Abstract
Apoptosis is a major mechanism for cell death in the nervous system during development. P2X7 nucleotide receptors are ionotropic ATP receptors that mediate cell death under pathological conditions. We developed an in vitro protocol to investigate the expression and functional responses of P2X7 nucleotide receptors during retinoic acid (RA)-induced neuronal differentiation of human SH-SY5Y neuroblastoma cells. Neuronal differentiation was examined measuring cellular growth arrest and neuritic processes elongation. We found that SH-SY5Y cells treated for 5 days with RA under low serum content exhibited a neuron-like phenotype with neurites extending more than twice the length of the cell body and cell growth arrest. Concurrently, we detected the abolishment of intracellular-free calcium mobilization and the down-regulation of P2X7 nucleotide receptor protein expression that protected differentiated cells from neuronal cell death and reduced caspase-3 cleavage-induced by P2X7 nucleotide receptor agonist. The role of P2X7 nucleotide receptors in neuronal death was established by selectively antagonizing the receptor with KN-62 prior to its activation. We assessed the involvement of protein kinases and found that p38 signaling was activated in undifferentiated after nucleotide stimulation, but abolished by the differentiating RA pretreatment. Importantly, P2X7 receptor-induced caspase-3 cleavage was blocked by the p38 protein kinase specific inhibitor PD169316. Taken together, our results suggest that RA treatment of human SH-SY5Y cells leads to decreased P2X7 nucleotide receptor protein expression thus protecting differentiated cells from extracellular nucleotide-induced neuronal death, and p38 signaling pathway is critically involved in this protection of RA-differentiated cells.
Keywords: Cell death, Differentiation, Human SH-SY5Y neuroblastoma cells, P2X7 nucleotide receptors, Retinoic acid
Introduction
Balance among cell proliferation, differentiation, and cell death is of pivotal importance for the development and maintenance of biological systems. During the course of normal development of the central nervous system (CNS), programmed cell death (PCD) or apoptotic cell death has a critical role eliminating cells that are redundant, damaged, or infected [1, 2]. In the nervous system, roughly half of the neurons initially generated cease division and die by apoptotic cell death in a well-defined interval, which coincide with the establishment of synaptic connections [3–5]. As a result, the adequate number of neurons and proper synaptic connections are established.
P2 nucleotide receptors, activated by extracellular nucleotides, have been attributed to mediate long-term (trophic) effects, which include cell death, differentiation, and cell proliferation as well as short-term effects, including neurotransmission by fast excitatory currents [6–10]. Based on transduction mechanisms and cloning studies, P2 nucleotide receptors have been categorized in two major families: G protein-coupled P2Y1,2,4,6,11,12,13,14 and ionotropic P2X1–7 nucleotide receptors [11–13].
P2X nucleotide receptors are ligand-gated channels activated upon ATP binding and their activation allows the influx of Na+ and Ca2+ and the efflux of K+ leading to membrane depolarization [14]. Among the P2X nucleotide receptor family, P2X7 nucleotide receptor is unique because its prolonged activation elicits the opening of a transmembrane nonselective pore that is permeable to large weight molecules up to 900 Da [15]. According to its channel/pore-forming property, activation of P2X7 nucleotide receptor by extracellular nucleotides promotes the accumulation of calcium ions in the cytoplasm and it has been strongly implicated in cell death responses [16, 17]. For example, P2X7 nucleotide receptor activation triggers apoptotic cell death that has been suggested to play an important role in removing cancerous and infected cells from tissues [14, 18]. In addition, P2X7 nucleotide receptor expression and functionality have been observed in pathological models of the CNS [19–21]. Although the role of P2X7 nucleotide receptor in mediating trophic effects under pathological conditions as well as short-term effects are broadly recognized, little is known about its expression and trophic role during the course of normal development. Recent studies have revealed developmental changes in P2X nucleotide receptors expression, including P2X7 nucleotide receptors [22, 23]. These studies suggest that the P2X nucleotide receptors may play a trophic role in the course of normal development.
The human SH-SY5Y neuroblastoma cell line is a well-established in vitro cell model of neuronal differentiation. Previous studies have shown that SH-SY5Y cells acquire a neuron-like phenotype when treated with differentiating agents such as RA. Upon neuronal differentiation, the SH-SY5Y cells exhibit elongated neuritic processes, cell growth arrest, and expression of neuron-specific markers [3, 5, 24, 25]. It is of importance to note that Larsson et al. [26] recently showed that human SH-SY5Y neuroblastoma cells express functional P2X7 nucleotide receptors. Furthermore, it was recently demonstrated that RA regulates the expression and functionality of P2 nucleotide receptors in rat pheochromocytoma PC-12 cells [27], normal human epidermal keratinocytes (NHEKs) [28], and human cervical epithelial cells [29]. However, no relationship has been established yet among RA treatment, P2X7 nucleotide receptor expression and cell death during neuronal differentiation.
In the present study, we developed an in vitro protocol to investigate RA-induced neuronal differentiation of the human SH-SY5Y neuroblastoma cell line, and the expression and functional responses of P2X7 nucleotide receptor during this process. Our results showed that RA treatment induces a significant down-regulation of P2X7 nucleotide receptors protein expression in SH-SY5Y cells that mediated the protection of RA-differentiated cells from cell death induced by exposure to extracellular nucleotides. In addition, our results indicated that the p38 protein kinase signaling pathway is critically involved in such protection of RA-differentiated cells from nucleotide-induced neuronal death. Taken together, this study is the first to suggest that RA-induced differentiation of neuronal cells has a specific functional impact on P2X7 nucleotide receptors mediating cell death responses.
Materials and methods
Materials
BzATP, 2′, 3′-O-(4-benzoylbenzoyl)-ATP, propidium iodide (PI), all-trans retinoic acid (RA), and the mouse monoclonal β-actin antibody were obtained from Sigma–Aldrich (St Louis, MO, USA). The polyclonal antibodies against cleaved-caspase-3 (Asp175), SAPK/JNK and p38 Thr180/Tyr182, the monoclonal antibodies against SAPK/JNK Thr183/Thy185 and p38, and staurosporine were purchased from Cell Signaling Technology (Beverly, MA, USA). 1-[N,O-bis (5-isoquinolinesulfonyl)-N-methyl-l-tyrosyl]-4-phenylpiperazine (KN-62) was obtained from Calbiochem (San Diego, CA, USA). The polyclonal antibody against human P2X7 nucleotide receptor was obtained from Alomone Labs (Jerusalem, Israel). The polyclonal rabbit anti-growth-associated protein 43 (GAP-43) antibody was obtained from Millipore Corp. (Billerica, MA, USA). The secondary AlexaFluor-488 labeled goat anti-rabbit, AlexaFluor-680 labeled rabbit anti-mouse, and AlexaFluor-680 labeled goat anti-rabbit antibodies were purchased from Invitrogen Corp. (Carlsbad, CA, USA). The IRDye-800 secondary goat anti-mouse and goat anti-rabbit antibodies were obtained from Rockland Immunochemicals (Gilbertsville, PA, USA).
Cell culture
Human SH-SY5Y neuroblastoma cells (American Type Culture Collection, Manassas, VA) were cultured in Dulbecco’s modified Eagle’s/F12 medium (DMEM/F-12) (Invitrogen Corp.) containing 10% (v/v) fetal clone III serum (FCS III) (Invitrogen Corp.), 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were maintained at 37°C in a humidified atmosphere of 5% CO2 and 95% air.
In vitro differentiation of SH-SY5Y cells
Human SH-SY5Y neuroblastoma cells were grown to 80% confluency onto cell culture Petri dishes in DMEM/F-12 medium with 10% FCS III (normal serum content). Then, cells were subjected to medium replacement containing 1% FCS III (low serum content) or 10% FCS III (normal serum content). Both cultures were supplemented with 15 μM RA (Sigma–Aldrich) at the experimental day 0. Chronic RA treatment was performed by replacing the corresponding medium with fresh medium supplemented with 15 μM RA every 2 days. After 5 days of treatment, a homogeneous population of differentiated cells was established that exhibited a neuron-like phenotype determined by neuritic extensions that achieved more than twice the length of the cell body according to Oppenheimer et al. [30]. Therefore, every experimental response tested (RA-induced differentiation morphological changes, cell growth arrest, intracellular-free calcium mobilization measurement, mRNA expression, neuronal cell death, protein kinase signaling, and caspase-3 activation) was determined at experimental day 5. Control experiments were performed in parallel where undifferentiated cells were pretreated with the corresponding medium without RA but containing an equivalent amount of vehicle (DMSO).
Intracellular-free calcium mobilization measurement
Fluorescence experiments with Fura-2 AM were performed following the protocol and analyzed as previously described [31, 32]. In brief, SH-SY5Y cells at a density of 1 × 106 cells/ml were incubated for 30 min with 2 μM acetoxymethyl ester fura-2 (Fura-2 AM) dissolved in DMSO. Then, cells were pelleted by centrifugation and resuspended in 2 ml HBS buffer (15 mM HEPES, pH 7.4, 120 mM NaCl, 4 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 1 mM CaCl2, 1 mM MgCl2, 0.1% (w/v) bovine serum albumin (BSA) (Fisher Scientific, Hampton, NH, USA), and 0.18% (w/v) glucose. The fura-2-loaded cells were transferred to a spectrophotometric cell containing a magnetic stirrer to allow constant sample agitation during fluorescence measurements. Activation of P2 nucleotide receptors was followed using a computer-controlled dual-excitation DeltaScan spectrofluorimeter (Photon Technology International, Newark, NJ, USA) that detects changes in intracellular-free calcium concentration in Fura-2 labeled cells, using an excitation wavelength of 340 and 380 nm, with emission detected at 505 nm. After a 5-min equilibration, an aliquot of agonist was added. The ratio of fluorescence intensities (R = F340nm/F380nm) as a function of time was determined. The maximum (Rmax) and minimum (Rmin) ratios of fluorescence were determined by addition of 100 μL of 5% Triton X-100 and 300 μL of a 100-mM EGTA in 250 mM Tris–HCl (pH 7.4), respectively. Rmax and Rmin values were used to calculate the intracellular-free Ca2+ concentration ([Ca2+]i) from the following equation:
where KD is the dissociation constant for the Ca2+/fura-2 complex (224 nM) and (Sf380 and Sb380) are the fluorescence intensities at 380 nm of Ca2+-free fura-2 and Ca2+-saturated fura-2, respectively.
Western blot analysis
On the experimental day, the cells were washed twice with cold phosphate-buffered saline (PBS) [137 mM NaCl, 3 mM KCl, 10 mM Na2HPO4, and 2 mM KH2PO4] and lysed with 150 μl of lysis buffer [25 mM Tris–HCl, pH 7.4, 25 mM NaCl, 1 mM Na3VO4, 10 mM NaF, 10 mM Na4P2O7, 25 mM β-glycerophosphate, 25 mM p-nitrophenylphosphate, 0.5 mM EDTA, 0.5% (v/v) Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 10 nM okadaic acid]. The cell extracts were centrifuged at 13,000g for 10 min at 4°C to remove insoluble material. Then, 12.5 μl of 3× Laemmli sample buffer [187.5 mM Tris–HCL, pH 6.8, 6% (w/v) sodium dodecyl sulfate (SDS), 1.8% (v/v) β-mercaptoethanol, and 0.003% (w/v) bromophenol blue] was added to 25 μl of supernatant. The mixture was heated for 5 min at 96°C, and 100 μg of cell lysate protein, determined by advanced protein assay reagent (Cytoskeleton Inc., Denver, CO, USA), was subjected to SDS–polyacrylamide gel electrophoresis. Proteins were transferred to nitrocellulose membranes for western blot analysis.
Immunodetection of GAP-43, P2X7 receptor, SAPK/JNK, phospho-SAPK/JNK, p38, phospho-p38 and β-actin on nitrocellulose membranes was performed using a 1:2000 dilution of primary rabbit polyclonal anti-human GAP-43 (Millipore Corp.), rabbit polyclonal P2X7 receptor (Alomone Labs), rabbit polyclonal SAPK/JNK and rabbit polyclonal phospho-p38 (Cell signaling Technology) antibodies, and 1:5000 dilution of primary mouse monoclonal anti-human phospho-SAPK/JNK and mouse monoclonal p38 (Cell signaling Technology) antibodies. Primary mouse monoclonal anti-β-actin antibody (Sigma–Aldrich) was used at a 1:25000 dilution. The specificity of the primary antibody against the P2X7 nucleotide receptor was tested utilizing neutralizing peptide obtained from the antibody manufacturer (Alomone Labs) at a mass ratio 1:1 between peptide and antibody. Furthermore, the functionality of the antibody in western blots was validated assessing the human HL-60 promyelocyte cell line known to expresses endogenous P2X7 nucleotide receptors [33]. Fluorescent IRDye-800 goat anti-rabbit secondary antibody (Rockland Immunochemicals) was used at 1:10000 (GAP-43) and 1:15000 (P2X7) dilutions, 1:20000 (phospho-SAPK/JNK) and 1:10000 (p38) dilutions for IRDye-800 goat anti-mouse antibody (Rockland Immunochemicals), 1:25000 (β-actin) dilution for AlexaFluor-680 labeled rabbit anti-mouse antibody (Invitrogen Corp.) and 1:5000 (SAPK/JNK) and 1:2500 (phospho-p38) dilutions for AlexaFluor-680 labeled goat anti-rabbit antibody (Invitrogen Corp.). Dilutions were prepared in PBS supplemented with 0.1% (v/v) Tween-20 (PBS-T) and Odyssey Blocking Buffer (LICOR Biosciences, Lincoln, NE, USA). Exposure of primary and secondary antibodies to nitrocellulose membranes was performed for 1 h. GAP-43, P2X7 receptor, SAPK/JNK, phospho-SAPK/JNK, p38, phospho-p38, and β-actin proteins were visualized and quantitated using the Odyssey Infrared Imaging System (LICOR Biosciences).
RNA isolation
Total RNA was isolated from treated cells using the Quiagen RNeasy System (Quiagen, Valencia, CA, USA) according to manufacturer’s instructions. RNA integrity was assessed by observing the integrity of 28S and 18S rRNA after ethidium bromide staining of total RNA samples subjected to 1% agarose gel electrophoresis.
Semi-quantitative RT–PCR analysis
RT–PCR analysis was performed with purified total RNA isolated as described above by using a Reverse Transcription System kit (Promega, Madison, WI, USA), following the manufacturer’s instructions with minor modifications. In brief, 4 μg of RNA was reversed transcribed in 20 μl of reverse transcription buffer containing 10 mM MgCl2, 0.5 μg Oligo(dT)15 primer, 1 mM dNTP mixture, and 25 U of avian myeloblastosis virus reverse transcriptase in the presence of recombinant RNasin® ribonuclease inhibitor. The cDNA synthesis was performed in a Bio-Rad Thermal iCycler at 42°C for 60 min followed by incubation at 95°C for 2 min before stopping the cDNA synthesis by cooling at 4°C. The amplification reaction was performed using 1 μM of the following human P2X7 nucleotide receptor primers and indicated amount of cDNA template: P2X7 sense, 5′-ACTCCTAGATCCAGGGATAGCC-3′; P2X7 antisense, 5′-TCACTCTTCGGAAACTCTTTCC-3′ (accession number: Y09561; primers corresponding to 1425–1446 and 1780–1759 nt, 4 μl of cDNA template), as designed by Wiley et al. [34]. Primers and volume of cDNA template for human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were GAPDH sense, 5′-TGTTGCCATCAATGACCCCTT-3′; GAPDH antisense, 5′-CTCCACGACGTACTCAGCG-3′ (accession number: NM002046; primers corresponding to 90–110 and 291–273 nt, 1 μl of cDNA template). GAPDH was used as positive control for the presence and integrity of the cDNA. The conditions for the PCR reaction were as follows: an initial denaturation step at 95°C for 5 min for both cDNA’s; 25 cycles of denaturation at 95°C for 1 min, annealing at 54°C for 1 min and extension at 72°C for 1 min, and a final 10 min extension step at 72°C for P2X7 gene amplification; 30 cycles of denaturation at 95°C for 1 min, annealing at 58°C for 1 min and extension at 72°C for 1 min, and a final 7 min extension step at 72°C for GAPDH gene amplification. Each PCR product was electrophoresed in 1× TAE buffer (40 mM Tris Acetate, 1 mM EDTA, pH 8.0) on 1% (w/v) agarose gel containing 0.6 μg/ml ethidium bromide. A 100-bp DNA ladder (Promega) was used as a marker for cDNA fragment size: P2X7 (356-bp) and GAPDH (200-bp). The bands were visualized by Bio-Rad Versa Doc™ and quantitated by Quantity One© software (Bio-Rad, Philadelphia, PA, USA) in a Macintosh Workstation (Cupertino, CA, USA).
Sulforhodamine B (SRB) colorimetric assay
Human SH-SY5Y neuroblastoma cells were grown onto 96-well plates for 24 h. Then, cells were subjected to the differentiation procedure previously described. The experimental day, cells were fixed in situ for 90 min at 4°C with 50% (w/v) trichloroacetic acid (TCA) to produce a final concentration of 16% (w/v) TCA. Then, cells were washed five times with water and air-dried. Subsequently, cells were stained with a solution of 0.4% (w/v) sulforhodamine B in 1% (v/v) acetic acid for 15 min, washed with 1% (v/v) acetic acid, air-dried, and incubated with 10 mM Tris-base (pH 10.4) for 15 min at room temperature. The results were based on the relative absorbance at 490 nm of the solubilized stain measured on a microplate reader (MRX II; Dynex Technologies, Chantilly, VA).
Confocal immunofluorescence microscopy
Human SH-SY5Y neuroblastoma cells were grown to 80% confluency onto poly d-lysine-coated glass dishes (Mat Tek Corporation, Ashland, MA), following the differentiation procedure previously described. Confocal microscopy was performed as previously described in Cheah et al. [35] with minor modifications. In brief, after treatments, cells were fixed in PBS containing 4% (w/v) paraformaldehyde for 30 min, washed three times with PBS, and further fixed with chilled (−20° C) 100% methanol for 10 min. Thereafter, cells were blocked and permeabilized for 1 h in PBS containing 1% (w/v) bovine serum albumin (PBS/BSA) and 0.3% (v/v) Triton X-100 at room temperature and incubated overnight with rabbit polyclonal anti-human cleaved-caspase-3 antibody at a 1:250 dilution (Cell Signaling Technology). After three washes with PBS/BSA, cells were further blocked with 10% goat serum for 30 min before staining with AlexaFluor-488 goat anti-rabbit antibody at a 1:100 dilution (Invitrogen Corp.) for 2 h in the dark at room temperature. In order to obtain a total cell count, cells were counter stained with propidium iodide (1:500 dilution) for 10 min before the 2 h of secondary antibody incubation was finished. Then, cells were PBS washed, air-dried and mounted for visualization. Images were acquired using a Zeiss LSM-5 Pascal scanning confocal microscope (Thornwood, NY, USA). An argon laser was used for excitation of AlexaFluor 488, and emission was detected using BP 505-530 filter. A helium–neon laser was used for excitation of propidium iodide and emission was detected using LP 560 filter. Final image composites were created using the Zeiss LSM5 PASCAL Image software, version 3.2. Cells were scored without knowledge of their prior treatment to obtain unbiased counting. The percentage of caspase-3 activation was calculated for each field using the ratio of caspase-3 positive cells to the total number of cells in the field.
Trypan blue exclusion assay
Trypan Blue exclusion assay was performed as previously described in Cavaliere et al. [36]. Cell number was determined using a hemocytometer and trypan blue staining. In brief, after treatments, cells were detached and stained for 5 min with 4 μg/ml Trypan Blue in culture medium. Cells were counted with a light microscope. The number of dead cells was determined for each field calculating the difference between the total number of cells counted and the number of viable stained cells.
Statistical analysis
One-way multiple Tukey comparison post-test ANOVA was used for comparison of multiple groups. P < 0.05 between control and experimental groups was considered to be statistically significant. All analyses were performed with InStat software, version 3.06 (GraphPad Software Inc., San Diego, CA, USA).
Results
Differentiation of human SH-SY5Y neuroblastoma cells
Previous studies have demonstrated that RA-induced SH-SY5Y cells show neuronal differentiation characteristics such as cell growth arrest, extension of neuritic processes, and expression of neuron-specific markers (i.e., NSE, neuron-specific enolase; GAP-43, growth-associated protein-43) [3, 5, 24, 25]. Accordingly, the neuronal differentiation procedures have been established using different combinations of serum supplement content (low to normal) in order to enhance the differentiating effects of RA and to establish a homogeneous population of differentiated cells exhibiting a neuron-like phenotype [3, 5, 25]. An initial goal for this study was to establish a homogenous population of differentiated human SH-SY5Y neuroblastoma cells that exhibited elongated neuritic processes and cell growth arrest. Since, SH-SY5Y cells express several phenotypes: neuroblasts, Schwann or melanocytic cells that may be interconvertible in vitro [37], it is essential to determine the optimal concentration of differentiation agent to induce efficient neuronal differentiation. The dose–response pattern of the data, i.e., intracellular calcium mobilization, morphological characterization, and cell viability assay indicated that 15 μM as the most efficient concentration for RA as differentiation agent (data not shown).
Using light microscopic examination, RA-treated SH-SY5Y cells under normal and low serum content were observed for neuronal differentiating characteristics as described above. Our results showed that RA treatment under low serum content promotes morphological changes in SH-SY5Y cells such as long and thin cell bodies as well as an abundant and branched neurites extending more than twice the length of the cell body (Fig. 1). These morphological changes were time dependent and a homogeneous population of SH-SY5Y cells with neuron-like phenotype was established at experimental day 5. In contrast, RA treatment under normal serum content displayed a heterogeneous population of SH-SY5Y cells that exhibited a large number of cells with neurites extending less than twice the length of the cell body and cells that did not exhibit extension of neuritic processes (Fig. 1). Vehicle/serum content was also assessed to investigate whether the vehicle/serum content had affected the extension of neuritic processes. Results showed that neither vehicle/low nor vehicle/high serum content promoted neurite extension in human SH-SY5Y neuroblastoma cells (Fig. 1). Collectively, these results demonstrate that at experimental day 5 RA-treated SH-SY5Y cells under low serum content show morphological characteristics associated with neuronal differentiation.
Fig. 1.
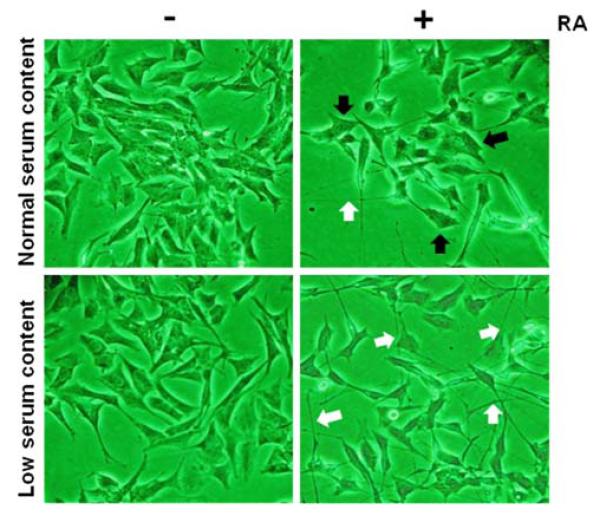
Retinoic acid treatment under normal and low serum content promotes morphological changes in human SH-SY5Y neuroblastoma cells. Human SH-SY5Y neuroblastoma cells were grown to 80% confluency onto cell culture Petri dishes in normal serum. Then, cells were subjected to medium replacement containing normal serum or low serum. Both cultures were supplemented with 15 μM RA as described in “Materials and methods” section. Chronic RA treatment was performed by replacing the corresponding medium with fresh medium supplemented with 15 μM RA every 2 days. On the experimental day 5, cells were photographed under a light microscope at magnification of 20×. Non-RA treated cells (undifferentiated cells) under normal and low serum content served as controls. White arrows represent neurites extending more than twice the length of the cell body and black arrows represent cell bodies without neurite elongation. It is important to note that RA-treated cultures under normal serum content were photographed in a field of low-density growth to capture the heterogeneous neurite outgrowth. Results shown are representative from five independent experiments
In order to quantitate the effects of RA in cell growth, experiments were performed using sulforhodamine B colorimetric assay. RA-treated SH-SY5Y cell cultures under low and normal serum content showed a significant cell growth arrest (Fig. 2). However, cells maintained in normal serum content produced cell overgrowth in the cultures in comparison to control cell cultures at experimental day 0. It is important that at all condition assessed, cell growth was never significantly below that of control cells prior to initiation of treatments (day 0). These results suggest that RA treatment did not cause cell death, but promoted cell growth arrest. In agreement with previous reports [5], our results revealed that RA treatment induces cell growth arrest of SH-SY5Y cells in comparison to vehicle/serum content.
Fig. 2.
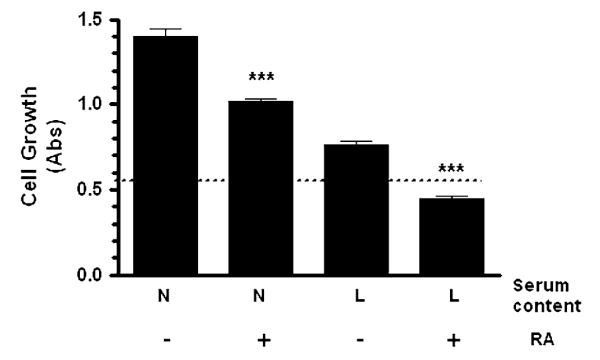
RA treatment promotes cell growth arrest in human SH-SY5Y neuroblastoma cells. Human SH-SY5Y neuroblastoma cells were grown onto 96-well plates in normal serum for 24 h. Then, cells were subjected to medium replacement containing N: normal serum or L: low serum. Both cultures were supplemented with 15 μM RA as described in “Materials and methods” section. Chronic RA treatment was performed by replacing the corresponding medium with fresh medium supplemented with 15 μM RA every 2 days. On the experimental day 5, SRB assay was performed as described in “Materials and methods” section. Non-RA treated cells (undifferentiated cells) served as controls. Dashed line represents the cell growth at experimental day 0 (n = 24; 0.53 ± 0.02). Data are the mean ± SEM (n = 24) of values from four independent experiments. RA-treated cells were compared to parallel cultures without RA treatment in the corresponding medium (*** P < 0.001 one-way ANOVA)
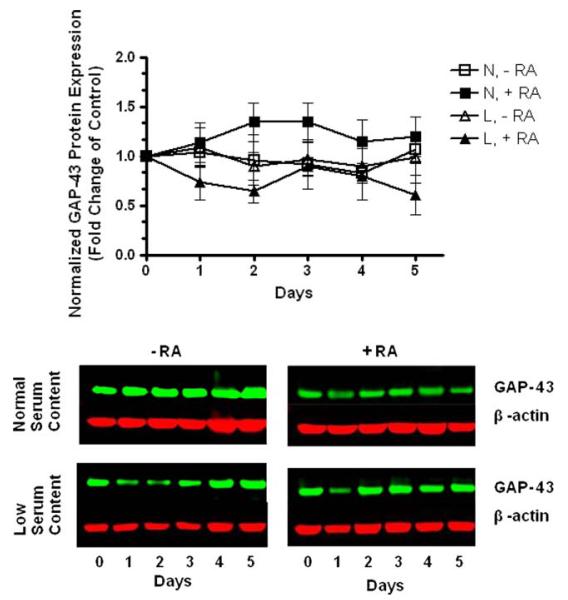
In order to further characterize the differentiation of SH-SY5Y cells induced by RA treatment, GAP-43 protein levels were also assessed since it is the most abundant neuron-specific protein in the grown cones of neurons [25]. Our results indicated that GAP-43 was abundantly present, even before RA-induced differentiation and there was the little change in the expression of GAP-43 over the period of RA treatment under both normal and low serum content from experimental day 0 to day 5 (Fig. 3). Our results are similar to results reported by Encinas et al. that demonstrated the expression of neuron-specific markers in SH-SY5Y cells even without exposure to differentiating agent treatment [3].
Fig. 3.
Human SH-SY5Y neuroblastoma cells express the neuron-specific marker GAP-43. Human SH-SY5Y neuroblastoma cells were grown to 80% confluency onto cell culture Petri dishes in normal serum. Then, cells were subjected to medium replacement containing N: normal serum or L: low serum. Both cultures were supplemented with 15 μM RA as described in “Materials and methods” section. Chronic RA treatment was performed by replacing the corresponding medium with fresh medium supplemented with 15 μM RA every 2 days. On the corresponding experimental day, cells were lysed and the GAP-43 protein levels were determined by western blot analysis of whole-cell lysates. Non-RA treated cells (undifferentiated cells) at experimental day 0 served as controls. GAP-43 expression was normalized to β-actin protein. Data are the means ± SEM of values from three independent experiments. There was no significant change in the expression of GAP-43 protein over the period and conditions assessed (P > 0.05 one-way ANOVA). Representative gels are shown
In summary, RA treatment under normal serum content promoted culture overgrowth and a large number of cells with neurites extending less than twice the length of the cell body and cells that did not exhibit extension of neuritic processes. These results support previous studies suggesting that cell cultures maintained in low serum medium conditions have the advantage over serum-supplemented cultures that those conditions reduce experimental variations associated with heterogeneous populations of differentiated cells [3, 5]. Thus, RA treatment under low serum content produced a homogeneous population of SH-SY5Y cells that exhibited significant morphological changes and cell growth arrest, and based on these results we identified RA treatment under low serum content as optimal conditions for neuronal differentiation of SH-SY5Y in vitro cell model in our hands.
BzATP-promoted intracellular-free calcium mobilization in SH-SY5Y cells is abolished by RA treatment
In order to investigate the functional impact of neuronal differentiation on P2 nucleotide receptors coupled to activation of Ca2+ channels, we performed intracellular-free calcium mobilization studies. In order to evoke activation of P2X7 nucleotide receptors, we used 2′,3′-O-(4-benzoylbenzoyl)-ATP (BzATP), a potent agonist of the receptor [12, 18, 38]. The treatment of undifferentiated SH-SY5Y cells (vehicle under low serum content) with 300 μM BzATP resulted in significant intracellular-free calcium mobilization (Fig. 4). In order to address the question of whether neuronal differentiation prevents intracellular-free calcium mobilization, RA-differentiated SH-SY5Y cells (low serum) were also examined with 300 μM of BzATP in parallel experiments. Our results indicate that differentiating RA treatment abolishes intracellular-free calcium mobilization by BzATP (Fig. 4). In order to establish that the observed intracellular-free calcium mobilization of undifferentiated cells was dependent on P2X7 nucleotide receptor, we examined the effect of 1-[N,O-bis(5-isoquinolinesulfonyl)-N-methyl-l-tyrosyl]-4-phenylpiperazine (KN-62), a widely used selective P2X7 nucleotide receptor antagonist [12, 39, 40] and also known as a CAM-kinase-II inhibitor [16]. Accordingly, undifferentiated cells were pretreated with 0.5 μM KN-62 for 20 min followed by exposure to 300 μM BzATP. Our results showed that P2X7 nucleotide receptor blockage abolishes intracellular-free calcium mobilization (Fig. 4). Such results were specific for KN-62 since non-selective P2 receptors antagonist suramin did not have an effect on BzATP-induced intracellular-free calcium mobilization (data not shown). Also, RA-differentiated cells pretreated with 0.5 μM KN-62 followed by 300 μM BzATP did not evoke intracellular-free calcium mobilization (Fig. 4) and were comparable to the effects of RA treatment alone. Furthermore, we also used Uridine 5′ - triphosphate (UTP), a potent P2Y nucleotide receptor agonist [8]. Importantly, our results indicate that UTP did not evoke intracellular-free calcium mobilization of undifferentiated SH-SY5Y cells (data not shown). In line with the findings of Larsson et al. [26], our results suggest that the BzATP-promoted responses are principally mediated by P2X7 nucleotide receptors [26].
Fig. 4.
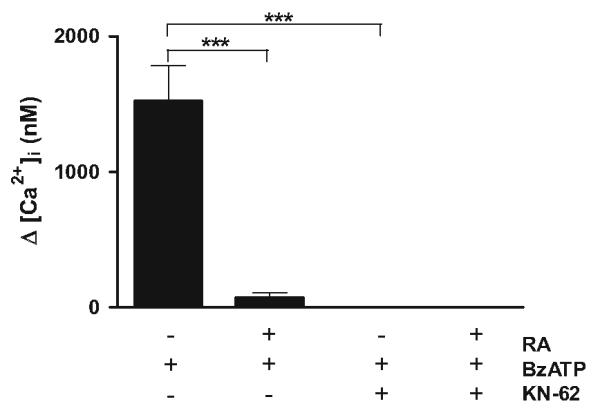
BzATP-promoted intracellular-free calcium mobilization in SH-SY5Y cells is abolished by RA treatment. Human SH-SY5Y neuroblastoma cells were grown to 80% confluency onto cell culture Petri dishes in normal serum. Then, cells were subjected to medium replacement containing low serum and supplemented with 15 μM RA as described in “Materials and methods” section. Chronic RA treatment was performed by replacing the medium with fresh medium supplemented with 15 μM RA every 2 days. On the experimental day 5, fura-2-loaded cells (2.0 × 106 cells/ml) were pretreated with 0.5 μM KN-62 for 20 min. Then, cells were challenged with 300 μM BzATP followed by intracellular-free calcium mobilization measurement as described in “Materials and methods” section. Data are presented as intracellular calcium mobilization concentration (nM). Data are the means ± SEM of values from three independent experiments (*** P < 0.001 one-way ANOVA)
P2X7 nucleotide receptor mRNA expression in SH-SY5Y cells is not regulated by RA treatment
Previous reports have described a regulated expression of P2X nucleotide receptors during differentiation, including the P2X7 nucleotide receptor [23, 41]. Based on those findings, our previous functional results and reports indicating that RA has been implicated in the regulation of P2 nucleotide receptors functionality and expression [27–29], we tested the possibility that P2X7 nucleotide receptor mRNA expression is regulated by RA treatment under differentiating conditions (low serum content) in SH-SY5Y cells.
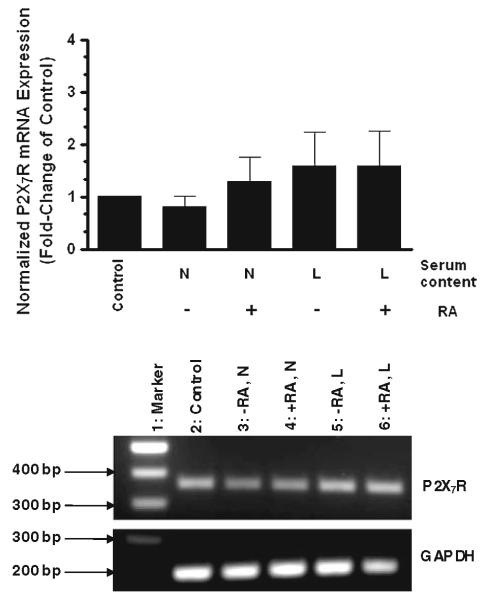
RT–PCR analysis revealed that the steady-state levels of P2X7 nucleotide receptor mRNA did not change by RA treatment under non-differentiating (normal serum) or differentiating (low serum) conditions (Fig. 5, lanes 4 and 6, respectively). Undifferentiated cells (vehicle) under normal and low serum content were also evaluated (Fig. 5, lanes 3 and 5, respectively). Cells at experimental day 0 served as control (Fig. 5, lane 2). These results showed that P2X7 nucleotide receptor was transcriptionally active in human SH-SY5Y neuroblastoma cells under all the conditions evaluated. Thus, the in vitro differentiation procedure established for SH-SY5Y cells did not induce significant changes in the mRNA expression of P2X7 nucleotide receptor suggesting that its transcription was not regulated by RA treatment.
Fig. 5.
mRNA expression of P2X7 nucleotide receptors is not regulated by retinoic acid treatment. Human SH-SY5Y neuroblastoma cells were grown to 80% confluency onto cell culture Petri dishes in normal serum. Then, cells were subjected to medium replacement containing N: normal serum or L: low serum. Both cultures were supplemented with 15 μM RA as described in “Materials and methods” section. Chronic RA treatment was performed by replacing the corresponding medium with fresh medium supplemented with 15 μM RA every 2 days. On the experimental day 5, mRNA levels of P2X7 nucleotide receptors were determined by semi-quantitative RT–PCR analysis as described in “Materials and methods” section. P2X7 nucleotide receptors mRNA expression was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Non-RA treated cells (undifferentiated cells) served as controls. For reference, the first bar on graph and lane 2 on gel represent P2X7 nucleotide receptors mRNA expression at the experimental day 0 (control). Data are the means ± SEM of values of four independent experiments. Representative gels are shown
P2X7 nucleotide receptor protein expression is regulated in RA-differentiated SH-SY5Y cells
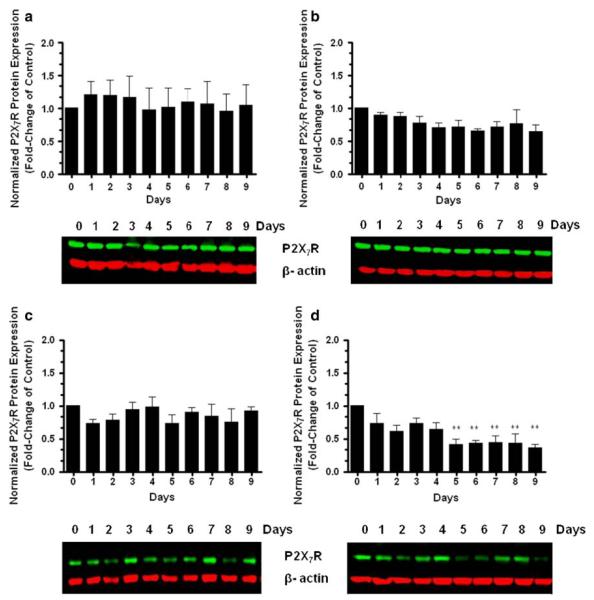
Previous studies have reported the regulation of P2X7 nucleotide receptor protein expression during human blood monocyte differentiation in the absence of detectable differences in P2X7 nucleotide receptor mRNA [41], suggesting that the differentiation process triggers post-transcriptional mechanisms for the regulation of P2X7 receptors. Based on such findings and our previous results, we decided to investigate and correlate the level of P2X7 nucleotide receptor protein expression with the differentiation state of SH-SY5Y cells. Accordingly, RA-treated SH-SY5Y cells were cultured under differentiating (low serum) and non-differentiating (normal serum) conditions during 9 days. Each day, cultures were analyzed to measure the quantitative change in P2X7 nucleotide receptor protein levels. Western blot analysis revealed that the protein levels of P2X7 nucleotide receptor did not change significantly after RA treatment under non-differentiating (normal serum) conditions throughout the 9-day assessment period (Fig. 6b). In contrast, cells exposed to RA treatment under differentiating (low serum) conditions displayed significant decreases in the protein levels of P2X7 nucleotide receptor after day 5 of treatment (Fig. 6d). Undifferentiated cells were also examined to investigate whether vehicle/serum content had affected the P2X7 nucleotide receptor expression changes observed in RA-differentiated cells. Our results indicate that the protein levels of P2X7 nucleotide receptor did not change significantly in undifferentiated cells during treatment under normal (Fig. 6a) or low (Fig. 6c) serum content conditions. These results may suggest that RA-induced neuronal differentiation in human SH-SY5Y neuroblastoma cells triggers post-transcriptional mechanisms to regulate P2X7 nucleotide receptor protein expression analogous to the differentiation of human blood monocytes [41]. The specificity of P2X7 nucleotide receptor antibody was confirmed using both HL-60 that expresses endogenous P2X7 nucleotide receptor [33] and SH-SY5Y cell lines in the presence and absence of a competitive peptide antigen (data not shown).
Fig. 6.
P2X7 nucleotide receptor protein expression is regulated in RA-differentiated SH-SY5Y cells. Human SH-SY5Y neuroblastoma cells were grown to 80% confluency onto cell culture Petri dishes in normal serum. Then, cells were subjected to medium replacement containing b normal serum or d low serum. Both cultures were supplemented with 15 μM RA as described in “Materials and methods” section. Chronic RA treatment was performed by replacing the corresponding medium with fresh medium supplemented with 15 μM RA every 2 days. On the corresponding experimental day, cells were lysed and the P2X7 nucleotide receptors protein levels were determined by western blot analysis of whole-cell lysates. Non-RA treated cells (undifferentiated cells) under a normal and c low serum content served as controls. P2X7 nucleotide receptor protein expression was normalized to β-actin protein. Data are the means ± SEM of values from four independent experiments (** P < 0.01 one-way ANOVA). Representative gels are shown
These data demonstrate that RA treatment under differentiating (low serum) conditions promotes significant down-regulation of P2X7 nucleotide receptor protein levels concurrently with the establishment of a homogenous population of differentiated human SH-SY5Y neuroblastoma cells to a neuron-like phenotype. These results suggest an important link between neuronal differentiation and P2X7 nucleotide receptor protein expression. Therefore, these experimental conditions were chosen in all further experiments to examine P2X7 nucleotide receptor functionality (i.e., neuronal cell death, protein kinase signaling, and caspase-3 activation) in RA-induced SH-SY5Y cells.
Inhibition of neuronal cell death by RA treatment (differentiating conditions)
Activated P2X7 nucleotide receptor has been mainly implicated in cell death responses under pathological conditions in the CNS [16]. In order to investigate the functional impact of the down-regulated expression of P2X7 nucleotide receptor after neuronal differentiation on cell death, we performed a trypan blue exclusion assay. In order to evoke activation of P2X7 nucleotide receptors, we used BzATP. A 48-h treatment of undifferentiated SH-SY5Y cells with 300 μM BzATP resulted in significant cell death (Fig. 7, column 3), comparable to the effects of 4 μM staurosporine for 2 h (dashed line on graph), a well-known inducer of predominantly apoptotic cell death [42, 43]. In order to address the question of whether the down-regulation of P2X7 nucleotide receptor protein prevents cell death, RA-differentiated SH-SY5Y cells were also examined with 300 μM of BzATP in parallel experiments. Importantly, our results indicate that differentiating RA treatment prevents cell death by P2X7 nucleotide receptor agonist (Fig. 7, column 4). In order to establish that the observed cell death of undifferentiated cells was dependent on P2X7 nucleotide receptor, we examined the effect of the selective P2X7 nucleotide receptor antagonist KN-62 on the cell death. Cultures were pretreated with 0.5 μM KN-62 for 20 min followed by exposure to 300 μM BzATP for 48 h. Our results showed that P2X7 nucleotide receptor blockage protects undifferentiated cells from cell death (Fig. 7, column 5). Also, RA-differentiated cells pretreated with 0.5 μM KN-62 followed by 300 μM BzATP for 48 h did not undergo increased cell death (Fig. 7, column 6) and were comparable to the effects of RA treatment alone. Therefore, these results demonstrate that regulation of P2X7 nucleotide receptor functionality by either direct blockage with the antagonist KN-62 or down-regulation of the P2X7 nucleotide receptor protein expression by differentiating RA treatment prevented the cell death responses evoked by the activation of this receptor in SH-SY5Y cells.
Fig. 7.
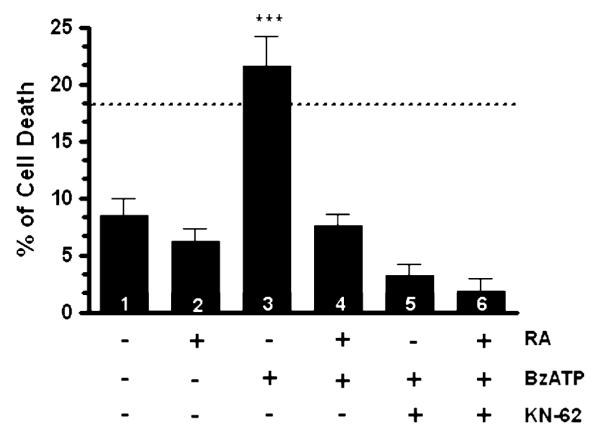
RA treatment (differentiating conditions) protects differentiated cells from neuronal cell death. Human SH-SY5Y neuroblastoma cells were grown to 80% confluency onto cell culture Petri dishes in normal serum. Then, cells were subjected to medium replacement containing low serum and supplemented with 15 μM RA as described in “Materials and methods” section. Chronic RA treatment was performed by replacing the medium with fresh medium supplemented with 15 μM RA every 2 days. On the experimental day 5, cells were pretreated with 0.5 μM KN-62 for 20 min followed by 300 μM BzATP for 48 h. After treatments, trypan blue exclusion assay was performed as described in “Materials and methods” section. Non-RA treated cells (undifferentiated cells) and RA-treated cells (differentiated cells) without additional treatments served as controls. Dashed line represents cultured cells treated with 4 μM staurosporine for 2 h as positive control (n = 15; 18.2 ± 2.6). Data are the means ± SEM of values from at least three independent experiments. BzATP- and KN-62-treated cells were compared to non-RA treated cells (undifferentiated cells) and RA-treated cells (differentiated cells) without additional treatments (*** P < 0.001 one-way ANOVA)
Inhibition of caspase-3 activation by RA treatment (differentiating conditions)
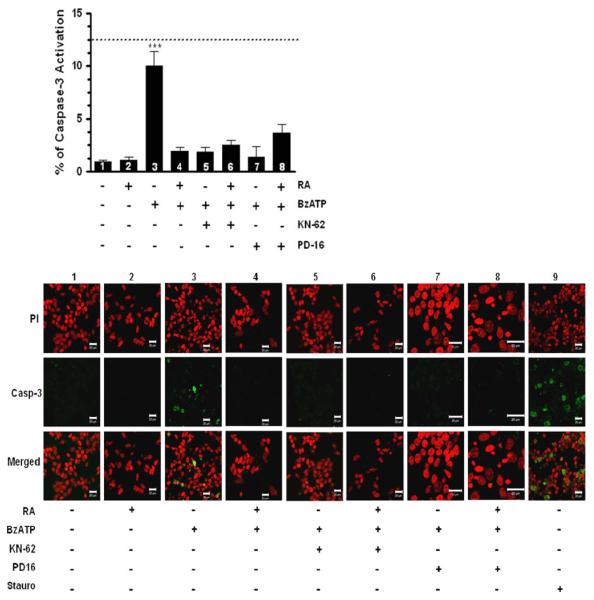
It has been well-documented that activation of the executioner protease caspase-3 is an important biochemical hallmark of apoptotic cell death [35, 44, 45]. Previous studies have demonstrated that caspase-3 activation is observed in nucleotide-induced neuronal apoptotic cell death through P2X7 nucleotide receptors [45]. Accordingly, we examined caspase-3 activation using an immunofluorescence confocal microscopy assay. A 48-h treatment of undifferentiated SH-SY5Y cells with 300 μM BzATP resulted in significant caspase-3 activation (Fig. 8, column 3), comparable to the effects of staurosporine (Fig. 8, dashed line on graph and column 9). In order to address the question of whether the down-regulation of P2X7 nucleotide receptor protein prevents caspase-3 activation, RA-differentiated SH-SY5Y cells were also examined after treatment with 300 μM BzATP in parallel experiments. Significantly, we found that differentiating RA treatment prevented caspase-3 activation by P2X7 nucleotide receptor agonist (Fig. 8, column 4). It is important to mention that the caspase-3 activation is not dependent on activation of uridine sensitive P2Y nucleotide receptors (data not shown). In order to establish that the observed caspase-3 activation of undifferentiated cells was dependent on P2X7 nucleotide receptor, cultures were pretreated with 0.5 μM KN-62 for 20 min prior to exposure to 300 μM BzATP for 48 h. Our results revealed that P2X7 nucleotide receptor blockage with KN-62 protected undifferentiated cells from caspase-3 activation (Fig. 8, column 5). Furthermore, RA-differentiated cells treated with 0.5 μM KN-62 followed by 300 μM BzATP for 48 h did not activate caspase-3 (Fig. 8, column 6), similar to the effects of RA treatment alone. These results are in agreement with the observed protection of neuronal cell death depicted in Fig. 7 and establish that regulation of P2X7 nucleotide receptor functionality by either direct KN-62-blockage or down-regulation of the P2X7 nucleotide receptor protein expression by differentiating RA treatment prevents caspase-3 activation evoked by extracellular nucleotides. In addition, our results suggest that caspase-3 might play a role in P2X7 nucleotide receptor-induced cell death of undifferentiated SH-SY5Y cells.
Fig. 8.
RA treatment (differentiating conditions) abolishes caspase-3 activation in human SH-SY5Y neuroblastoma cells. Human SH-SY5Y neuroblastoma cells were grown to 80% confluency onto poly d-lysine-coated glass dishes in normal serum. Then, cells were subjected to medium replacement containing low serum and supplemented with 15 μM RA as described in “Materials and methods” section. Chronic RA treatment was performed by replacing the medium with fresh medium supplemented with 15 μM RA every 2 days. On the experimental day 5, cells were pretreated with 0.5 μM KN-62 for 20 min followed by 300 μM BzATP for 48 h. Also, some cultures were pretreated with 10 μM PD169316 (PD16) for 10 min followed by 300 μM BzATP for 48 h. After treatments, confocal immunofluorescence microscopy assays were performed as described in “Materials and methods” section. Non-RA treated cells (undifferentiated cells) and RA-treated cells (differentiated cells) without additional treatments served as controls. Dashed line represents cultured cells treated with 4 μM staurosporine for 2 h as positive control (n = 15; 12.4 ± 1.4). All counts were performed using a range of 53–1882 cells from different fields. Data are the means ± SEM of values from at least three independent experiments. BzATP- and KN-62-treated cells were compared to non-RA treated cells (undifferentiated cells) and RA-treated cells (differentiated cells) without additional treatments (*** P < 0.001 one-way ANOVA). Representative images are shown, scale bar = 20 μm. (PI: propidium iodide; Casp-3: cleaved-caspase-3)
P2X7 nucleotide receptor activates caspase-3 via p38
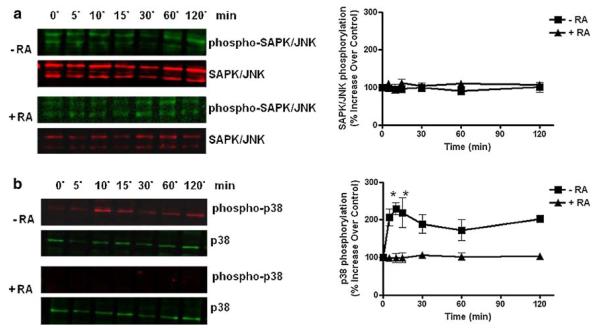
P2 nucleotide receptors are known to activate signaling protein kinases cascades involving mitogen-activated protein kinases (MAPKs) that regulate diverse trophic responses [46, 47]. Among the MAP kinases, activation of p38 and SAPK/JNK signaling pathways downstream of P2X7 nucleotide receptors have been suggested to play key roles in promoting apoptotic cell death [45, 48, 49]. In order to determine the molecular mechanisms underlying the cell death responses evoked by P2X7 nucleotide receptor in undifferentiated cells, the activation of these SAPK/JNK and p38 protein kinases was examined. Results showed that the SAPK/JNK signaling pathway was not activated by P2X7 nucleotide receptor neither in differentiated cells nor undifferentiated cells (Fig. 9a). On the other hand, undifferentiated cells that express P2X7 nucleotide receptors induced p38 phosphorylation in a time-dependent manner upon exposure to extracellular nucleotides (Fig. 9b). Nucleotide-induced p38 activity was sustained between 10 and 15 min but began to decline gradually after 30 min of P2X7 nucleotide receptor activation. Importantly, we showed that differentiating RA treatment abolished the phosphorylation of p38 promoted by P2X7 nucleotide receptor agonist (Fig. 9b).
Fig. 9.
RA treatment (differentiating conditions) abolishes MAPK activation in human SH-SY5Y neuroblastoma cells. Human SH-SY5Y neuroblastoma cells were grown to 80% confluency onto cell culture Petri dishes in normal serum. Then, cells were subjected to medium replacement containing low serum and supplemented with 15 μM RA as described in “Materials and methods” section. Chronic RA treatment was performed by replacing the medium with fresh medium supplemented with 15 μM RA every 2 days. On the experimental day 5, cultures were treated with 300 μM BzATP for the indicated times before analysis. Cells were lysed and a SAPK/JNK and b p38 phosphorylation was determined by western blot analysis of whole-cell lysates. Non-RA treated cells (undifferentiated cells) served as controls. Data are the mean ± SEM of values from three independent experiments. RA-treated cells were compared to parallel cultures without RA treatment (* P < 0.05 one-way ANOVA). Representative gels are shown
In order to establish a role of p38 in the nucleotide-cell death responses observed in undifferentiated cells, we used the specific p38 inhibitor PD169316. Accordingly, cultures were pretreated with 10 μM PD169316 for 10 min followed by 300 μM BzATP for 48 h. Remarkably, the presence of the p38 inhibitor caused a significant reduction of BzATP-induced caspase-3 activation in undifferentiated cells (Fig. 8, column 7), even though these cells express P2X7 nucleotide receptors. In parallel control experiments, RA-differentiated cells were pretreated with 10 μM PD169316 followed by 300 μM BzATP for 48 h, and activation of caspase-3 was not detected (Fig. 8, column 8). Collectively, our results strongly suggest that activation of p38 through P2X7 nucleotide receptors is a critical molecular regulator that induces caspase-3 activation as a possible cell death mechanism of undifferentiated SH-SY5Y cells, and RA treatment abolishes p38 activation protecting differentiated cells from cell death responses.
Discussion
The proper development and maintenance of the CNS depend on a regulated homeostasis among cell proliferation, death, and differentiation. Malfunction in any of these processes may contribute to pathological disorders such as cancer and neurodegenerative diseases [1, 30, 50]. In the nervous system, P2 nucleotide receptors (activated by extracellular nucleotides) have been shown to play a trophic role regulating these biological processes in both glial cells and neurons [47]. RA, a well-documented differentiating agent both in vivo [51] and in vitro [30], has been demonstrated to promote regulated expression of P2 nucleotide receptors during differentiation [23]. Among the P2 nucleotide receptors, the P2X7 nucleotide receptor has been mainly identified as a mediator of cell death responses under pathological conditions in the CNS [16], but little is known about the possible RA-mediated regulation of the expression of P2X7 nucleotide receptor and the trophic effects of this phenomenon during neuronal differentiation. The present study has clearly demonstrated that neuronal cell death diminishes when protein expression of the P2X7 nucleotide receptors decreases after RA-induced neuronal differentiation. Importantly, we included in all the experiments presented in this report several control experiments with vehicle and the corresponding serum content (undifferentiated cells) since serum content itself, especially low serum, has been reported to promote differentiation [52] and even cell death responses in some cells [44, 53]. In all tested assays in this study, neither vehicle nor serum content by itself affected the cell responses of P2X7 nucleotide receptor. Therefore, we conclude that RA is the agent primarily responsible for the modulation of the effects dependent on P2X7 nucleotide receptor studied in this article and that the p38 protein kinase signaling pathway is critically involved in such protection of RA-differentiated cells from nucleotide-induced neuronal death.
In the present study, we developed a simple protocol to investigate RA-induced neuronal differentiation in vitro, and the expression and functional responses of P2X7 nucleotide receptors at the cellular level using one of the well-established in vitro cell model of neuronal differentiation. Due to the complexity of the nervous system, the use of in vitro models of differentiated cells has become a widespread and convenient practice to examine specific molecular and biochemical mechanisms at a cellular level. Thus, complex developmental processes can be studied in a simplified environment using in vitro cell models [23] such as SH-SY5Y cell line. Consequently, we established a homogenous population of differentiated cells based on chronic exposure of human SH-SY5Y neuroblastoma cells to 15 μM RA. The differentiating effect of RA was enhanced under low serum content as evidenced by cell growth arrest (Fig. 2) and a neuronal phenotype with abundant and branched neurites extending more than twice the length of the cell body (Fig. 1). These observations were in accord with previous reports by others [3, 5, 24, 25]. Therefore, we demonstrated that RA treatment under low serum content provided optimal conditions for significant neuronal differentiation of SH-SY5Y in vitro in our cell model system.
It is recognized that the pharmacological discrimination between P2X receptors and their functionality depends on coupling to intracellular calcium concentration ([Ca2+]i) triggered by receptor’s agonists and antagonists. In agreement with others [26], we obtained a strong BzATP-induced intracellular Ca2+ signal in undifferentiated SH-SY5Y cells (Fig. 4). Importantly, in RA-differentiated cells BzATP-induced Ca2+ influx was significantly lower comparable to the effects of KN-62 in both undifferentiated and RA-differentiated SH-SY5Y cells (Fig. 4). BzATP is not a selective agonist for P2X7 nucleotide receptors since can bind P2X1, P2X2, and P2X3 nucleotide receptors [16, 18]. However, KN-62, an inhibitor of Ca2+calmodulin II kinase [16], has been recognized as selective antagonist at human P2X7 nucleotide receptors by many [12, 16, 39, 40]. Taking this information into account including the pharmacological properties of P2 nucleotide receptors agonists (BzATP and UTP) and antagonists (KN-62 and suramin), our results suggest that the BzATP-promoted responses along neuronal differentiation are principally mediated by P2X7 nucleotide receptors.
Using the SH-SY5Y cell line as in vitro model for neuronal differentiation, we then found a significant down-regulation of the expression of P2X7 nucleotide receptor at the protein level (Fig. 6d), but to our surprise the mRNA steady-state levels of P2X7 nucleotide receptor were not regulated by RA treatment (Fig. 5). It is known that RA can directly and/or indirectly affect gene expressions [54]. Direct effects result from modulation of the transcriptional activity of direct targets while indirect effects, which reflect the actions of intermediate transcription factors, non-classical associations of receptors with other proteins, or even more distant mechanisms [54]. Our results demonstrated the post-transcriptional control of P2X7 nucleotide receptor in RA-differentiated SH-SY5Y cells, supporting recent studies in RA-differentiated Neuro-2a neuroblastoma cells that have shown that RA decreases the protein expression of P2X7 nucleotide receptors [55]. Moreover, it has been found that RA increases EGF receptor expression in epithelial cells [56] and that EGF enhances the epinephrine-induced decrease of P2X7 nucleotide receptor protein expression in cervical epithelial cells [57]. Based on our results demonstrating RA-induced decrease of P2X7 nucleotide receptor protein expression and previous studies indicating EGF receptor protein expression in human SH-SY5Y neuroblastoma cell line [58], it is tempting to speculate that RA increases EGF receptor expression leading to a down-regulation of P2X7 nucleotide receptor protein expression in our in vitro cell model of neuronal differentiation. Although we do not established a mechanism of RA-induced down-regulation of P2X7 nucleotide receptors, the current expression data can be readily compared with or supplemented with in vivo expression data that have shown regulated expression of P2X7 nucleotide receptors during in vivo neuronal development [22, 59]. Thus, RA triggers a novel mechanism to control P2X7 nucleotide receptors expression in an indirect fashion at a post-transcriptional level and clarification of this mechanism requires further investigation.
Recently, Oppenheimer et al. showed that RA-induced differentiation of neuroblastic tumor cell lines (i.e., SH-SY5Y cell line) regulates the expression of genes related to play a role in neurogenesis and differentiation [30]. It is important to note that the down-regulated expression of P2X7 nucleotide receptors was associated to the differentiation state of our in vitro SH-SY5Y cell model since the cells exposed to RA under non-differentiating conditions (normal serum), as well as those exposed to vehicle/serum content without RA (undifferentiated cells), showed no significant down-regulation of P2X7 nucleotide receptor protein. These results may lend support to previous in vivo studies by others that have shown a regulated expression of subtypes of P2X nucleotide receptors during a specific stage in the rat brain development [22]. The authors have suggested that the regulated expression of P2X receptor subtypes is related to their trophic role during a specific stage in the development of the nervous system [22]. Post-transcriptional control of P2X7 nucleotide receptor expression was observed in RA-differentiated cells, analogous to what happens in interferon γ-differentiated human blood monocytes [41]. The regulation may represent an adaptive mechanism to prevent biological responses associated to the P2X7 nucleotide receptor. Activation of P2X7 nucleotide receptors has been implicated in the activation of protease-based signaling cascades (i.e., caspases-1, 8, 9, 3) which are not readily reversible [41, 45, 60, 61]. Thus, the expression of P2X7 nucleotide receptor must be rigorously regulated to prevent apoptosis-related cell death after neuronal differentiation.
Apoptotic cell death has been recognized as the major mechanism for cell death in the nervous system [1, 3, 45]. This study clearly shows that regulation of the P2X7 nucleotide receptor protein expression by RA treatment may modulate cell death responses of differentiated cells. Undifferentiated cells express P2X7 nucleotide receptor protein, promote intracellular-free calcium mobilization (Fig. 4) following agonist stimulation, and undergo cell death (Fig. 7) presenting significant increases of caspase-3 activation (Fig. 8). On the other hand, RA-differentiated cells showed the down-regulation of P2X7 nucleotide receptor protein, thus abolishing intracellular-free calcium mobilization (Fig. 4) and preventing cell death (Fig. 7) and caspase-3 activation (Fig. 8) when exposed to extracellular nucleotide. It is well known that the activation of P2X7 nucleotide receptors by extracellular nucleotides promotes the accumulation of calcium ions in the cytoplasm due to the channel/pore-forming property and the subsequent activation of voltage-gated calcium channels following the membrane depolarization [16, 17, 62]. Consequently, excessive influx of Ca2+ triggers a variety of intracellular-free neurotoxic mechanisms such as mitochondrial Ca2+ overload and free radical formation, which finally lead to cell death [64, 65]. These findings suggest that cell death responses induced by P2X7 nucleotide receptor activation can be mediated by intracellular-free Ca2+ accumulation. Taken all together, our results indicate that intracellular-free calcium mobilization and caspase-3 activation are concurrent events that might play a role in P2X7 nucleotide receptor-induced cell death of undifferentiated SH-SY5Y cells. Similar apoptotic cell death of rat primary cortical neurons has been reported to be mediated by P2X7 nucleotide receptor via a caspase-3 activation pathway [45].
The involvement of P2X7 nucleotide receptor in neuronal death was established by antagonizing the receptor with KN-62 prior to its activation. Our results showed that the P2X7 nucleotide receptor blockage abolished intracellular-free calcium mobilization (Fig. 4) and protected undifferentiated cells from cell death (Fig. 7) and caspase-3 activation (Fig. 8), comparable to the effects of RA-differentiated cells. These results implicate the P2X7 nucleotide receptor as a major player in the RA-evoked protection of SH-SY5Y cells from cell death responses. Furthermore, undifferentiated cells that express P2X7 nucleotide receptors induced p38 phosphorylation (Fig. 9) and a selective inhibitor of p38 protected undifferentiated cells from P2X7 receptor-induced caspase-3 activation. These results demonstrate that activation of the p38 signaling pathway downstream of P2X7 nucleotide receptor is a critical molecular regulator that induces caspase-3 activation as a possible cell death mechanism in undifferentiated cells. Most importantly, RA treatment abolished p38 activation thus protecting differentiated SH-SY5Y cells from death responses involving caspase-3 activation. These results support previous studies in human THP-1 monocytic cells demonstrating that functional responses of P2X7 nucleotide receptors depend on the activation of p38 [63]. Altogether, our data suggest an important link among the state of differentiation, P2X7 nucleotide receptor protein expression, and cell death responses.
P2X7 nucleotide receptor-mediated cell death may be a physiologically important event during the course of normal neuronal development. Accordingly, previous studies have shown that P2X7 nucleotide receptors activated by endogenous extracellular ATP trigger the death of retinal cholinergic neurons during normal development [59]. These outcomes were shown to be important for controlling the total number, the local density, and the regular spacing of neurons for the proper formation of synaptic connections [59]. It should also be noted that the role of P2X7 nucleotide receptor during differentiation has been previously suggested [66]. Recent studies using neural progenitor cells (NPCs) from the adult rat hippocampus have demonstrated that NPCs express functional P2X7 nucleotide receptors before any other cell surface receptor during NPCs differentiation [66]. Collectively, these observations suggest an emerging significant trophic role of P2X7 nucleotide receptors in the course of normal development of the nervous system.
Neurite outgrowth and the subsequent neuronal polarization that lead to the formation of the axon and dendrites establishing proper synaptic connections are required events during normal development of the nervous system [67]. Hence, the regulated expression of P2X7 nucleotide receptors found in our study could represent an adaptive mechanism of biological systems to maintain a regulated homeostasis and to modulate the trophic role of this receptor during the course of normal development. Similarly, recent studies have shown that inhibition of P2X7 nucleotide receptors promotes axonal branching and elongation in cultured hippocampal neurons [68]. Based on our results and previous findings, it may be suggested that P2X7 nucleotide receptors negatively modulate the process of establishing synaptic connections.
In conclusion, we identified RA as an important modulator of P2X7 nucleotide receptor protein expression during neuronal differentiation. RA-induced down-regulation of P2X7 nucleotide receptor protein expression was shown to be a concurrent event with the protection of differentiated cells from neuronal death induced by P2X7 nucleotide receptor activation. Also important, we identified p38 signaling pathway as a critical molecular regulator of the cell death responses observed in P2X7 receptor activated undifferentiated cells. It is well known that RA is a morphogen, having profound effects during the course of normal development [51, 69] as well as it continues to play a prominent role in the adult CNS [70]. Thus, recognition of the modulation of the signaling pathways of P2X7 nucleotide receptors by RA will surely impact our understanding of the multiplicity of trophic effects mediated by P2X7 nucleotide receptors during development as well as in the adult CNS.
Acknowledgments
The authors are grateful to Dr. Michelle Burgos (BD, Biosciences) for useful discussion and for critical reading of the manuscript. We appreciate the excellent technical assistance of Cydmarie Cruz Quintana. The project described was supported by Grant P20 RR016470 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Center for Research Resources or National Institutes of Health. Elsie A. Orellano has a pre-doctoral fellowship from Puerto Rico Industrial Development Company (PRIDCO).
Contributor Information
Elsie A. Orellano, Department of Biology, University of Puerto Rico, Río Piedras Campus, San Juan, PR, USA; Department of Biochemistry, School of Medicine, University of Puerto Rico, San Juan, PR, USA
Omayra J. Rivera, Department of Chemistry, University of Puerto Rico, Río Piedras Campus, PO Box 23346, San Juan, PR 00931-3346, USA
Migdalia Chevres, Department of Biochemistry, School of Medicine, University of Puerto Rico, San Juan, PR, USA.
Nataliya E. Chorna, Department of Chemistry, University of Puerto Rico, Río Piedras Campus, PO Box 23346, San Juan, PR 00931-3346, USA
Fernando A. González, Department of Biochemistry, School of Medicine, University of Puerto Rico, San Juan, PR, USA; Department of Chemistry, University of Puerto Rico, Río Piedras Campus, PO Box 23346, San Juan, PR 00931-3346, USA
References
- 1.Deshmukh M, Johnson EM., Jr Programmed cell death in neurons: focus on the pathway of nerve growth factor deprivation-induced death of sympathetic neurons. Mol Pharmacol. 1997;51:897–906. doi: 10.1124/mol.51.6.897. [DOI] [PubMed] [Google Scholar]
- 2.Gao L, Abu Kwaik Y. Hijacking of apoptotic pathways by bacterial pathogens. Microbes Infect. 2000;2:1705–1719. doi: 10.1016/s1286-4579(00)01326-5. [DOI] [PubMed] [Google Scholar]
- 3.Encinas M, Iglesias M, Liu Y, Wang H, Muhaisen A, Cena V, Gallego C, Comella JX. Sequential treatment of SH-SY5Y cells with retinoic acid and brain-derived neurotrophic factor gives rise to fully differentiated, neurotrophic factor-dependent, human neuron-like cells. J Neurochem. 2000;75:991–1003. doi: 10.1046/j.1471-4159.2000.0750991.x. [DOI] [PubMed] [Google Scholar]
- 4.Oppenheim RW. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- 5.Prince JA, Oreland L. Staurosporine differentiated human SH-SY5Y neuroblastoma cultures exhibit transient apoptosis and trophic factor independence. Brain Res Bull. 1997;43:515–523. doi: 10.1016/s0361-9230(97)00328-6. [DOI] [PubMed] [Google Scholar]
- 6.Arthur DB, Akassoglou K, Insel PA. P2Y2 receptor activates nerve growth factor/TrkA signaling to enhance neuronal differentiation. Proc Natl Acad Sci USA. 2005;102:19138–19143. doi: 10.1073/pnas.0505913102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arthur DB, Georgi S, Akassoglou K, Insel PA. Inhibition of apoptosis by P2Y2 receptor activation: novel pathways for neuronal survival. J Neurosci. 2006;26:3798–3804. doi: 10.1523/JNEUROSCI.5338-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 9.Chorna NE, Santiago-Perez LI, Erb L, Seye CI, Neary JT, Sun GY, Weisman GA, Gonzalez FA. P2Y receptors activate neuroprotective mechanisms in astrocytic cells. J Neurochem. 2004;91:119–132. doi: 10.1111/j.1471-4159.2004.02699.x. [DOI] [PubMed] [Google Scholar]
- 10.Fumagalli M, Brambilla R, D’Ambrosi N, Volonte C, Matteoli M, Verderio C, Abbracchio MP. Nucleotide-mediated calcium signaling in rat cortical astrocytes: role of P2X and P2Y receptors. Glia. 2003;43:218–230. doi: 10.1002/glia.10248. [DOI] [PubMed] [Google Scholar]
- 11.Abbracchio MP, Burnstock G. Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol Ther. 1994;64:445–475. doi: 10.1016/0163-7258(94)00048-4. [DOI] [PubMed] [Google Scholar]
- 12.Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 14.White N, Burnstock G. P2 receptors and cancer. Trends Pharmacol Sci. 2006;27:211–217. doi: 10.1016/j.tips.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 15.North RA, Verkhratsky A. Purinergic transmission in the central nervous system. Pflugers Arch. 2006;452:479–485. doi: 10.1007/s00424-006-0060-y. [DOI] [PubMed] [Google Scholar]
- 16.Sperlagh B, Vizi ES, Wirkner K, Illes P. P2X7 receptors in the nervous system. Prog Neurobiol. 2006;78:327–346. doi: 10.1016/j.pneurobio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Zhang M, Laties AM, Mitchell CH. Stimulation of P2X7 receptors elevates Ca2+ and kills retinal ganglion cells. Invest Ophthalmol Vis Sci. 2005;46:2183–2191. doi: 10.1167/iovs.05-0052. [DOI] [PubMed] [Google Scholar]
- 18.Burnstock G, Williams M. P2 purinergic receptors: modulation of cell function and therapeutic potential. J Pharmacol Exp Ther. 2000;295:862–869. [PubMed] [Google Scholar]
- 19.Ballerini P, Giulinai P, Buccella S, Nargi E, Santavenere C, Scemens E, Rathbone MP, Caciagli F. P2X7 ATP receptor-mediated modulation of astrocytes proliferation and coupling. Drug Dev Res. 2000;50:108. [Google Scholar]
- 20.Franke H, Grosche J, Schadlich H, Krugel U, Allgaier C, Illes P. P2X receptor expression on astrocytes in the nucleus accumbens of rats. Neuroscience. 2001;108:421–429. doi: 10.1016/s0306-4522(01)00416-x. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Arcuino G, Takano T, Lin J, Peng WG, Wan P, Li P, Xu Q, Liu QS, Goldman SA, Nedergaard M. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med. 2004;10:821–827. doi: 10.1038/nm1082. [DOI] [PubMed] [Google Scholar]
- 22.Cheung KK, Chan WY, Burnstock G. Expression of P2X purinoceptors during rat brain development and their inhibitory role on motor axon outgrowth in neural tube explant cultures. Neuroscience. 2005;133:937–945. doi: 10.1016/j.neuroscience.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 23.Resende RR, Majumder P, Gomes KN, Britto LR, Ulrich H. P19 embryonal carcinoma cells as in vitro model for studying purinergic receptor expression and modulation of N-methyl-d-aspartate-glutamate and acetylcholine receptors during neuronal differentiation. Neuroscience. 2007;146:1169–1181. doi: 10.1016/j.neuroscience.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 24.Pahlman S, Hoehner JC, Nanberg E, Hedborg F, Fagerstrom S, Gestblom C, Johansson I, Larsson U, Lavenius E, Ortoft E, et al. Differentiation and survival influences of growth factors in human neuroblastoma. Eur J Cancer. 1995;31A:453–458. doi: 10.1016/0959-8049(95)00033-f. [DOI] [PubMed] [Google Scholar]
- 25.Singh US, Pan J, Kao YL, Joshi S, Young KL, Baker KM. Tissue transglutaminase mediates activation of RhoA and MAP kinase pathways during retinoic acid-induced neuronal differentiation of SH-SY5Y cells. J Biol Chem. 2003;278:391–399. doi: 10.1074/jbc.M206361200. [DOI] [PubMed] [Google Scholar]
- 26.Larsson KP, Hansen AJ, Dissing S. The human SH-SY5Y neuroblastoma cell-line expresses a functional P2X7 purinoceptor that modulates voltage-dependent Ca2+ channel function. J Neurochem. 2002;83:285–298. doi: 10.1046/j.1471-4159.2002.01110.x. [DOI] [PubMed] [Google Scholar]
- 27.Tozaki-Saitoh H, Koizumi S, Sato Y, Tsuda M, Nagao T, Inoue K. Retinoic acids increase P2X2 receptor expression through the 5′-flanking region of P2rx2 gene in rat phaeochromocytoma PC-12 cells. Mol Pharmacol. 2006;70:319–328. doi: 10.1124/mol.105.020511. [DOI] [PubMed] [Google Scholar]
- 28.Fujishita K, Koizumi S, Inoue K. Upregulation of P2Y2 receptors by retinoids in normal human epidermal keratinocytes. Purinergic Signal. 2006;2:491–498. doi: 10.1007/s11302-005-7331-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorodeski GI. Expression, regulation, and function of P2X(4) purinergic receptor in human cervical epithelial cells. Am J Physiol Cell Physiol. 2002;282:C84–C93. doi: 10.1152/ajpcell.2002.282.1.C84. [DOI] [PubMed] [Google Scholar]
- 30.Oppenheimer O, Cheung NK, Gerald WL. The RET oncogene is a critical component of transcriptional programs associated with retinoic acid-induced differentiation in neuroblastoma. Mol Cancer Ther. 2007;6:1300–1309. doi: 10.1158/1535-7163.MCT-06-0587. [DOI] [PubMed] [Google Scholar]
- 31.Santiago-Perez LI, Flores RV, Santos-Berrios C, Chorna NE, Krugh B, Garrad RC, Erb L, Weisman GA, Gonzalez FA. P2Y(2) nucleotide receptor signaling in human monocytic cells: activation, desensitization and coupling to mitogen-activated protein kinases. J Cell Physiol. 2001;187:196–208. doi: 10.1002/jcp.1063. [DOI] [PubMed] [Google Scholar]
- 32.Velazquez B, Garrad RC, Weisman GA, Gonzalez FA. Differential agonist-induced desensitization of P2Y2 nucleotide receptors by ATP and UTP. Mol Cell Biochem. 2000;206:75–89. doi: 10.1023/a:1007091127392. [DOI] [PubMed] [Google Scholar]
- 33.Suh BC, Kim JS, Namgung U, Ha H, Kim KT. P2X7 nucleotide receptor mediation of membrane pore formation and superoxide generation in human promyelocytes and neutrophils. J Immunol. 2001;166:6754–6763. doi: 10.4049/jimmunol.166.11.6754. [DOI] [PubMed] [Google Scholar]
- 34.Wiley JS, Gargett CE, Zhang W, Snook MB, Jamieson GP. Partial agonists and antagonists reveal a second permeability state of human lymphocyte P2Z/P2X7 channel. Am J Physiol. 1998;275:C1224–C1231. doi: 10.1152/ajpcell.1998.275.5.C1224. [DOI] [PubMed] [Google Scholar]
- 35.Cheah FC, Hampton MB, Darlow BA, Winterbourn CC, Vissers MC. Detection of apoptosis by caspase-3 activation in tracheal aspirate neutrophils from premature infants: relationship with NF-kappaB activation. J Leukoc Biol. 2005;77:432–437. doi: 10.1189/jlb.0904520. [DOI] [PubMed] [Google Scholar]
- 36.Cavaliere F, Dinkel K, Reymann K. Microglia response and P2 receptor participation in oxygen/glucose deprivation-induced cortical damage. Neuroscience. 2005;136:615–623. doi: 10.1016/j.neuroscience.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 37.Preis PN, Saya H, Nadasdi L, Hochhaus G, Levin V, Sadee W. Neuronal cell differentiation of human neuroblastoma cells by retinoic acid plus herbimycin A. Cancer Res. 1988;48:6530–6534. [PubMed] [Google Scholar]
- 38.Gever JR, Cockayne DA, Dillon MP, Burnstock G, Ford AP. Pharmacology of P2X channels. Pflugers Arch. 2006;452:513–537. doi: 10.1007/s00424-006-0070-9. [DOI] [PubMed] [Google Scholar]
- 39.Chessell IP, Michel AD, Humphrey PP. Effects of antagonists at the human recombinant P2X7 receptor. Br J Pharmacol. 1998;124:1314–1320. doi: 10.1038/sj.bjp.0701958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Humphreys BD, Virginio C, Surprenant A, Rice J, Dubyak GR. Isoquinolines as antagonists of the P2X7 nucleotide receptor: high selectivity for the human versus rat receptor homologues. Mol Pharmacol. 1998;54:22–32. doi: 10.1124/mol.54.1.22. [DOI] [PubMed] [Google Scholar]
- 41.Gudipaty L, Humphreys BD, Buell G, Dubyak GR. Regulation of P2X(7) nucleotide receptor function in human monocytes by extracellular ions and receptor density. Am J Physiol Cell Physiol. 2001;280:C943–C953. doi: 10.1152/ajpcell.2001.280.4.C943. [DOI] [PubMed] [Google Scholar]
- 42.Burgos M, Neary JT, Gonzalez FA. P2Y2 nucleotide receptors inhibit trauma-induced death of astrocytic cells. J Neurochem. 2007;103:1785–1800. doi: 10.1111/j.1471-4159.2007.04872.x. [DOI] [PubMed] [Google Scholar]
- 43.Harms C, Lautenschlager M, Bergk A, Katchanov J, Freyer D, Kapinya K, Herwig U, Megow D, Dirnagl U, Weber JR, Hortnagl H. Differential mechanisms of neuroprotection by 17 beta-estradiol in apoptotic versus necrotic neurodegeneration. J Neurosci. 2001;21:2600–2609. doi: 10.1523/JNEUROSCI.21-08-02600.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Charles I, Khalyfa A, Kumar DM, Krishnamoorthy RR, Roque RS, Cooper N, Agarwal N. Serum deprivation induces apoptotic cell death of transformed rat retinal ganglion cells via mitochondrial signaling pathways. Invest Ophthalmol Vis Sci. 2005;46:1330–1338. doi: 10.1167/iovs.04-0363. [DOI] [PubMed] [Google Scholar]
- 45.Kong Q, Wang M, Liao Z, Camden JM, Yu S, Simonyi A, Sun GY, Gonzalez FA, Erb L, Seye CI, Weisman GA. P2X(7) nucleotide receptors mediate caspase-8/9/3-dependent apoptosis in rat primary cortical neurons. Purinergic Signal. 2005;1:337–347. doi: 10.1007/s11302-005-7145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Franke H, Illes P. Involvement of P2 receptors in the growth and survival of neurons in the CNS. Pharmacol Ther. 2006;109:297–324. doi: 10.1016/j.pharmthera.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 47.Neary JT, Rathbone MP, Cattabeni F, Abbracchio MP, Burnstock G. Trophic actions of extracellular nucleotides and nucleosides on glial and neuronal cells. Trends Neurosci. 1996;19:13–18. doi: 10.1016/0166-2236(96)81861-3. [DOI] [PubMed] [Google Scholar]
- 48.Noguchi T, Ishii K, Fukutomi H, Naguro I, Matsuzawa A, Takeda K, Ichijo H. Requirement of reactive oxygen species-dependent activation of ASK1-p38 MAPK pathway for extracellular ATP-induced apoptosis in macrophage. J Biol Chem. 2008;283:7657–7665. doi: 10.1074/jbc.M708402200. [DOI] [PubMed] [Google Scholar]
- 49.Parvathenani LK, Tertyshnikova S, Greco CR, Roberts SB, Robertson B, Posmantur R. P2X7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of Alzheimer’s disease. J Biol Chem. 2003;278:13309–13317. doi: 10.1074/jbc.M209478200. [DOI] [PubMed] [Google Scholar]
- 50.Reed JC. Mechanisms of apoptosis. Am J Pathol. 2000;157:1415–1430. doi: 10.1016/S0002-9440(10)64779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zile MH. Function of vitamin A in vertebrate embryonic development. J Nutr. 2001;131:705–708. doi: 10.1093/jn/131.3.705. [DOI] [PubMed] [Google Scholar]
- 52.Carraro-Lacroix LR, Ramirez MA, Zorn TM, Reboucas NA, Malnic G. Increased NHE1 expression is associated with serum deprivation-induced differentiation in immortalized rat proximal tubule cells. Am J Physiol Renal Physiol. 2006;291:F129–F139. doi: 10.1152/ajprenal.00290.2005. [DOI] [PubMed] [Google Scholar]
- 53.De Chiara G, Marcocci ME, Torcia M, Lucibello M, Rosini P, Bonini P, Higashimoto Y, Damonte G, Armirotti A, Amodei S, Palamara AT, Russo T, Garaci E, Cozzolino F. Bcl-2 Phosphorylation by p38 MAPK: identification of target sites and biologic consequences. J Biol Chem. 2006;281:21353–21361. doi: 10.1074/jbc.M511052200. [DOI] [PubMed] [Google Scholar]
- 54.Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res. 2002;43:1773–1808. doi: 10.1194/jlr.r100015-jlr200. [DOI] [PubMed] [Google Scholar]
- 55.Wu PY, Lin YC, Chang CL, Lu HT, Chin CH, Hsu TT, Chu D, Sun SH. Functional decreases in P2X7 receptors are associated with retinoic acid-induced neuronal differentiation of Neuro-2a neuroblastoma cells. Cell Signal. 2009;21:881–891. doi: 10.1016/j.cellsig.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 56.Raymond VW, Grisham JW, Earp HS. Characterization of epidermal growth factor receptor induction by retinoic acid in a chemically transformed rat liver cell line. Cell Growth Differ. 1990;1:393–399. [PubMed] [Google Scholar]
- 57.Wang L, Feng YH, Gorodeski GI. Epidermal growth factor facilitates epinephrine inhibition of P2X7-receptor-mediated pore formation and apoptosis: a novel signaling network. Endocrinology. 2005;146:164–174. doi: 10.1210/en.2004-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Michaelis M, Bliss J, Arnold SC, Hinsch N, Rothweiler F, Deubzer HE, Witt O, Langer K, Doerr HW, Wels WS, Cinatl J., Jr Cisplatin-resistant neuroblastoma cells express enhanced levels of epidermal growth factor receptor (EGFR) and are sensitive to treatment with EGFR-specific toxins. Clin Cancer Res. 2008;14:6531–6537. doi: 10.1158/1078-0432.CCR-08-0821. [DOI] [PubMed] [Google Scholar]
- 59.Resta V, Novelli E, Di Virgilio F, Galli-Resta L. Neuronal death induced by endogenous extracellular ATP in retinal cholinergic neuron density control. Development. 2005;132:2873–2882. doi: 10.1242/dev.01855. [DOI] [PubMed] [Google Scholar]
- 60.Aga M, Johnson CJ, Hart AP, Guadarrama AG, Suresh M, Svaren J, Bertics PJ, Darien BJ. Modulation of monocyte signaling and pore formation in response to agonists of the nucleotide receptor P2X(7) J Leukoc Biol. 2002;72:222–232. [PubMed] [Google Scholar]
- 61.Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, Griffiths RJ, Gabel CA. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- 62.Erb L, Liao Z, Seye CI, Weisman GA. P2 receptors: intracellular signaling. Pflugers Arch. 2006;452:552–562. doi: 10.1007/s00424-006-0069-2. [DOI] [PubMed] [Google Scholar]
- 63.Donnelly-Roberts DL, Namovic MT, Faltynek CR, Jarvis MF. Mitogen-activated protein kinase and caspase signaling pathways are required for P2X7 receptor (P2X7R)-induced pore formation in human THP-1 cells. J Pharmacol Exp Ther. 2004;308:1053–1061. doi: 10.1124/jpet.103.059600. [DOI] [PubMed] [Google Scholar]
- 64.Limke TL, Bearss JJ, Atchison WD. Acute exposure to methylmercury causes Ca2+ dysregulation and neuronal death in rat cerebellar granule cells through an M3 muscarinic receptor-linked pathway. Toxicol Sci. 2004;80:60–68. doi: 10.1093/toxsci/kfh131. [DOI] [PubMed] [Google Scholar]
- 65.Volonte C, Ciotti MT, D’Ambrosi N, Lockhart B, Spedding M. Neuroprotective effects of modulators of P2 receptors in primary culture of CNS neurones. Neuropharmacology. 1999;38:1335–1342. doi: 10.1016/s0028-3908(99)00034-9. [DOI] [PubMed] [Google Scholar]
- 66.Hogg RC, Chipperfield H, Whyte KA, Stafford MR, Hansen MA, Cool SM, Nurcombe V, Adams DJ. Functional maturation of isolated neural progenitor cells from the adult rat hippocampus. Eur J Neurosci. 2004;19:2410–2420. doi: 10.1111/j.0953-816X.2004.03346.x. [DOI] [PubMed] [Google Scholar]
- 67.da Silva JS, Dotti CG. Breaking the neuronal sphere: regulation of the actin cytoskeleton in neuritogenesis. Nat Rev Neurosci. 2002;3:694–704. doi: 10.1038/nrn918. [DOI] [PubMed] [Google Scholar]
- 68.Diaz-Hernandez M, Del Puerto A, Diaz-Hernandez JI, Diez-Zaera M, Lucas JJ, Garrido JJ, Miras-Portugal MT. Inhibition of the ATP-gated P2X7 receptor promotes axonal growth and branching in cultured hippocampal neurons. J Cell Sci. 2008;121:3717–3728. doi: 10.1242/jcs.034082. [DOI] [PubMed] [Google Scholar]
- 69.Chambon P. The retinoid signaling pathway: molecular and genetic analyses. Semin Cell Biol. 1994;5:115–125. doi: 10.1006/scel.1994.1015. [DOI] [PubMed] [Google Scholar]
- 70.Werner EA, Deluca HF. Retinoic acid is detected at relatively high levels in the CNS of adult rats. Am J Physiol Endocrinol Metab. 2002;282:E672–E678. doi: 10.1152/ajpendo.00280.2001. [DOI] [PubMed] [Google Scholar]