Abstract
Purpose
Early-stage osteoarthritis (OA) includes glycosaminoglycan (GAG) loss and collagen disruption that cannot be seen on morphological magnetic resonance imaging (MRI). T1ρ MRI is a measurement that probes the low-frequency rate of exchange between protons of free water and those from water associated with macromolecules in the cartilage’s extracellular matrix. While it has been hypothesized that increased water mobility resulting from early osteoarthritic changes cause elevated T1ρ MRI values, there remain several unknown mechanisms influencing T1ρ measurements in cartilage. The purpose of this work was to relate histological and biochemical metrics directly measured from osteochondral biopsies and fluid specimens with quantitative MRI-detected changes of in vivo cartilage composition.
Patients and methods
Six young patients were enrolled an average of 41 days after acute anterior cruciate ligament (ACL) rupture. Femoral trochlear groove osteochondral biopsies, serum, and synovial fluid were harvested during ACL reconstruction to complement a presurgery quantitative MRI study (T1ρ, T2, delayed gadolinium-enhanced MRI of cartilage [dGEMRIC] relaxation times). A high-resolution MRI scan of the excised osteochondral biopsy was also collected. Analyses of in vivo T1ρ images were compared with ex vivo T1ρ imaging, GAG assays and histological GAG distribution in the osteochondral biopsies, and direct measures of bone and cartilage turnover markers and “OA marker” 3B3 in serum and synovial fluid samples.
Conclusion
T1ρ relaxation times in patients with a torn ACL were elevated from normal, indicating changes consistent with general fluid effusion after blunt joint trauma. Increased chondrogenic progenitor cell (CPC) production of chondroprotective lubricin may relate to cartilage surface disruption by blunt trauma and CPC amplification of joint inflammation. Disparity between ex vivo and matched in vivo MRI of trochlear cartilage suggests MRI signal differences that may be related to the synovial fluid environment. T1ρ is emerging as a promising MRI biomarker to relate noninvasive measures of whole-joint condition and cartilage composition to direct measures of cartilage changes in the acute phase of joint injuries.
Keywords: proteoglycan, osteochondral biopsy, T1ρ, biomarker
Introduction
T1ρ is emerging as a promising magnetic resonance imaging (MRI) biomarker to relate noninvasive measures of whole joint condition and cartilage composition, to direct measures of cartilage changes in the acute phase of joint injuries, which may portend osteoarthritis (OA). Early signs of OA include glycosaminoglycan (GAG) loss and collagen disruption not seen on morphological MRI.1 While it is hypothesized that increasing water mobility resulting from early osteoarthritic changes in collagen and proteoglycan (PG) content cause elevated T1ρ values,2 unknown mechanisms influencing T1ρ measurements remain. Several studies have correlated the biochemical and biomechanical content of cartilage to T1ρ in animal models3–5 and in end-stage OA explants.6–9 While important, these models are still not exemplary of early OA development, particularly during the acute phase of posttraumatic OA events.
We collected osteochondral and fluid specimens to directly assess the complementary roles of biomarkers in defining the joint environment early after acute anterior cruciate ligament (ACL) rupture in young patients. Thirteen acute ACL rupture patients were each imaged during a 4-hour presurgery work-up to acquire a fast-spin-echo (FSE)-based T1ρ sequence, a multi-echo spin-echo T2 sequence, and a T1-weighted inversion recovery sequence with a gadolinium contrast agent (delayed gadolinium-enhanced MRI of cartilage [dGEMRIC]) an average of 55.7 days postinjury.10 Knee-site-specific differences were evinced, but nonsignificant differences in mean relaxation time between cartilage layers of the same region and sequence were observed. No appreciable focal GAG loss was detected by dGEMRIC, and T2 was generally elevated in the early acute phase of this blunt trauma injury. General and focal elevations in T1ρ relaxation times were identified; further suggesting changes not only in GAG, but, perhaps more aptly, the ratio of water-to-PG content in the matrix. T1ρ is a relaxation measurement that probes the low-frequency rate of exchange between protons of free water and those from water associated with macromolecules in the cartilage’s extracellular matrix, yielding longer relaxation times in which components of the extracellular matrix, especially PGs, are disrupted.11
In this study, we report complementary metrics from osteochondral biopsies and fluid specimens that may offer insight into mechanisms underlying these previously reported in vivo quantitative MRI-detected changes of cartilage composition. The current study examined biologic specimens from six of the 13 patients who underwent reconstructive surgery after ACL rupture, a known injury event that often progresses to posttraumatic OA (PTOA). Analyses of in vivo and ex vivo T1ρ imaging, GAG assays and automated GAG distribution from Safranin O histology of the osteochondral biopsies, and direct measures of bone and cartilage turnover markers and “OA marker” 3B3 in serum and synovial fluid samples were combined to better describe the injury environment.
Materials and methods
As a means of assessing the reproducibility and validity of our T1ρ imaging protocol, the following experiments were conducted. T1ρ maps of normal non-OA knees on three separate days (Monday, Wednesday, Friday) demonstrate reproducibility (Figure 1). A phantom-based validation experiment tested the equivalence of T1ρ measurements acquired on both 3.0 T and 4.7 T platforms. A circular plastic phantom of 25 mm diameter was designed with four compartments. Each compartment was filled with agarose gel of differing concentrations (1%, 2%, 3%, and 4%) to vary the expected T1ρ relaxation time. The phantom was scanned at both field strengths, using FSE pulse sequences equivalent to those used for in vivo (3.0 T) and ex vivo (4.7 T) biopsy specimen scans. After generation of T1ρ maps with nonlinear least-squares fitting, corresponding regions of the four gel concentrations were selected and compared.
Figure 1.
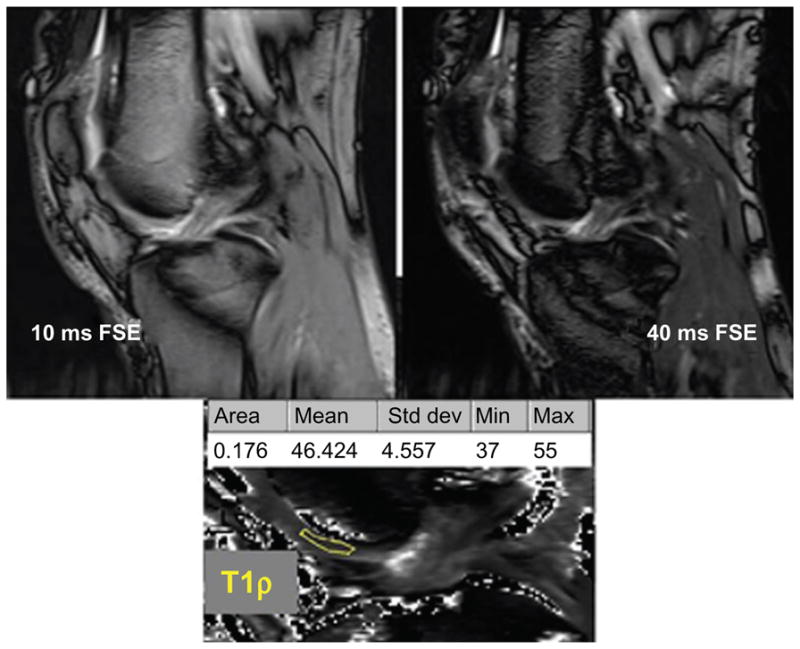
In vivo 3.0 T sagittal slices through the distal femoral trochlea.
Notes: Fast spin-echo (FSE) images at different spin-lock times are combined to create a map of T1ρ relaxation times. The region of interest (ROI) is outlined in yellow. Cartilage surface and bone interface voxel exclusion avoids partial-volume effects.
Six patients (2 male, 4 female) were enrolled an average of 40.8 days after acute ACL rupture injury (range: 12–81 days). In addition to an extended 4-hour quantitative MRI knee imaging study, these young patients (range: 18–29 years old at time of injury) participated in the complementary biological specimen protocol. Institutional Review Board approval and informed consent were obtained to harvest 3.5 mm diameter cylindrical femoral cartilage biopsies, serum and synovial fluid during ACL reconstructive surgery.
In total, six trochlear groove biopsies were harvested. The femoral trochlear groove rather than the condyles was utilized as a representative specimen of the ACL joint injury to minimize possibly furthering any traumatic injury to a weight bearing surface. After harvest, the osteochondral biopsies were immersed in a vial of Dulbecco’s modified Eagle medium (DMEM) for transport to the laboratory, where they were immediately embedded in a tray containing 1% agarose (Sigma-Aldrich, St Louis, MO, USA) dissolved in phosphate-buffered saline (pH 6.9–7.1).
Image acquisition and processing
The clinical MRI knee imaging sessions were performed on a 3.0 T Siemens TIM Trio scanner (Siemens Healthcare, Erlangen, Germany) using a single-channel transmit–receive extremity coil. A previously reported preoperative quantitative cartilage imaging study (T1ρ, T2, and dGEMRIC) was collected in two sessions within a 4-hour period.10 For T1ρ imaging, a magnetization preparation preceded a standard FSE imaging protocol (Repetition time [TR] = 3000 ms, echo time [TE] = 9.5 ms, in-plane resolution = 0.55 mm, field of view [FOV] = 140 mm, slice thickness = 4 mm, spacing between slice centers = 8 mm). The T1ρ magnetization preparation step consisted of a +90°x tip-down, 400 Hz self-compensating spin-lock radio frequency (RF) of variable duration (5, 10, 20, 40, 60, 80 ms) for T1ρ weighting, and a 90°x tip-up and final crusher gradient.12 Mean T1ρ relaxation times were extracted using region of interest (ROI) analysis (outermost voxels were excluded to avoid partial volume effects). The mean relaxation times from the full-thickness ROI were recorded. The ex vivo T1ρ imaging of the whole osteochondral cylinder along its sagittal midline was performed on a small-bore Varian 4.7 T scanner (Varian Medical Systems, Inc, Palo Alto, CA, USA) with a 38 mm diameter coil. The T1ρ sequence was a series of magnetization preparation and FSE images at spin-lock times of 5, 10, 20, 30, 40, 60, and 80 ms, with ω = 400 Hz, TR/TE = 3000/12 ms, an echo train length of 8, and in-plane resolution of 0.1 ×0.2 mm with 1.0 mm slice thickness.6
All T1ρ maps were computed on a voxel-by-voxel basis with robust nonlinear least-squares fits (with bisquare weights) of a mono-exponential decay curve to derive relaxation time using MATLAB (MathWorks, Natick, MA, USA).13 Cartilage zones’ mean relaxation times were calculated using normalized cartilage thickness analysis.13 The zones’ names and locations with respect to the bone cartilage interface (BCI) were demarcated as deep (0%–13% of thickness), radial (13%–53%), transitional (53%–89%), and superficial (89%–100%) and relate directly to the site-specific zonal definitions used during the Mankin-based PG analysis.14,15 To divide the initial full-thickness ROI into these zonal areas, the articular and BCI surfaces were recorded, and normal vectors from both surfaces were found (Figure 2). A fifth-order polynomial was fit to both normal vectors’ sets of zonal intervals, thereby creating normalized-thickness zonal partitions, which reflected the local contours of the articular surface and the BCI. Voxels within these borders were assigned their appropriate zonal labels and mean relaxation times of the four zones were recorded. Border voxels were assigned only one zonal label based on minimum Euclidean distance from their center to the location of the zonal partitions, similar to Carballido-Gamio et al’s laminar methodology used for in vivo T1ρ imaging.2
Figure 2.
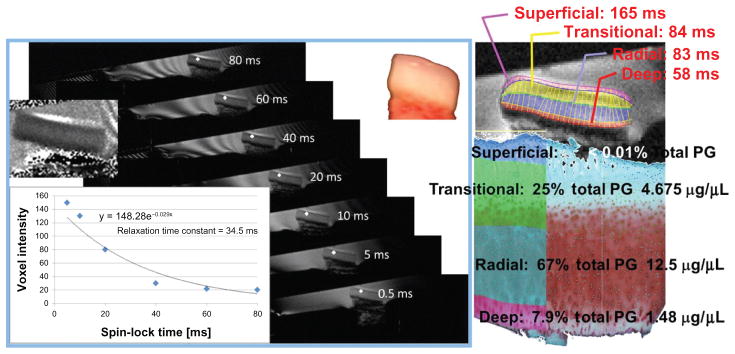
The voxel’s value at each spin-lock time is identified.
Notes: T1ρ maps were computed on a voxel-by-voxel basis with robust nonlinear least-squares fits (with bisquare weights) of a mono-exponential decay curve to derive relaxation times in this ex vivo 4.7 T magnetic resonance imaging series of biopsy fast spin echo images. Histologically defined cartilage zones are imposed and zonal T1ρ relaxation times extracted for direct comparison with zonal proteoglycan (PG) distributions from automated Mankin scoring of Safranin O red intensity.
Biopsy histology processing
After ex vivo T1ρ imaging, the cartilage specimen was cut in half along the sagittal midline imaging plane. One portion of the specimen was measured for volume by water displacement using a micropipette. The tissue was solubilized using papain (Sigma-Aldrich) and sulfated GAG content (μg/μL) was measured by dimethyl methylene blue assay (DMMB).16 This yielded the total PG content for each chondral specimen.
The directly apposed hemicylindrical portion was cryo-embedded, cut into 5 μm-thick sections and stained for histological analysis, using Weigert’s hematoxylin to blacken cell nuclei, Fast Green counter-stain, and Safranin O, which is a cationic dye that binds specifically to sulfated GAGs. The intensity of the red stain is indicative of PG content.17 Automated Mankin scoring14,15 was used to extract Safranin O red hue distribution and saturation within the four normalized cartilage zones (same physical normalization as T1ρ map analysis). PG content for each zone was found by dividing the mean zonal saturation value by the full-thickness cartilage mean saturation value (to find a PG-to-density ratio) and multiplying by the total PG assay value. When multiple histological slides were available for a particular specimen, the average Safranin O zonal staining across the sections was used.
Biological assays of fluid specimens
Blood draw and synovial fluid aspiration at the time of surgery were stored at −80°C until analysis. Enzyme linked immunosorbent assays (ELISAs) were performed for five potential markers: tartrate-resistant alkaline phosphatase (TRAP5b), a bone mineral-degrading enzyme synthesized by osteoclasts (bone degradation marker); bone sialoprotein (BSP), normally trapped within bone, which is released by osteoclast activity (bone degradation); bone alkaline phosphatase (BAP), an enzyme involved in matrix mineralization by osteoblasts (bone synthesis); collagen 2 cleavage neoepitope (C2C) (also known as Col2-3/4C long), a marker of type II collagen degradation by collagenases; and the OA marker chondroitin sulfate epitope (CS3B3[−]),made in immature and in osteoarthritic cartilage. Blood urea nitrogen (BUN) was used for normalization. To identify the source of CS3B3, the 3B3(−) epitope and total GAG were measured in medium samples from two primary cultures of chondrogenic progenitor cells (CPCs) and from two primary cultures of normal chondrocytes.18
Results
Phantom T1ρ times increase linearly with increasing water content on both scanners (Table 1), and normal in vivo trochlear regions of interest (Figure 1) exhibit T1ρ values similar to the 4%–3% gels. T1ρ relaxation times for ACL patients’ biopsies at 4.7 T (Table 2) correlate with PG distributions. On average, greater PG content is associated with shorter T1ρ relaxation times. The in vivo relaxation times, extracted from biopsy locations in preoperative 3.0 T T1ρ maps using adapted histology-based methods, are longer than normal trochlea reference times, indicating a greater free water-to-PG ratio. Ex vivo biopsies, all immersed in DMEM for 2 hours before potting in 1% agarose and imaged at 4.7 T, no longer demonstrate a linear relationship to their matched 3.0 T maps. This suggests there may be in vivo differences in the synovial fluid environment. Serum biomarker results for ACL and normal subjects (Table 3) are complemented by ACL patients’ knee synovial fluid assays normalized to mg of BUN. The CS3B3-to-GAG ratios for medium from four separate CPC and normal chondrocyte cultures are plotted at 24- and 72-hour end points (Figure 3).
Table 1.
Reference T1ρ values
| T1ρ relaxation time (ms) | Trochlear region | |||
|---|---|---|---|---|
|
| ||||
| Phantom comparison | Normals | 3.0 T (in vivo) | ||
| Agarose | 4.7 T (ex vivo) | 3.0 T (in vivo) | Norm-1 | 49.88 ± 6.44 |
| 1% | 99.2 ± 18.0 | 105.3 ± 7.0 | Norm-2 | 47.55 ± 6.61 |
| 2% | 73.0 ± 15.2 | 67.4 ± 9.7 | Norm-3 | 47.28 ± 7.60 |
| 3% | 49.1 ± 3.7 | 46.7 ± 3.6 | Norm-4 | 39.30 ± 6.59 |
| 4% | 32.1 ± 3.0 | 27.0 ± 1.9 | Norm-5 | 39.48 ± 7.68 |
| Average | 44.70 ± 6.98 | |||
Note: Mean ± 1 standard deviation.
Table 2.
Anterior cruciate ligament biopsy cartilage T1ρ values (± 1 S.D.) on two scanners
| Trochlear region
|
In vivo
|
Ex vivo
|
Proteoglycan
|
|---|---|---|---|
| Preoperative biopsy | 3.0 T mean T1ρ (ms) | 4.7 T mean T1ρ (ms) | PG (mg/mL) |
| Transitional zone | |||
| 1_Troch | 49.28 | 151.66 | 2.91 |
| 2_Troch | 70.55 | 76.17 | 7.48 |
| 3_Troch | 55.17 | 88.01 | 4.83 |
| 5_Troch | 43.11 | 63.85 | 23.17 |
| 6_Troch | 52.51 | 97.37 | 17.67 |
| 7_Troch | 46.56 | 67.47 | 27.76 |
| Transitional average | 52.9 (±9.7) | 90.8 (±32.4) | 13.97 (±10.35) |
| Radial zone | |||
| 1_Troch | 43.94 | 110.22 | 29.59 |
| 2_Troch | 66.11 | 72.00 | 59.38 |
| 3_Troch | 56.48 | 84.74 | 44.62 |
| 5_Troch | 42.02 | 68.43 | 39.28 |
| 6_Troch | 46.00 | 81.29 | 48.67 |
| 7_Troch | 45.45 | 65.86 | 41.07 |
| Radial average | 50.0 (±9.4) | 80.4 (±16.3) | 43.8 (±9.97) |
| Transitional + radial | 55.38 (±13.13) | 85.2 (±23.0) | 28.9 (±16.86) |
Table 3.
Fluid assay results
| Biomarker
|
Acute ACL patients’ serum (n = 4)
|
Normal subjects’ serum
|
ACL patients’ synovial fluid (n= 4)
|
|||||
|---|---|---|---|---|---|---|---|---|
| Marker | Units | Mean | SD | Mean | SD | n | Mean | SD |
| Trap5b | U/L | 0.229 | 0.067 | 3.17 | 0.73 | 28 | 3.98 | 2.21 |
| BSP | ng/mg | 84.1 | 31.0 | 32.3 | 21.9 | 28 | 73 | 31 |
| BAP | mg/mg | 0.095 | 0.021 | 0.072 | 0.031 | 10 | 0.009 | 0.006 |
| C2C | ng/mg | 705 | 99 | 759 | 156 | 15 | 802 | 416 |
| CS3B3 | ng/mg | 28.4 | 8.48 | 4.74 | 2.24 | 27 | 1.07 | 0.72 |
Notes: ACL serum change from normal: TRAP5b down (P = 0.001); BSP up (P < 0.001); BAP up (P = 0.137); C2C down (P = 0.520); CS3B3 up (P < 0.001).
Abbreviations: ACL, anterior cruciate ligament; BAP, bone alkaline phosphatase; BSP, bone sialoprotein; C2C, collagen 2 cleavage neoepitope; CS3B3, chondroitin sulfate epitope; SD, standard deviation; Trap5b, tartrate-resistant alkaline phosphatase.
Figure 3.
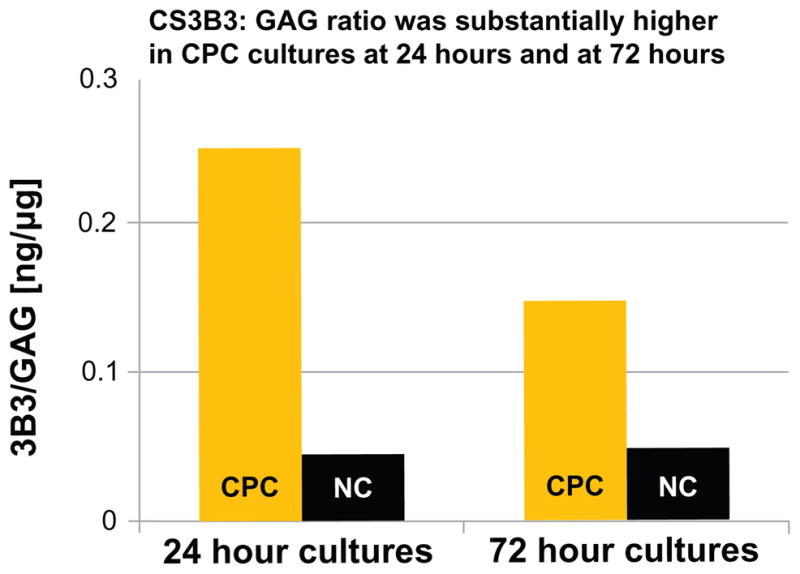
The chondroitin sulfate epitope (CS3B3[−]) epitope and total glycosaminoglycan (GAG) in medium samples from two chondrogenic progenitor cell (CPC) cultures, and from two normal chondrocyte (NC) cultures.
Note: Each column is one primary culture’s CS3B3-to-GAG ratio.
Discussion
Osteochondral biopsies from young ACL rupture patients shortly after injury are a unique research study cohort. Any PG loss observed and subsequent elevated T1rho relaxation times were assumed not to be from age-related cartilage degradation effects in these young patients, but rather the effect of the traumatic injury. The trochlear region of normal knees showed a narrow range of T1ρ relaxation times in vivo (45 ± 7 ms). The phantom T1ρ times showed a linear relationship with increasing gel content (r2 = 0.97) at 3.0 T and 4.7 T, both independently and combined. However, there was a distinct disparity between 3.0 T in vivo and 4.7 T ex vivo values (57 ± 15 ms and 85 ± 23 ms, respectively). The PG assay values varied between specimens (average: 24.4 μg/μL, range: 15.7–31.9 μg/μL). PG content per cartilage zone was determined by multiplying the Safranin O PG-to-density ratio (from Mankin analysis) by the total PG content (from DMMB assays). The resulting PG values were then matched to the corresponding cartilage zonal mean T1ρ relaxation times. Mean T1ρ relaxation times for zonal analyses showed much greater variability than found in the normal knees’ trochlear region. Clearly, the inverse relationship to PG content demonstrates that this sequence is sensitive to higher water-to-PG ratio, and supports others’ findings where PG loss or increasing OA grades positively correlated with increasing T1ρ relaxation times.4,5,7,8,19
While not necessarily a limitation, reported absolute T1ρ relaxation times vary. In this study, the range across osteochondral specimens’ mean T1ρ relaxation times was 58.3–165.5 ms. Trochlear cartilage values in normal subjects averaged 45 ± 7 ms (14–78 ms range). In comparison, recent 3.0 T studies found nonsymptomatic subjects’ mean relaxation times in the weight-bearing lateral femoral condyle to be 45.0 ± 7.0 ms13 and 53.3 ± 10.6 ms.20 The post-ACL rupture values in this current study are higher than the normal subjects’, and would likely indicate the changes in biochemistry, but other factors may also affect these values. The magnetic field strength, type of tissue sampled, and other imaging environment conditions affect these absolute values, causing discrepancies between studies (range of values in 3.0 T studies: 38.3–50.4 ms,8 37–101 ms,19 30–170 ms;9 range of values in 4.0 T studies: 56–100 ms,6 62–90 ms,7 107–163 ms6). With such variability, the overall relationship between healthy cartilage and injured/damaged cartilage should be examined by each institution.
This study utilized a biofidelic phantom for cross-platform T1ρ characterization that demonstrated a linear relationship between matched gel relaxation times (Table 1). Osteochondral specimens were imaged in a common environment (ex vivo transport in DMEM, encased in 1% agarose gel and scanned in a 4.7 T magnet) 2 hours after harvest. Disparity from the matched in vivo scans may be related to differences in the synovial fluid environment. While bone degradation markers were significantly different from normal, they gave conflicting signals, with TRAP5b levels down and BSP up. Bone synthesis marker BAP and collagen degradation C2C were within normal levels. A five-fold increase in OA marker CS3B3 may be linked to significantly increased activity of CPCs. Increased CPC production of chondroprotective lubricin may relate to cartilage surface disruption by blunt trauma, but CPC amplification of joint inflammation factors may account for the variability between the in vivo and ex vivo T1ρ times.
Conclusion
The early time frame after injury during which these samples were harvested demonstrates T1ρ’s effectiveness as a clinical biomarker prior to irreversible PG loss and OA onset. T1ρ relaxation times were elevated from normal, indicating increased unbound water in ACL rupture patients’ articular cartilage consistent with general fluid effusion after blunt joint trauma. CS3B3 was significantly higher in ACL patients, consistent with significant cartilage progenitor cell activation following blunt joint trauma. T1ρ’s sensitivity to early cartilage changes, complemented with serum biomarker changes in the first month after ACL rupture trauma found in this study, enhance the appeal of T1ρ as a clinically feasible imaging biomarker of cartilage health.
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number AR055533 and by the American Orthopaedic Society for Sports Medicine (AOSSM).
Footnotes
This is an Open Access article which permits unrestricted noncommercial use, provided the original work is properly cited.
Disclosure
The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors report no conflicts of interest in this work.
References
- 1.Blumenkrantz G, Majumdar S. Quantitative magnetic resonance imaging of articular cartilage in osteoarthritis. Eur Cell Mater. 2007;13:76–86. doi: 10.22203/ecm.v013a08. [DOI] [PubMed] [Google Scholar]
- 2.Carballido-Gamio J, Stahl R, Blumenkrantz G, Romero A, Majumdar S, Link TM. Spatial analysis of magnetic resonance T1rho and T2 relaxation times improves classification between subjects with and without osteoarthritis. Med Phys. 2009;36(9):4059–4067. doi: 10.1118/1.3187228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wheaton AJ, Dodge GR, Elliott DM, Nicoll SB, Reddy R. Quantification of cartilage biomechanical and biochemical properties via T1rho magnetic resonance imaging. Magn Reson Med. 2005;54(5):1087–1093. doi: 10.1002/mrm.20678. [DOI] [PubMed] [Google Scholar]
- 4.Duvvuri U, Kudchodkar S, Reddy R, Leigh JS. T(1rho) relaxation can assess longitudinal proteoglycan loss from articular cartilage in vitro. Osteoarthritis Cartilage. 2002;10(11):838–844. doi: 10.1053/joca.2002.0826. [DOI] [PubMed] [Google Scholar]
- 5.Duvvuri U, Reddy R, Patel SD, Kaufman JH, Kneeland JB, Leigh JS. T1rho-relaxation in articular cartilage: effects of enzymatic degradation. Magn Reson Med. 1997;38(6):863–867. doi: 10.1002/mrm.1910380602. [DOI] [PubMed] [Google Scholar]
- 6.Wheaton AJ, Casey FL, Gougoutas AJ, et al. Correlation of T1rho with fixed charge density in cartilage. J Magn Reson Imaging. 2004;20(3):519–525. doi: 10.1002/jmri.20148. [DOI] [PubMed] [Google Scholar]
- 7.Regatte RR, Akella SV, Lonner JH, Kneeland JB, Reddy R. T1rho relaxation mapping in human osteoarthritis (OA) cartilage: comparison of T1rho with T2. J Magn Reson Imaging. 2006;23(4):547–553. doi: 10.1002/jmri.20536. [DOI] [PubMed] [Google Scholar]
- 8.Nishioka H, Hirose J, Nakamura E, et al. T1ρ and T2 mapping reveal the in vivo extracellular matrix of articular cartilage. J Magn Reson Imaging. 2012;35(1):147–155. doi: 10.1002/jmri.22811. [DOI] [PubMed] [Google Scholar]
- 9.Tang SY, Souza RB, Ries M, Hansma PK, Alliston T, Li X. Local tissue properties of human osteoarthritic cartilage correlate with magnetic resonance T(1) rho relaxation times. J Orthop Res. 2011;29(9):1312–1319. doi: 10.1002/jor.21381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klocke NF, Amendola A, Thedens DR, et al. Comparison of T1ρ, dGEMRIC, and quantitative T2 MRI in preoperative ACL rupture patients. Acad Radiol. 2013;20(1):99–107. doi: 10.1016/j.acra.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thedens DR, Martin JA, Pedersen DR. Magnetic resonance imaging of cartilage: new imaging and clinical approaches. In: Buckwalter JA, Lotz M, Stoltz J-F, editors. Osteoarthritis, Inflammation and Degradation: A Continuum. Vol. 70. Amsterdam: IOS Press; 2007. pp. 239–253. [Google Scholar]
- 12.Charagundla SR, Borthakur A, Leigh JS, Reddy R. Artifacts in T(1rho)-weighted imaging: correction with a self-compensating spin-locking pulse. J Magn Reson. 2003;162(1):113–121. doi: 10.1016/s1090-7807(02)00197-0. [DOI] [PubMed] [Google Scholar]
- 13.Pedersen DR, Klocke NF, Thedens DR, Martin JA, Williams GN, Amendola A. Integrating carthage-specific T1rho MRI into knee clinic diagnostic imaging. Iowa Orthop J. 2011;31:99–109. [PMC free article] [PubMed] [Google Scholar]
- 14.Moussavi-Harami SF, Pedersen DR, Martin JA, Hillis SL, Brown TD. Automated objective scoring of histologically apparent cartilage degeneration using a custom image analysis program. J Orthop Res. 2009;27(4):522–528. doi: 10.1002/jor.20779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pedersen DR, Goetz JE, Kurriger GL, Martin JA. Comparative digital histology for human and common osteoarthritis models. Orthop Res Rev. 2013;5:13–20. doi: 10.2147/ORR.S38400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barbosa I, Garcia S, Barbier-Chassefière V, Caruelle JP, Martelly I, Papy-García D. Improved and simple micro assay for sulfated glycosaminoglycans quantification in biological extracts and its use in skin and muscle tissue studies. Glycobiology. 2003;13(9):647–653. doi: 10.1093/glycob/cwg082. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg L. Chemical basis for the histological use of safranin O in the study of articular cartilage. J Bone Joint Surg Am. 1971;53(1):69–82. [PubMed] [Google Scholar]
- 18.Seol D, McCabe DJ, Choe H, et al. Chondrogenic progenitor cells respond to cartilage injury. Arthritis Rheum. 2012;64(11):3626–3637. doi: 10.1002/art.34613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keenan KE, Besier TF, Pauly JM, et al. Prediction of glycosaminoglycan content in human cartilage by age, T1rho and T2 MRI. Osteoarthritis Cartilage. 2011;19(2):171–179. doi: 10.1016/j.joca.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goto H, Iwama Y, Fujii M, et al. A preliminary study of the T1rho values of normal knee cartilage using 3T-MRI. Eur J Radiol. 2012;81(7):e796–e803. doi: 10.1016/j.ejrad.2012.03.022. [DOI] [PubMed] [Google Scholar]


