Abstract
Background
Chemotherapy treatment for pediatric acute lymphoblastic leukemia (ALL) has been associated with long-term cognitive impairments in some patients. However, the neurobiologic mechanisms underlying these impairments, particularly in young survivors, are not well understood. This study aimed to examine intrinsic functional brain connectivity in pediatric ALL and its relationship with cognitive status.
Procedure
We obtained resting state functional magnetic resonance imaging (rsfMRI) and cognitive testing data from 15 ALL survivors age 8–15 years and 14 matched healthy children. The ALL group had a history of intrathecal chemotherapy treatment but were off-therapy for at least 6 months at the time of enrollment. We used seed-based analyses to compare intrinsic functional brain network connectivity between the groups. We also explored correlations between connectivity and cognitive performance, demographic, medical, and treatment variables.
Results
We demonstrated significantly reduced connectivity between bilateral hippocampus, left inferior occipital, left lingual gyrus, bilateral calcarine sulcus, and right amygdala in the ALL group compared to controls. The ALL group also showed regions of functional hyperconnectivity including right lingual gyrus, precuneus, bilateral superior occipital lobe, and right inferior occipital lobe. Functional hypoconnectivity was associated with reduced cognitive function as well as younger age at diagnosis in the ALL group.
Conclusions
This is the first study to demonstrate that intrinsic functional brain connectivity is disrupted in pediatric ALL following chemotherapy treatment. These results help explain cognitive dysfunction even when objective test performance is seemingly normal. Children diagnosed at a younger age may show increased vulnerability to altered functional brain connectivity.
Keywords: chemotherapy, cognition, fMRI, leukemia, resting state
INTRODUCTION
Chemotherapy treatment for pediatric acute lymphoblastic leukemia (ALL) is associated with cognitive difficulties. The most common cognitive domains affected include executive function, memory, attention, visual processing, and visuomotor skills [1]. These difficulties persist decades later into adulthood and negatively impact occupational and educational achievement [2–5]. Candidate mechanisms for cognitive impairment following ALL include disruption of neural progenitor cells and neurogenesis, inflammatory response, microvascular damage, and genetic vulnerabilities [6–8].
Neuroimaging studies that used volumetric magnetic resonance imaging (MRI), diffusion weighted imaging and task-based functional MRI (fMRI) have demonstrated alterations of brain structure and function in ALL survivors [4,9–16]. These studies identified biomarkers of cognitive outcome that provide insights into the neurobiologic mechanisms underlying cognitive impairment in ALL. However, there have been very few neuroimaging studies to date, particularly in young survivors (<16 years).
Resting state functional MRI (rsfMRI) provides measurement of intrinsic brain networks. Intrinsic network connectivity depends on stable structural networks [17,18]. The widespread alterations in brain structure that have previously been observed in ALL suggest that resting state networks are likely disrupted in ALL. For example, Zeller et al. [9] demonstrated significantly reduced volumes of cortical gray matter and cerebral white matter as well as reduced regional volumes in amygdala, caudate, hippocampus, and thalamus in adult survivors of pediatric ALL compared to healthy controls. Our group previously showed significantly reduced organization of large-scale structural brain networks in young ALL survivors compared to healthy children [19]. However, to date, no studies have evaluated resting state intrinsic brain networks in ALL.
We therefore aimed to determine if functional connectivity of resting state networks is altered in young survivors of ALL compared to typically developing children. We hypothesized that ALL would be associated with diffuse dysconnectivity of intrinsic networks and that these abnormalities would correlate with cognitive measures. We also sought to explore the impact on resting state functional connectivity of demographic variables (age and maternal education) and known risk factors for cognitive impairment in ALL including cognitive reserve (represented by maternal education), gender, treatment intensity, time since treatment, and age at diagnosis [12].
PATIENTS AND METHODS
Participants
We enrolled 29 children, 15 children with a history of ALL who were off-therapy for at least 6 months at the time of enrollment and 14 healthy children. ALL participants were recruited through physician referrals and a recruitment liaison in the local clinics, while control subjects were recruited through community postings. There were no between group differences in age, gender or minority status (Table I). Participants with ALL were excluded for history of cranial radiation, CNS involvement, or gross neuropathologies (e.g., leukomalacia, ventriculomegaly). All participants were excluded for major sensory impairments, MRI contraindications, or any significant medical or psychiatric condition known to affect cognitive function (diagnosed before or unrelated to ALL for the ALL group). Participants with ALL received intrathecal chemotherapy as per POG/COG protocols 9904 (N = 2), 9905 (N = 3), AALL0331 (N = 8), and AALL0434 (N = 2). There were 12 participants who received standard dose treatment and three who received high dose. Informed consent was obtained from the parent/legal guardian and assent was obtained from all participants. Stanford University’s Institutional Review Board approved this study.
TABLE I.
Demographic and Medical Data Shown as Mean (Standard Deviation) Unless Otherwise Indicated
| ALL (N = 15) | Controls (N = 14) | t/ χ 2 | P | |
|---|---|---|---|---|
| Age | 11.5 (2.0), range 8.9–15.9 | 11.5 (2.0), range: 8.0–14.6 | 0.60 | 0.95 |
| Grade | 6.1 (2.2), range 3–10 | 6.2 (2.0), range 2–9 | 0.11 | 0.91 |
| Maternal education (years) | 12.9 (4.3), range 6–18 | 14.2 (3.1), range 6–21 | 0.91 | 0.37 |
| Male | 60% | 43% | 0.32 | 0.57 |
| Minority status | 53% | 43% | 0.42 | 0.52 |
| Age at diagnosis | 4.4 (1.8), range 1.5–8 | |||
| Time since treatment (months) | 43.8 (29.4), range: 9–110 |
rsfMRI Acquisition
rsfMRI data were obtained using a GE Discovery MR750 3.0 T whole-body scanner (GE Medical Systems, Milwaukee, WI) while participants rested in the scanner with their eyes closed. We used a T2*-weighted gradient echo spiral pulse sequence: TR=2,000 milliseconds, TE=30 milliseconds, flip angle=80°, field of view=22 cm,matrix=64×64, slice thickness=4.0mm, spacing=1.0mm, 150 volumes, scan time=5:00. Subjects were monitored visually via a mirror in the head coil and physiologic recordings of heart and respiratory rate. An automated high-order shimming method was used to reduce field inhomogeneity. We also acquired a high-resolution, 3D inversion-recovery prepared fast spoiled gradient echo anatomical scan with the following parameters: TR=minimum, TE=minimum, flip=11 degrees, inversion time=300 milliseconds, bandwidth=±31.25 kHz, field of view=24 cm, phase field of view=0.75, slice thickness=1.5mm, 125 slices, 256×256 at 1 excitation, scan time=4:26.
rsfMRI Analysis
Image preprocessing was performed using Statistical Parametric Mapping 8 (SPM8, Wellcome Trust Centre, London, UK) as described in detail in our previous publications [20–22]. Resting state functional connectivity analysis was performed using a seed-based approach within the CONN toolbox [23]. Seeds were defined by 90 cortical and subcortical regions of interest (ROIs) from the automated anatomical labeling (AAL) atlas [24], re-sliced in SPM8 to match the image dimensions of the structural and functional images (91 × 109 × 91). To reduce the influence of non-neuronal noise, the preprocessed images were motion-regressed, corrected via the CompCor strategy [25] and band-pass filtered to 0.008–0.09 Hz. Pearson’s correlation coefficients were calculated between seed time courses and the time courses of all other voxels in the brain. Correlation coefficients were then normalized using Fisher’s r–z transformation resulting in a corrected correlation map for each individual. Second-level analysis was performed using the general linear model within CONN to determine between group differences in correlation maps [23] using false discovery rate (FDR) correction for multiple comparisons.
Cognitive Performance
We administered the following standardized measures to all participants on the same day as the MRI scanning session: Information, Matrix Reasoning, Letter-Number Sequencing and Coding subtests of the Wechsler Intelligence Scale for Children, 4th edition (WISC-IV) [26], Verbal Learning and Picture Memory subtests of the Wide Range Assessment of Learning and Memory, 2nd edition [27], Letter Fluency and Color-Word Interference subtests of the Delis-Kaplan Executive Function System [28], Reading Fluency and Math Fluency subtests of the Woodcock Johnson Tests of Achievement, 3rd edition [29], and the Behavioral Rating Inventory of Executive Function (BRIEF) [30]. We calculated an intelligence quotient (IQ) estimate using the average scaled score from the WISC-IV subtests. For the BRIEF, we used the Global Executive Composite (GEC).
Tests were chosen based on their psychometric properties including available normative data for the entire age range of our sample and their measurement of cognitive skills known to be affected in pediatric cancer while keeping the battery feasibly brief for young participants. Tests were administered by research staff trained and supervised by a clinical neuropsychologist (S.K.) and all tests were doubled scored by raters blinded to participant group membership. We used two-tailed t-tests to determine between group differences in cognitive performance with FDR correction. We also calculated effect sizes using Cohen’s d [31].
Correlations
Two-tailed exploratory Pearson or Spearman correlations (as appropriate) were performed within each group separately between significant functional connections (represented by normalized z score), demographic (age, gender, maternal education), and cognitive variables. Only those cognitive measures that differed between groups as defined by a medium (0.50) [31] or higher effect size were examined. Within the ALL group, correlations between significant functional connections and medical/treatment variables (time since treatment, age at diagnosis, treatment intensity) were also calculated.
RESULTS
Group Differences in Intrinsic Functional Connectivity
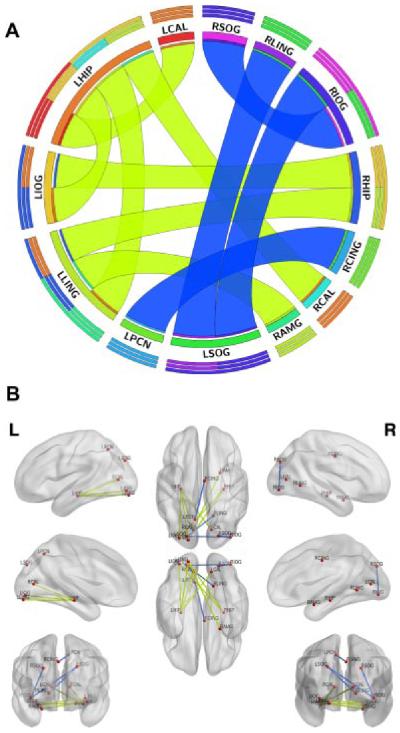
As shown in Table II and Figure 1, the ALL group-demonstrated regions of both functional hyper- and hypo-connection compared to controls. Functional hyperconnectivity was observed within peristriate cortex regions including right lingual gyrus, bilateral superior occipital lobe and right inferior occipital lobe. The functional connectivity between right middle cingulate gyrus and left precuneus also was increased in ALL.
TABLE II.
Between Group Differences in Intrinsic Functional Connectivity
| P (FDR-corrected) | |
|---|---|
| Functionally hyperconnected in ALL compared to controls | |
| Right lingual | |
| Left superior occipital | 0.016 |
| Right middle cingulum | |
| Left precuneus | 0.031 |
| Right inferior occipital | |
| Left superior occipital | 0.022 |
| Right superior occipital | 0.027 |
| Functionally hypoconnected in ALL compared to controls | |
| Left hippocampus | |
| Left inferior occipital | 0.038 |
| Left lingual | 0.038 |
| Right calcarine | 0.038 |
| Left calcarine | 0.043 |
| Left lingual | |
| Right amygdala | 0.035 |
| Right hippocampus | 0.035 |
| Left inferior occipital | |
| Right hippocampus | 0.047 |
ALL, acute lymphoblastic leukemia; FDR, false discovery rate.
Fig. 1.
Between group differences in intrinsic functional connectivity. Regions of significant (P < 0.05, FDR corrected) functional hyperconnectivity in the ALL group compared to controls are indicated in the circular graph (A) by blue ribbons while regions of functional hypoconnectivity are indicated by yellow-green ribbons. Concentric bars above the region label, color-coded by segment, show relative contribution of each connection for (from outer to inner) total outgoing and incoming connections, incoming connections, and outgoing connections. Circular graph created using Circos (http://circos.ca) [51]. The brain graph (B) also shows regions of functional hyperconnectivity in blue and functional hypoconnectivity in yellow-green. Brain graph created using BrainNet Viewer (http://www.nitrc.org/projects/bnv/) [52]. RLING, right lingual gyrus; RSOG, right superior occipital gyrus; RCING, right middle cingulate; LPCN, left precuneus; RIOG, right inferior occipital gyrus; LSOG, left superior occipital gyrus; LHIP, left hippocampus; LLING, left lingual gyrus; RCAL, right calcarine sulcus; LCAL, left calcarine sulcus; RAMG, right amygdala; RHIP, right hippocampus; LIOG, left inferior occipital gyrus.
Reduced functional connectivity in ALL was observed between left hippocampus and left inferior occipital, left lingual and bilateral calcarine sulcus, left lingual and right amygdala and right hippocampus and left inferior occipital and right hippocampus.
Group Differences in Cognitive Performance
As shown in Table III, the ALL group showed reduced performance on several measures. However, these did not survive multiple comparisons correction.
TABLE III.
Cognitive Data Shown as Mean (Standard Deviation)
| ALL (N = 13) | Controls (N = 14) | t | P (FDR adjusted) | d | |
|---|---|---|---|---|---|
| IQ estimate | 98.5 (11.5) | 105 (8.7) | 1.59 | 0.40 | 0.64 |
| Information | 10.3 (3.4) | 10.1 (2.3) | 0.11 | 0.91 | 0.07 |
| Matrix reasoning | 9.7 (3.2) | 11.1 (3.1) | 1.28 | 0.40 | 0.44 |
| LNS | 9.1 (2.8) | 11.3 (1.9) | 2.54 | 0.23 | 0.92 |
| Coding | 9.6 (3.2) | 10.9 (2.5) | 1.23 | 0.40 | 0.45 |
| Verbal learning | 10.7 (2.9) | 10.9 (2.8) | 0.25 | 0.90 | 0.07 |
| Picture memory | 8.2 (1.7) | 9.2 (2.6) | 1.23 | 0.40 | 0.46 |
| Letter fluency | 9.7 (3.1) | 10.6 (3.5) | 0.80 | 0.59 | 0.27 |
| Color naming | 10.1 (3.2) | 12.3 (2.8) | 1.93 | 0.30 | 0.73 |
| Word reading | 11.6 (2.2) | 12.5 (1.7) | 1.23 | 0.40 | 0.46 |
| Inhibition | 10.6 (1.9) | 12.4 (2.4) | 2.29 | 0.23 | 0.83 |
| Inhibition/switching | 11.1 (2.7) | 11.3 (3.1) | 0.20 | 0.90 | 0.07 |
| Reading fluency | 101.9 (16.8) | 107.5 (10.8) | 1.03 | 0.47 | 0.40 |
| Math fluency | 101.5 (12.5) | 105.2 (15.0) | 0.71 | 0.60 | 0.27 |
| BRIEF GECa | 56.3 (11.3) | 51.9 (6.6) | 1.22 | 0.40 | 0.48 |
FDR, false discovery rate; IQ, intelligence quotient; LNS, letter-number sequencing; BRIEF GEC, Behavioral Rating Inventory of Executive Function Global Executive Composite.
aHigher BRIEF score = greater impairment. For all other measures, lower score = lower performance.
Correlations Between Intrinsic Connectivity and Cognitive Performance
For regions of functional hypoconnection in ALL, lower IQ was associated with lower connectivity between left hippocampus and left lingual gyrus in the ALL group (r=0.541, P=0.037). Also, reduced Color Naming score was associated with reduced connectivity between left lingual gyrus and right amygdala (r = 0.524, P = 0.045). Controls showed no significant associations between regions of functional hypoconnectivity and cognitive performance. For regions of functional hyperconnection, neither group showed any significant correlations with cognitive measures.
Correlations Between Intrinsic Connectivity and Demographic Variables
Neither group showed any significant correlations between connectivity and age, maternal education, or gender.
Correlations Between Intrinsic Connectivity and Medical/Treatment Variables (ALL Only)
Younger age at diagnosis was associated with lower connectivity between left hippocampus and left lingual gyrus (r=0.609, P = 0.016) as well as between left hippocampus and left calcarine sulcus (r = 0.681, P = 0.005). There were no correlations between regions of functional hypoconnection and time since treatment or treatment intensity.
DISCUSSION
Using rsfMRI, we demonstrated disrupted connectivity in several intrinsic brain network regions among young survivors of pediatric ALL compared with healthy children. Regions that were functionally hypoconnected in ALL included bilateral hippocampus, left inferior occipital, left lingual gyrus, bilateral calcarine sulcus, and right amygdala. The ALL group also showed regions of functional hyperconnectivity including right lingual gyrus, precuneus, bilateral superior occipital lobe, and right inferior occipital lobe. Cognitive performance was reduced in the ALL group compared to controls particularly on measures of global intelligence, working memory, visual processing of color, and response inhibition. In exploratory analysis, functional hypoconnectivity between some regions was associated with decreased cognitive performance.
Intrinsic functional networks are believed to modulate allocation of neural resources toward goal-oriented processes [32]. This dynamic resource allocation depends critically on stable structural connectivity [33–35]. Therefore, these findings are consistent with reports showing abnormal white matter connectivity following ALL [14,15,36] including our previous study showing disrupted gray matter structural network connectivity in ALL survivors [19]. Together, these neuroimaging findings may suggest that ALL is associated with diffuse disconnection of neural networks. This suggests a reduction in overall information processing efficiency, consistent with the subtle versus pronounced profile of cognitive difficulties and learning delays observed in survivors of pediatric ALL [37].
Together, the regions of reduced connectivity in ALL are known to be involved in memory, attention and/or processing of visual information [38–40]. Previous studies have demonstrated deficits in these cognitive domains among survivors of ALL [41,42]. Our results suggest that reduced Color Naming performance may be associated with reduced connectivity between left lingual gyrus and right amygdala. Color Naming performance is highly associated with Attention Deficit Hyperactivity Disorder in children and is believed to indicate impairments in visual perception and visual selective attention [43,44]. However, our correlational findings were exploratory and uncorrected and therefore areas of functional hypoconnection require further study in larger samples.
There were no correlations between cognitive performance and regions of functional hyperconnectivity. The specific regions of functional hyperconnectivity included right lingual gyrus, bilateral superior occipital lobe and right inferior occipital lobe, which are also involved in attention and visual processing. The functional significance of these regions might be better evaluated with additional cognitive–behavioral measures that focus more specifically on attention and integrated visual–spatial skills. Alternatively, functional hyperconnectivity may reflect a compensatory neural mechanism as we have demonstrated in survivors of adult-onset cancer treated with chemotherapy [45]. Additionally, we previously showed that young survivors of ALL show reorganization of white matter regions following treatment-related brain injury [12]. Compensatory neural mechanisms may mask underlying cognitive difficulties [46]. For example, our ALL sample demonstrated cognitive testing scores within the “average” range despite scores being lower than that of their peers. However, it is difficult to determine if functional hyperconnectivity is compensatory in the present sample given the lack of correlation with cognitive outcome. It is possible that a change in cognitive function over time is associated with altered connectivity and therefore longitudinal studies are required.
Younger age at diagnosis may be associated with reduced connectivity between left hippocampus and left lingual gyrus as well as between left hippocampus and left calcarine sulcus. This is consistent with previous studies suggesting that younger age at diagnosis is predictive of poorer cognitive and neurobiologic outcome following treatment for ALL [47–49]. Studies of early brain injury in children suggest nonlinear effects of age with potential critical periods interacting with neural plasticity mechanisms to influence outcome [50]. Longitudinal studies of brain development in larger samples of children treated for ALL are required to determine the specific ages or age ranges that are associated with the greatest neurobiologic vulnerability. Statistical power was likely inadequate to detect further relationships between connectivity measures and functional outcomes, demographics and treatment variables. These variables require further examination to determine factors that reliably predict neurobiologic outcome following ALL.
This study is limited by the small sample size and cross-sectional design. Additionally, there was likely not enough variance in treatment intensity to determine if this variable influenced neurobiologic or cognitive status. Our rsfMRI ROI scheme is a very common one but as with all rsfMRI studies, a different scheme might yield alternate results. Despite these limitations, our findings demonstrate unique insights regarding the neurobiologic mechanisms of cognitive dysfunction associated with ALL chemotherapy. We provide further evidence that intrathecal chemotherapy alone, without cranial radiation, can significantly impact brain development in young patients. rsfMRI can be used to evaluate multiple neural systems with one brief scan (e.g., 5 minutes) and does not have any behavioral requirements. It can also be obtained during sleep or sedation and is therefore ideal for evaluating young children. Using rsfMRI in multisite, cooperative studies would yield important information in larger samples of children.
Footnotes
Conflict of interest: Nothing to declare.
REFERENCES
- 1.Anderson FS, Kunin-Batson AS. Neurocognitive late effects of chemotherapy in children: The past 10 years of research on brain structure and function. Pediatr Blood Cancer. 2009;52:159–164. doi: 10.1002/pbc.21700. [DOI] [PubMed] [Google Scholar]
- 2.Krull KR, Zhang N, Santucci A, et al. Long-term decline in intelligence among adult survivors of childhood acute lymphoblastic leukemia treated with cranial radiation. Blood. 2013;122:550–553. doi: 10.1182/blood-2013-03-487744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahalley LS, Conklin HM, Tyc VL, et al. Slower processing speed after treatment for pediatric brain tumor and acute lymphoblastic leukemia. Psychooncology. 2013;22:1979–1986. doi: 10.1002/pon.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong GT, Reddick WE, Petersen RC, et al. Evaluation of memory impairment in aging adult survivors of childhood acute lymphoblastic leukemia treated with cranial radiotherapy. J Natl Cancer Inst. 2013;105:899–907. doi: 10.1093/jnci/djt089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winick N. Neurocognitive outcome in survivors of pediatric cancer. Curr Opin Pediatr. 2011;23:27–33. doi: 10.1097/MOP.0b013e32834255e9. [DOI] [PubMed] [Google Scholar]
- 6.Monje M, Dietrich J. Cognitive side effects of cancer therapy demonstrate a functional role for adult neurogenesis. Behav Brain Res. 2012;227:376–379. doi: 10.1016/j.bbr.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seigers R, Timmermans J, van der Horn HJ, et al. Methotrexate reduces hippocampal blood vessel density and activates microglia in rats but does not elevate central cytokine release. Behav Brain Res. 2010;207:265–272. doi: 10.1016/j.bbr.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Krull KR, Bhojwani D, Conklin HM, et al. Genetic mediators of neurocognitive outcomes in survivors of childhood acute lymphoblastic leukemia. J Clin Oncol. 2013;31:2182–2188. doi: 10.1200/JCO.2012.46.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeller B, Tamnes CK, Kanellopoulos A, et al. Reduced neuroanatomic volumes in long-term survivors of childhood acute lymphoblastic leukemia. J Clin Oncol. 2013;31:2078–2085. doi: 10.1200/JCO.2012.47.4031. [DOI] [PubMed] [Google Scholar]
- 10.Monje M, Thomason ME, Rigolo L, et al. Functional and structural differences in the hippocampus associated with memory deficits in adult survivors of acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013;60:293–300. doi: 10.1002/pbc.24263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson KE, Livesay KL, Campbell LK, et al. Working memory in survivors of childhood acute lymphocytic leukemia: Functional neuroimaging analyses. Pediatr Blood Cancer. 2010;54:585–590. doi: 10.1002/pbc.22362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kesler SR, Tanaka H, Koovakkattu D. Cognitive reserve and brain volumes in pediatric acute lymphoblastic leukemia. Brain Imaging Behav. 2010;4:256–269. doi: 10.1007/s11682-010-9104-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reddick WE, Glass JO, Johnson DP, et al. Voxel-based analysis of T2 hyperintensities in white matter during treatment of childhood leukemia. Am J Neuroradiol. 2009;30:1947–1954. doi: 10.3174/ajnr.A1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aukema EJ, Caan MW, Oudhuis N, et al. White matter fractional anisotropy correlates with speed of processing and motor speed in young childhood cancer survivors. Int J Radiat Oncol Biol Phys. 2009;74:837–843. doi: 10.1016/j.ijrobp.2008.08.060. [DOI] [PubMed] [Google Scholar]
- 15.Porto L, Preibisch C, Hattingen E, et al. Voxel-based morphometry and diffusion-tensor MR imaging of the brain in long-term survivors of childhood leukemia. Eur Radiol. 2008;18:2691–2700. doi: 10.1007/s00330-008-1038-2. [DOI] [PubMed] [Google Scholar]
- 16.Horska A, Laclair A, Mohamed M, et al. Low cerebellar vermis volumes and impaired neuropsychologic performance in children treated for brain tumors and leukemia. Am J Neuroradiol. 2010;31:1430–1437. doi: 10.3174/ajnr.A2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sporns O. The human connectome: A complex network. Ann N Y Acad Sci. 2011;1224:109–125. doi: 10.1111/j.1749-6632.2010.05888.x. [DOI] [PubMed] [Google Scholar]
- 18.Damoiseaux JS, Prater KE, Miller BL, et al. Functional connectivity tracks clinical deterioration in Alzheimer’s disease. Neurobiol Aging. 2012;33:828–e19. doi: 10.1016/j.neurobiolaging.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosseini SM, Hoeft F, Kesler SR. GAT: A graph-theoretical analysis toolbox for analyzing between-group differences in large-scale structural and functional brain networks. PLoS ONE. 2012;7:e40709. doi: 10.1371/journal.pone.0040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosseini SM, Kesler SR. Comparing connectivity pattern and small-world organization between structural correlation and resting-state networks in healthy adults. NeuroImage. 2013;78:402–414. doi: 10.1016/j.neuroimage.2013.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kesler SR, Wefel JS, Hosseini SM, et al. Default mode network connectivity distinguishes chemotherapy-treated breast cancer survivors from controls. Proc Natl Acad Sci USA. 2013;110:11600–11605. doi: 10.1073/pnas.1214551110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruno J, Hosseini SM, Kesler S. Altered resting state functional brain network topology in chemotherapy-treated breast cancer survivors. Neurobiol Dis. 2012;48:329–338. doi: 10.1016/j.nbd.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- 24.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 25.Behzadi Y, Restom K, Liau J, et al. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wechsler D. Wechsler Intelligence Scale for Children. 4th edition The Psychological Corporation; San Antonio: 2003. [Google Scholar]
- 27.Sheslow D, Adams W. Wide range assessment of memory and learning, 2nd edition (WRAML2) Psychological Assessment Resources; Lutz, FL: 2005. [Google Scholar]
- 28.Homack S, Lee D, Riccio CA. Test review: Delis–Kaplan executive function system. J Clin Exp Neuropsychol. 2005;27:599–609. doi: 10.1080/13803390490918444. [DOI] [PubMed] [Google Scholar]
- 29.Woodcock R, McGrew K, Mather N. Woodcock-Johnson tests of achievement, 3rd edition (WJ-III) Riverside Publishing; Itasca, IL: 2001. [Google Scholar]
- 30.Guy S, Isquith P, Gioia G. Behavioral Rating Inventory of Executive Function (BRIEF) Psychological Assessment Resources; Lutz, FL: 2000. [Google Scholar]
- 31.Cohen J. Statistical power analysis for the behavioral sciences. Lawrence Earlbaum Associates; Hillsdale, NJ: 1998. [Google Scholar]
- 32.Sambataro F, Murty VP, Callicott JH, et al. Age-related alterations in default mode network: Impact on working memory performance. Neurobiol Aging. 2010;31:839–852. doi: 10.1016/j.neurobiolaging.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bassett DS, Wymbs NF, Porter MA, et al. Dynamic reconfiguration of human brain networks during learning. Proc Natl Acad Sci U S A. 2011;108:7641–7646. doi: 10.1073/pnas.1018985108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sporns O, Chialvo DR, Kaiser M, et al. Organization, development and function of complex brain networks. Trends Cogn Sci. 2004;8:418–425. doi: 10.1016/j.tics.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 35.Damoiseaux JS, Greicius MD. Greater than the sum of its parts: A review of studies combining structural connectivity and resting-state functional connectivity. Brain Struct Funct. 2009;213:525–533. doi: 10.1007/s00429-009-0208-6. [DOI] [PubMed] [Google Scholar]
- 36.Khong PL, Leung LH, Fung AS, et al. White matter anisotropy in post-treatment childhood cancer survivors: Preliminary evidence of association with neurocognitive function. J Clin Oncol. 2006;24:884–890. doi: 10.1200/JCO.2005.02.4505. [DOI] [PubMed] [Google Scholar]
- 37.Buizer AI, de Sonneville LM, Veerman AJ. Effects of chemotherapy on neurocognitive function in children with acute lymphoblastic leukemia: A critical review of the literature. Pediatr Blood Cancer. 2009;52:447–454. doi: 10.1002/pbc.21869. [DOI] [PubMed] [Google Scholar]
- 38.Raschle NM, Chang M, Gaab N. Structural brain alterations associated with dyslexia predate reading onset. NeuroImage. 2011;57:742–749. doi: 10.1016/j.neuroimage.2010.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.An L, Cao QJ, Sui MQ, et al. Local synchronization and amplitude of the fluctuation of spontaneous brain activity in attention-deficit/hyperactivity disorder: A resting-state fMRI study. Neurosci Bull. 2013;29:603–613. doi: 10.1007/s12264-013-1353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- 41.Conklin HM, Krull KR, Reddick WE, et al. Cognitive outcomes following contemporary treatment without cranial irradiation for childhood acute lymphoblastic leukemia. J Natl Cancer Inst. 2012;104:1386–1395. doi: 10.1093/jnci/djs344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waber DP, Queally JT, Catania L, et al. Neuropsychological outcomes of standard risk and high risk patients treated for acute lymphoblastic leukemia on Dana-Farber ALL consortium protocol 95–01 at 5 years post-diagnosis. Pediatr Blood Cancer. 2012;58:758–765. doi: 10.1002/pbc.23234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wodka EL, Loftis C, Mostofsky SH, et al. Prediction of ADHD in boys and girls using the D-KEFS. Arch Clin Neuropsychol. 2008;23:283–293. doi: 10.1016/j.acn.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tannock R, Banaschewski T, Gold D. Color naming deficits and attention-deficit/hyperactivity disorder: A retinal dopaminergic hypothesis. Behav Brain Funct. 2006;2:1–8. doi: 10.1186/1744-9081-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hosseini SM, Kesler SR. Multivariate pattern analysis of fMRI in breast cancer survivors and healthy women. J Int Neuropsychol Soc. 2013;19:1–11. doi: 10.1017/S1355617713001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reuter-Lorenz PA, Cimprich B. Cognitive function and breast cancer: Promise and potential insights from functional brain imaging. Breast Cancer Res Treat. 2013;137:33–43. doi: 10.1007/s10549-012-2266-3. [DOI] [PubMed] [Google Scholar]
- 47.Khong PL, Kwong DL, Chan GC, et al. Diffusion-tensor imaging for the detection and quantification of treatment-induced white matter injury in children with medulloblastoma: A pilot study. Am J Neuroradiol. 2003;24:734–740. [PMC free article] [PubMed] [Google Scholar]
- 48.Mabbott DJ, Spiegler BJ, Greenberg ML, et al. Serial evaluation of academic and behavioral outcome after treatment with cranial radiation in childhood. J Clin Oncol. 2005;23:2256–2263. doi: 10.1200/JCO.2005.01.158. [DOI] [PubMed] [Google Scholar]
- 49.von der Weid N, Mosimann I, Hirt A, et al. Intellectual outcome in children and adolescents with acute lymphoblastic leukaemia treated with chemotherapy alone: Age- and sex-related differences. Eur J Cancer. 2003;39:359–365. doi: 10.1016/s0959-8049(02)00260-5. [DOI] [PubMed] [Google Scholar]
- 50.Anderson V, Spencer-Smith M, Wood A. Do children really recover better? Neurobehavioural plasticity after early brain insult. Brain. 2011;134:2197–2221. doi: 10.1093/brain/awr103. [DOI] [PubMed] [Google Scholar]
- 51.Krzywinski M, Schein J, Birol I, et al. Circos: An information aesthetic for comparative genomics. Genome Res. 2009;19:1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xia M, Wang J, He Y. BrainNet Viewer: A network visualization tool for human brain connectomics. PLoS ONE. 2013;8:e68910. doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]