric Unit; New York New York: 2005. [Google Scholar]
- 14.Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: The revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Br J Addict. 1989;84(11):1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 15.Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: Assessing normal drinkers’ reports of recent drinking and a comparative evaluation across several populations. Br J Addict. 1988;83(4):393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- 16.Anton RF, Moak DH, Latham PK. The obsessive compulsive drinking scale: A new method of assessing outcome in alcoholism treatment studies. Arch Gen Psychiatry. 1996;53(3):225–231. doi: 10.1001/archpsyc.1996.01830030047008. [DOI] [PubMed] [Google Scholar]
- 17.Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res. 1995;19(3):600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- 18.Watson PE, Watson ID, Batt RD. Prediction of blood alcohol concentrations in human subjects. Updating the Widmark Equation. J Stud Alcohol. 1981;42(7):547–556. doi: 10.15288/jsa.1981.42.547. [DOI] [PubMed] [Google Scholar]
- 19.O’Malley SS, Krishnaa-Sarin S, Farren C, Shina R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002;160(1):19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- 20.Martin CS, Earleywinw M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17(1):140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- 21.Smith A. Symbol Digits Modalities Test. Western Psychological Services; Los Angeles: 1982. [Google Scholar]
- 22.Benton AL, Hamsher KD. Multilingual Aphasia Examination. AJA; Iowa City: 1989. 1989. [Google Scholar]
- 23.Wechsler AS. Wechsler Memory Scales. The Psychological Corp; San Antonio: 1997. [Google Scholar]
- 24.Leppik IE. Zonisamide: Chemistry, mechanism of action, and pharmacokinetics. Seizure. 2004;13(Suppl 1):S5–9. doi: 10.1016/j.seizure.2004.04.016. discussion S10. [DOI] [PubMed] [Google Scholar]