Abstract
Multiple Endocrine Neoplasia Type I (MEN1), a familial tumor syndrome results from mutations in the MEN1 gene, which encodes a tumor suppressor, menin. It has been previously shown that menin plays an important role in both repressing and activating gene expression. However, it is not well understood how menin represses expression of multiple genes. Here we show that upon Men1 excision, Gli1 and its target genes including PTCH1 and C-MYC are elevated in the absence of an apparent Hedgehog (Hh) pathway-activating ligand or when Smoothened (SMO), a key component of the pathway, is inhibited. Menin binds to the Gli1 promoter and recruits PRMT5, a histone arginine methyltransferase associated with transcriptional repression. Both PRMT5 binding and histone H4 arginine 3 methylation (H4R3m2s) are decreased at the Gli1 promoter in Men1-excised cells. Moreover, Men1 ablation results in increased binding of transcriptionally active GLI1 at the Gli1 promoter, in a manner not influenced by the canonical Hedgehog signaling pathway. Inhibition of GLI1 by a small molecule inhibitor, GANT 61, leads to decrease in expression of Gli1, and its target genes in Men1-excised cells. Furthermore, GANT-61 more potently suppresses proliferation of Men1-excised cells than control wild type cells. These findings uncover a novel link whereby menin directly represses Gli1 expression independent of the canonical Hedgehog (Hh) signaling pathway epigenetically via PRMT5 and its repressive H4R3m2s mark. These results suggest that inhibition of Gli1 can lead to suppression of neuroendocrine tumors harboring mutations in the MEN1 gene.
Keywords: Menin, PRMT5, GLI1, H4R3m2s, Epigenetics
Introduction
Multiple Endocrine Neoplasia type 1 (MEN1) is an inherited tumor syndrome, with development of tumors in several endocrine organs including pancreatic islets (1-4). The gene mutated in this syndrome, MEN1, encodes a protein of 610 amino acid residues, menin (5, 6). Menin interacts with a variety of proteins including the transcription factors JunD (7-9), nuclear factor (NF)-κb (10) and SMAD3 (11), and represses the transcriptional activity of JunD and NF-κb.
The Hedgehog (Hh) pathway has previously been implicated in insulin production and secretion in INS1 cells (12), and it regulates insulin production in adult mice (13). The Hh signaling pathway regulates diverse biological processes ranging from embryonic development to cell cycle and tumorigenesis (14). Canonical Hh signaling is triggered by the binding of Hh ligands [Sonic, Indian or Desert Hedgehog] to its receptor Patched 1 (PTCH1), resulting in the release of PTCH1-mediated repression of the seven trans-membrane protein Smoothened (SMO) (15). Activated SMO triggers the dissociation of GLI proteins from the Sufu-Fused-Cos2 complex (16), resulting in the nuclear translocation of GLI1 and GLI2, degradation of the repressor GLI3, and subsequent increase in the transcription of Gli1 target genes including Ptch1 and Gli1 itself (14, 15). Constitutive activation of this signaling pathway, either through Ptch1 inactivating mutations or Smo activating mutations, has been reported in several malignancies including basal cell carcinoma and medulloblastoma (17, 18).
GLI1, the main effector of the Hh signaling pathway, can also be regulated independent of the canonical Hh signaling pathway (19, 20). The TGF-β signaling pathway activates Gli1 even in the presence of cyclopamine, a SMO antagonist (20), the Ewing's sarcoma (EWS)-Friend's leukemia insertion (FLI) activates Gli1 directly (21) and Gli1 mediates the survival and transformation of pancreatic ductal adenocarcinoma (PDAC) cells induced by activated K-RAS (19).
Acetylation of proteins is a dynamic process involving HAT's and HDAC's, and regulates many diverse functions including DNA recognition, protein-protein interaction and protein stability (22). HDAC's play a role in transcriptional regulation including suppression of several tumor suppressors including JunB, Prss11 and Plagl1(23). On the contrary, both HDAC activity and recruitment are required for transcriptional activation of genes including Gja1, Irf1, and Gbp2(23). Similarly, GLI1 physically interacts with HDAC, and HDAC-mediated deacetylation of GLI1 results in the transcriptionally active, deacetylated-GLI1 species (24).
Recently, we have shown that menin interacts with Protein Arginine Methyltransferase 5 (PRMT5), a repressive histone methyltransferase, and epigenetically represses canonical Hh signaling via regulation of GAS1 (25), a crucial co-factor required for binding of Hh ligand to the PTCH1 receptor (26-28). However, it is unclear whether the menin/PRMT5 axis also regulates other steps of the Hh signaling cascade. Here, we show that menin binds to the Gli1 promoter, and recruits PRMT5 and promotes repressive histone arginine methylation, H4R3m2s, at the promoter region, resulting in decreased Gli1 expression. Furthermore, binding of menin to the Gli1 promoter leads to reduced binding of GLI1 and HDAC1 to the Gli1 promoter. Pharmacological inhibition of GLI1 resulted in decreased expression of Gli1, PTCH1 and C-MYC.
Materials and Methods
Plasmids and Cell Culture
Retroviral plasmids expressing Flag-tagged or mutant menin have been described elsewhere (29). Men1-null MEF cells complemented with wild type or mutant menin, and 293 cells were cultured in Dulbecco's modified Eagle's Medium (DMEM) medium supplemented with 10 % fetal bovine serum (FBS) and 1% Pen/Strep (29).
RNA Extraction and Quantitative Real Time PCR (qRT-PCR)
Total RNA was extracted from cultured cells with Trizol and an RNeasy extraction kit from Qiagen. Details and primer sequences can be found in Supplementary Table 1. The Ct values for the target genes were normalized to Gapdh Ct values, and analysis was done using the relative quantification method according to instructions from ABI.
Antibodies and Western Blotting
Whole cell lysates were prepared using RIPA buffer (Sigma) supplemented with mammalian protease inhibitor cocktail (Sigma), and subjected to Western Blotting as previously described (29). The primary antibodies used were anti-menin (Bethyl, A300-105A), anti-PRMT5 (Abcam, ab31751 and ab109451), anti-PTCH1 (Protein Tech, 17520-1-AP), anti-C-MYC (Epitomics, 1472-1), and anti-β-Actin (Sigma, A5441).
Chromatin Immunoprecipitation (ChIP) Assay
ChIP assay was performed as previously described using a Quick ChIP kit from Imgenex (30). Briefly, cells were fixed with 1 % formaldehyde, lysed in ChIP lysis buffer supplemented with a protease inhibitor cocktail, and the genomic DNA was sheared into 200-1000 bp fragments by sonication with a Bioruptor sonicator (Diagenode), followed by incubation with either control anti-IgG antibody or a specific primary antibody at 4 °C overnight. The immunoprecipitated antibody-protein-DNA complex was collected using salmon sperm DNA-coated Protein G agarose beads. The eluted protein-DNA complex was reverse cross-linked at 65 °C overnight. q-PCR was performed on the precipitated DNA, and normalized to percent input of genomic DNA. Binding was quantitated as 2ΔCt where ΔCt equals Ct (input) - Ct (ChIP). Primer sequences are listed in Supplementary Material. Antibodies used for ChIP were anti-menin (Bethyl, A300-105A), anti-PRMT5 (Abcam, ab31751), anti-GLI1 (Rockland, 100-401-223), anti-Histone H4 Symmetric Di-Methyl R3 (Abcam, ab5823), anti-Histone H3 (Abcam, ab1791).
Luciferase assay
MEF cells were transfected with the indicated plasmids using Fugene (Roche). Dual-luciferase reporter assay (Promega, Madison, WI) kits were used according to the manufacturer's instructions. The GLI-binding site-driven luciferase reporter plasmid was kindly provided by Dr. Hiroshi Sasaki (31). Both firefly and renilla luciferase activities were measured by Luminoskan Ascent (Thermo Fisher). Results were representative of three independent experiments.
Statistical Analyses
Statistical analysis was performed using Graphpad Prism (v 5.0, Graphpad Software). The data are presented as the mean ± s.d. of n determinations unless noted otherwise. A two-tailed student's t test was used for measuring statistical differences.
Results
Menin represses GLI1 and its target genes independent of Hedgehog (Hh) ligand-mediated signaling
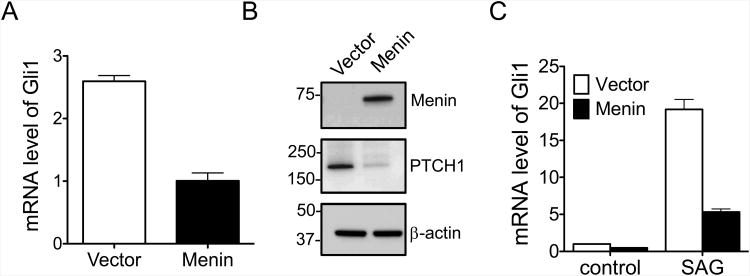
Our previous studies show that menin dampens canonical Hh signaling by epigenetically repressing GAS1 (25), a crucial co-factor, along with CDO and BOC (26, 28, 32), promoting Hh ligand binding to its receptor PTCH1 (14). Interestingly, in the absence of Hh ligand, and hence canonical signaling, Gli1 mRNA levels were reduced in Men1-null MEFs complemented with wild type (WT) menin as compared to control cells (Fig. 1A). To determine whether certain MEN1 disease-related point mutations affect Gli1 expression, we complemented menin-null MEFs with disease-related mutant menin, A242V, and showed that the mutant lost its ability to repress Gli1 (Supplementary Fig. S1), possibly due to the diminished interaction with PRMT5 as we demonstrated earlier (25). Furthermore, PTCH1, a direct GLI1 target, was reduced upon complementing Men1-null cells with menin (Fig. 1B). To further determine whether excision of endogenous Men1 yields similar results, we treated Men1l/l;CreER MEFs with either DMSO or 4-hydroxyl tamoxifen (4-OHT) and showed that Men1 excision results in six-fold increase of Gli1 (Supplementary Fig. S2). Notably, ectopic menin expression substantially repressed Smoothened Agonist (SAG)-induced Gli1 expression (Fig. 1C), suggesting that menin further represses Gli1 expression downstream of GAS1 and SMO, i.e. the canonical Hh ligand-induced signaling pathway. As such, it is plausible that menin-mediated suppression of GLI1 expression can be attributed to suppression of Gli1 expression independent of the canonical, GAS1/PTCH1/SMO-mediated Hh signaling.
Figure 1.
Menin inhibits GLI1 independent of the canonical Hedgehog (Hh) pathway. (A) Menin-null MEFs were complemented with either vector or wild type (WT) menin, and the mRNA levels of Gli1 were determined by qRT-PCR. Data is expressed as fold change over menin-expressing cells. (B) Whole cell lysates from menin-null MEFs complemented with either vector or WT menin were subjected to SDS-PAGE electrophoresis, and the protein levels of menin and PTCH1 were determined by immunoblotting. (C) Menin-null MEFs complemented with either vector or WT menin were cultured in the presence of 100 nM Smoothened Agonist (SAG) and the mRNA levels of Gli1 were determined by qRT-PCR. Data is expressed as fold change over menin-null cells cultured in the absence of SAG. Error bars indicate ± s.d.
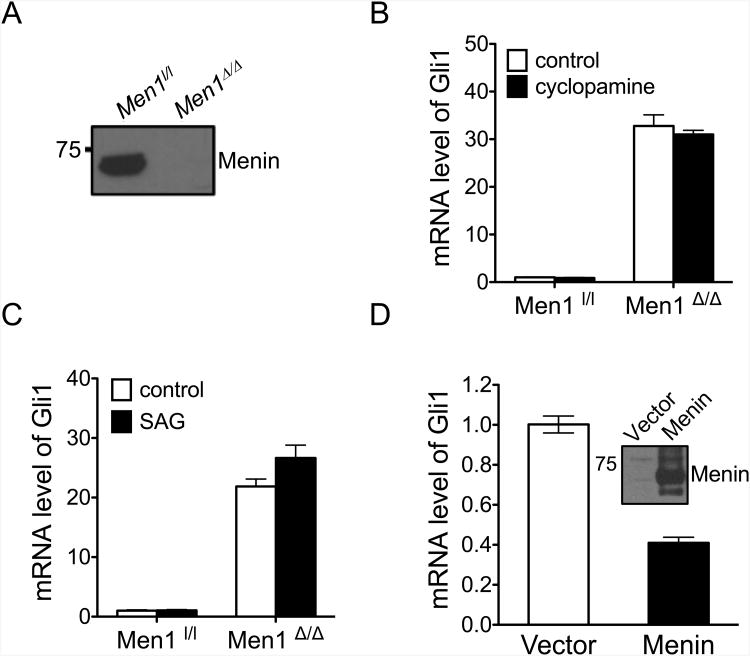
We found that PIME1 cells, a mouse pancreatic islet derived cell line in which floxed Men1(Men1l/l) can be conditionally excised by adding 4-OHT (33) (Fig. 2A), were not responsive to either cyclopamine, a chemical SMO inhibitor (Fig. 2B) or SAG (Fig. 2C), a SMO activator, based on unaltered Gli1 mRNA levels. These results indicate that PIME1 cells are not responsive to canonical Hh signaling. Many immortalized or tumor-derived cell lines have been shown to lose their response to Hh ligand stimulation due to perturbations in the Hh signaling pathway (34). In this regard, PIME1 cells serve as an ideal tool for studying the potential role of menin in regulating Gli1 expression independent of the canonical Hh signaling pathway. Men1 excision in PIME1 cells resulted in significant upregulation of Gli1 mRNA (Figs. 2B, 2C). To examine whether ectopic menin expression in Men1-excised PIME1 cells represses Gli1 expression, we complemented the Men1-excised cells with either control vector or retroviruses expressing menin. qRT-PCR showed that menin expression in the cells partially restored the repression of Gli1 mRNA levels (Fig. 2D). Consistent with these results, we found that ectopic expression of menin in BON cells, a human neuroendocrine carcinoid cell line that expresses low levels of endogenous menin (35) and are unresponsive to Hh signaling (data not shown), also reduced Gli1 mRNA levels (Supplementary Fig. S3). These findings demonstrate menin's role in repressing Gli1 expression independent of the canonical Hh signaling pathway.
Figure 2.
Menin represses Gli1 in PIME1 cells, a cell line lacking the canonical Hedgehog (Hh) signaling pathway. (A) Men1l/l;CreER PIME1 cells were treated with either DMSO or 4-hydroxytamoxifen (4-OHT), and menin levels were determined by immunoblotting. (B, C) qRT-PCR for Gli1 mRNA in the presence of 5 μM cyclopamine (B) and Smoothened Agonist (SAG) (C) in control Men1l/l and Men1Δ/Δ PIME1 cells. The data is expressed as fold change over menin-null cells cultured in the absence of cyclopamine or SAG. (D) qRT-PCR for Gli1 mRNA in Men1-excised PIME1 cells complemented with either empty vector or wild type menin. Inset, Immunoblotting showing expression of ectopic menin in Men1-excised PIME1 cells. Data is expressed as fold change over menin-null cells. Error bars indicate ± s.d.
Menin represses Gli1 via PRMT5-mediated histone methylation
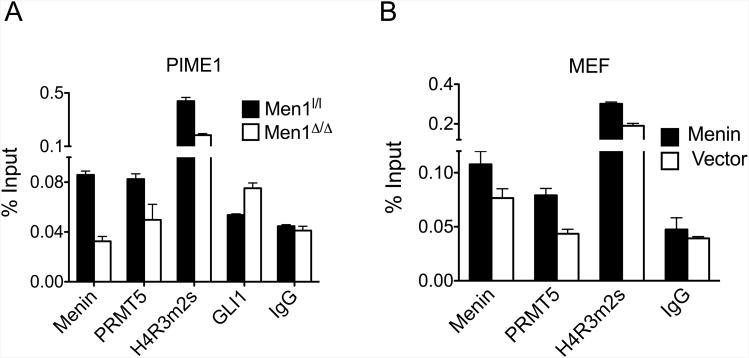
To determine how menin represses Gli1 expression in a Hh signaling-independent manner, downstream of SMO, we performed a ChIP assay to evaluate whether menin directly binds the Gli1 promoter to regulate its expression. ChIP assay in PIME1 cells showed that menin bound to the Gli1 promoter, and Men1-excision resulted in increased binding of GLI1 to the Gli1 promoter (Fig. 3A), suggesting partial overlap between menin and GLI1 binding regions at the Gli1 promoter. It has been reported that GLI1 can bind to its promoter and activate its own expression (36, 37). We have previously shown that menin directly interacts with and recruits PRMT5, a repressive histone arginine methyltransferase, and its histone H4 arginine 3 methylation (H4R3m2s) mark to the Gas1 promoter and represses its expression (25). To determine whether menin recruits PRMT5 to the Gli1 promoter and represses its expression, we performed a ChIP assay, and found that Men1 excision in PIME1 cells reduced PRMT5 binding and H4R3m2s at the Gli1 promoter (Fig. 3A). As a control, total H3 enrichment at the Gli1 promoter was not altered upon Men1 excision (Supplementary Fig. S4). These results indicate that Gli1 expression can be repressed epigenetically by PRMT5, likely via menin-mediated recruitment of PRMT5. Furthermore, in Hh-ligand responsive MEFs, we observed that in the absence of Hh ligand, menin bound to the Gli1 promoter using ChIP assay, and consistently, ectopic menin expression led to an increase in PRMT5 binding and H4R3m2s at the promoter (Fig. 3B). Together, these findings indicate that menin directly suppresses GLI1 partially by recruiting PRMT5 and its repressive histone methylation mark to the Gli1 promoter in both Hh signaling responsive MEFs and non-responsive PIME1 cells.
Figure 3.
Menin regulates Gli1 by recruiting PRMT5 and increasing symmetric histone H4 arginine dimethylation (H4R3m2s) at the Gli1 promoter. (A) Chromatin immunoprecipitation (ChIP) using antibodies against menin, PRMT5, H4R3m2s, GLI1 and control IgG at the Gli1 promoter in either DMSO (Men1l/l) or tamoxifen-treated Men1l/l; CreER (Men1Δ/Δ) PIME1 cells. (B) ChIP with antibodies against menin, PRMT5 and H4R3m2s at the Gli1 promoter in Men1-null MEFs complemented with either vector or wild type menin. Error bars indicate ± s.d.
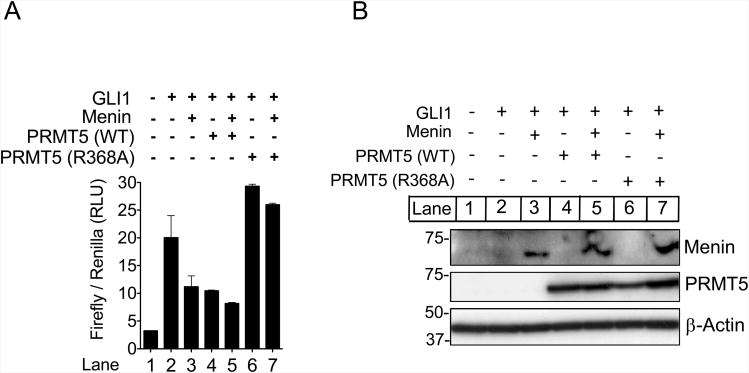
As the Gli1 gene can be activated by binding of GLI1 to its own promoter (36, 37), we performed luciferase reporter assays with a GLI1-binding site-driven luciferase reporter gene (31), and found that ectopic expression of either menin or PRMT5 in menin-null cells repressed expression of the reporter gene (Fig. 4A). Notably, a catalytically inactive PRMT5 mutant, R368A (38), not only failed to suppress reporter gene activity in the presence or absence of menin, but even modestly increased the expression of the reporter (Fig. 4A, lane 7 vs. lane 3). These results suggest that the PRMT5 catalytic mutant may exert a dominant negative effect on the endogenous wild type (WT) PRMT5 by blocking its binding to menin. As a control, both WT and mutant PRMT5 were expressed at comparable levels (Fig. 4B). These findings are consistent with the notion that menin directly represses Gli1 in a SMO or Hh ligand-independent manner via interacting with PRMT5.
Figure 4.
The menin-PRMT5 complex inhibits GLI1 transcriptional activity. (A) Relative luciferase activity in Men1-null MEFs expressing a luciferase reporter under control of the Gli1-responsive promoter co-transfected with cDNA's for GLI1, Menin, PRMT5-WT and PRMT5 (R368A) mutant as indicated. Values obtained were normalized to renilla luciferase activity. (B) Inputs for cell lysates used in Fig. 4A. Error bars indicate ± s.d.
Menin affects binding of the active GLI1-HDAC complex to its target genes
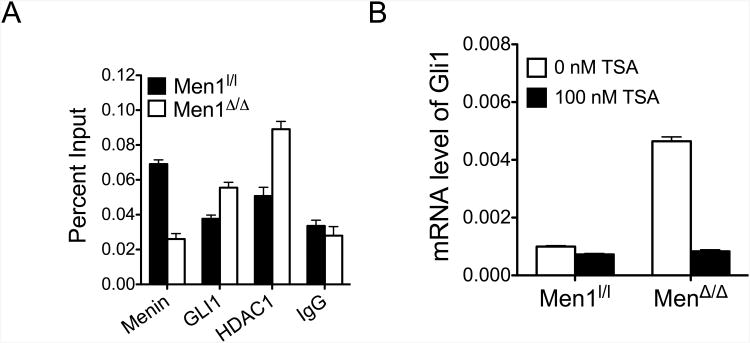
GLI1 is an acetylated protein, interacts physically with HDAC1 and HDAC2, and its deacetylation results in transcriptional activation of target genes including Gli1 and Ptch1(24). Similarly, menin interacts with HDAC1 and HDAC2 via mSin3A, a general transcription co-repressor (39). To determine whether menin affects binding of the active, deacetylated GLI1-HDAC1 complex to the Gli1 promoter, we performed a ChIP assay, and found that Men1 excision in PIME1 cells resulted in increased GLI1 binding (Fig. 5A). Similarly, HDAC1 binding at the Gli1 promoter was also increased upon Men1 excision, indicating that menin negatively affects binding of the active GLI1-HDAC1 complex to the Gli1 promoter, thereby suppressing transcriptional activation (Fig. 5A). GLI1 and HDAC are recruited to the promoters of Gli1 and its target genes as a complex as demonstrated by re-ChIP (24). To determine whether menin affects the GLI1-acetylation/deacetylation homeostasis and hence its activation status, we treated control and menin-null MEFs with a Class I and II HDAC inhibitor, trichostatin A (TSA). While treatment with TSA resulted in a minimal decrease of Gli1 in control WT cells, Men1-excison-induced Gli1 up-regulation was substantially reduced upon treatment with TSA (Fig. 5B). These findings are consistent with the notion that an increased population of transcriptionally active HDAC1-GLI1 complex exists in Men1-null cells, and are more susceptible to TSA-induced GLI1 deacetylation as compared to the control WT cells. It is worth noting that the total levels of HDAC1 were not altered upon Men1 excision (Supplementary Fig. S5), thus indicating that menin either affects the association of HDAC1 and GLI1 and/or interferes with binding of the transcriptionally active HDAC1-GLI1 complex to the promoter of target genes.
Figure 5.
Menin affects binding of transcriptionally active GLI1-HDAC1 complex to the Gli1 promoter. (A) Chromatin immunoprecipitation (ChIP) using antibodies against menin, GLI1 and HDAC1 in control and Men1-null cells. (B) qRT-PCR for Gli1 mRNA in control and Men1-excised cells after treatment with a HDAC inhibitor, trichostatin A (TSA). Error bars indicate ± s.d.
Inhibition of GLI1 by GANT-61 results in decreased expression of Gli1 mRNA and its target genes
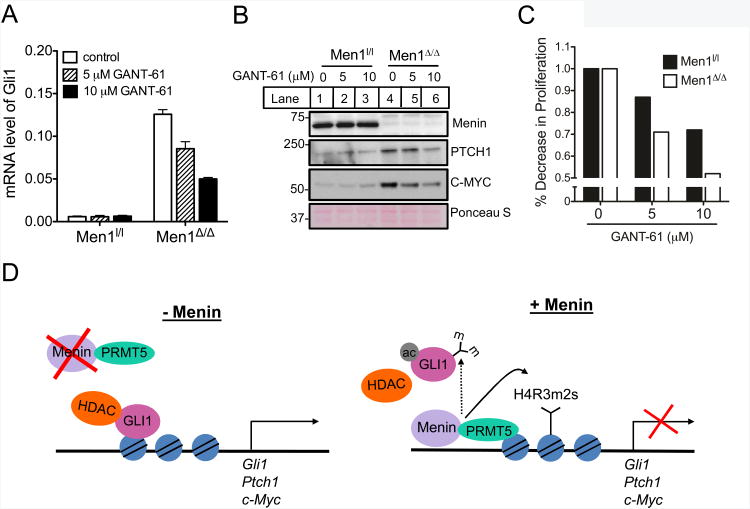
To determine the effects of increased GLI1 levels upon Men1 excision in PIME1 cells, we treated the cells with a small molecule GLI1 inhibitor, GANT-61, that reduces the transcriptional activity of GLI1 and GLI2 and interferes with the binding of GLI1 to DNA (40, 41). While no decrease in Gli1 mRNA levels was observed in menin-expressing control cells, Gli1 mRNA levels were reduced in a dose-dependant manner in Men1-excised PIME1 cells upon treatment with GANT-61 (Fig. 6A). It is likely that the exceedingly low basal levels of Gli1 in the wild-type PIME1 cells accounts for lack of GLI1 inhibition upon GANT-61 treatment. Furthermore, the protein levels of PTCH1, a direct Gli1 target was also reduced in the Men1-excised PIME1 cells upon treatment with GANT-61 in a dose-dependant manner (Fig. 6B, Supplementary Fig. S6). To further elucidate the effects of GLI1 inhibition by GANT-61, we examined the levels of C-MYC, another known GLI1 target (15). The basal level of C-MYC was significantly higher in Men1-excised PIME1 cells as compared to the control wild type cells (Fig. 6B). While the protein levels of C-MYC were unaltered in control cells, treatment of Men1-excised PIME1 cells with GANT-61 resulted in substantial reduction of C-MYC levels in a dose-dependant manner (Fig. 6B, Supplementary Fig. S6). Furthermore, treatment with GANT-61 resulted in a larger decrease of cell proliferation in Men1-excised PIME1 cells as compared to control wild type cells at similar concentrations (Fig. 6C). Although the decrease in cell proliferation upon GANT-61 treatment is moderate, it is likely that other factors besides increase in GLI1 account for menin-excision-induced increase in cell proliferation. Nonetheless, our findings suggest that elevated expression of Gli1 along with its target genes upon Men1 excision in PIME cells at least partially accounts for increased cell proliferation.
Figure 6.
GLI1 inhibition by GANT-61 results in decreased expression of Gli1 and its target genes. (A) PIME1 cells were cultured under varying concentrations of GANT-61 and the mRNA levels of Gli1 were determined by qRT-PCR. (B) Cell lysates from control Men1l/l and Men1Δ/Δ PIME cells were treated with GANT-61 and the levels of menin, PTCH1 and C-MYC were determined by immunoblotting. Ponceau S staining is included as a control. (C) Men1l/l and Men1Δ/Δ PIME1 cells were cultured in the presence of GANT-61 for 6 days, and the effects on cell proliferation was determined. Data are plotted as a percent decrease in cell number in the inhibitor-treated samples compared to the DMSO-treated cells. (D) A model for direct repression of GLI1 repression mediated by menin. Error bars indicate ± s.d.
Discussion
Here, we identify the role of menin in repressing Gli1 independent of the canonical Hh signaling pathway. First, we show that menin recruits PRMT5 and its repressive histone H4R3 symmetric dimethylation (H4R3m2s) mark to the Gli1 promoter (Fig. 3A). Second, menin negatively affects binding of the active GLI1-HDAC1 complex to the promoter of its target genes including Gli1 (Fig. 4A). Epigenetic regulation of Hh signaling has previously been reported including hyper-methylation of components of the Hh signaling pathway in tumors (42), and epigenetic repression of GLI1 by SNF5, a core component of the SWI/SNF chromatin remodeling complex (43). Our results indicate that menin-PRMT5-mediated epigenetic suppression of Gli1 is crucial for regulating the pro-oncogenic GLI1 expression.
Non-canonical GLI1 activation, independent of mutations in Hh pathway components, has previously been reported (19-21). In PDAC's, SMO is dispensable in the ductal epithelium, and GLI1 is regulated in a SMO-independent mechanism mediated partly by TGF-β and KRAS (19). However, we did not observe any changes in total K-RAS protein levels upon Men1 excision in both Hh ligand responsive MEFs and Hh ligand non-responsive PIME1 cells (data not shown). Furthermore, menin is necessary for TGF-β-mediated repression of parathyroid cell proliferation (44), and it is thus unlikely that increased cell proliferation and Gli1 upregulation upon Men1 excision results from enhanced TGF-β signaling. We have shown that menin represses Gli1 directly by recruiting PRMT5 and its histone methylation mark to the Gli1 promoter (Fig. 3A). Importantly, ectopic expression of catalytically inactive PRMT5 mutant results in increased GLI1 transcriptional activity (Fig. 4A), demonstrating that menin and PRMT5 act in concert to repress Gli1 at least partly via PRMT5-catalyzed histone arginine methylation. Previous reports have shown that the canonical Hh signaling pathway plays an important role in the development and maintenance of small-cell lung cancer (SCLC) (45) and in the pathophysiology of gastrointestinal neuroendocrine carcinomas (46). Thus, in addition to activated canonical Hh signaling observed in certain neuroendocrine tumors as detailed above, we uncover here that direct activation of GLI1 in an Hh ligand-independent manner upon Men1 mutation may play a role in tumorigenesis of endocrine organs.
It was recently reported that SNF5 localizes to GLI1-regulated promoters, and loss of SNF5 results in aberrant activation of Gli1, a process that is insensitive to the SMO-antagonist NVP-LDE225 but sensitive to the GLI1-antagonist HPI-1 (43). Furthermore, subunits of the mSin3A/HDAC2 co-repressor complex co-purify with hSWI/SNF complexes (47), and PRMT5 associates with Brg1 and hBrm-based hSWI/SNF complexes (48). Thus, the menin-PRMT5 complex might synergize with the mSin3A-HDAC and the SWI/SNF complex to repress the expression of GLI1 target genes. Moreover, it has been reported that hSWI/SNF-associated PRMT5 methylates hypo-acetylated histones H3 and H4 more efficiently than hyper-acetylated histones H3 and H4, thus demonstrating synergism between repressive histone deacetylation and PRMT5-mediated histone methylation. Similarly, it is possible that inactive, acetylated-GLI is modified by the menin-PRMT5 complex further reinforcing the repressed state, and decreasing the expression of GLI1 target genes.
Men1 excision did not affect the global protein levels of HDAC1 (Supplementary Fig. S5). Thus, it is unlikely that the mode of GLI1 repression by menin occurs at the level of REN-Cul3 E3 ubiquitin ligase complex that targets HDAC for proteasomal degradation (24). Menin associates with both HDAC1 and HDAC2 through mSin3A (39), and it is possible that menin compromises the interaction between HDAC and GLI1. This would result in an increase of the transcriptionally inactive, acetylated-GLI1 species, resulting in a decrease of Gli1 target gene expression. Furthermore, menin might interfere with binding of the active GLI1-HDAC complex to the promoters of GLI1 target genes as demonstrated by ChIP assays above (Fig. 5A), and partially supported by the observation that Gli1 mRNA levels in Men1-null cells are more sensitive to TSA-induced reduction than in control WT cells (Fig. 5B).
In conclusion, we show that menin epigenetically represses GLI1 by recruiting PRMT5 and its histone methylation mark to the Gli1 promoter, and interferes with binding of the active GLI1-HDAC complex to the Gli1 promoter (Fig. 6D). Our studies define a novel mechanism controlling the levels of GLI1 via a menin-mediated epigenetic pathway, and provide a rationale for directly inhibiting GLI1 for treating neuroendocrine tumors.
Supplementary Material
Acknowledgments
The 8× GLI1 binding site luciferase construct was a generous gift from Dr. Sasaki (RIKEN Center for Developmental Biology, Japan).
Grant Support: We appreciate support from AACR Care for Carcinoid Foundation (11-60-33XH) and the NIH (R01-CA-113962 and R01-DK085121 to XH).
Footnotes
Conflicts of Interest. The authors declare no conflict of interest.
References
- 1.Libe R, Bertherat J. Molecular genetics of adrenocortical tumours, from familial to sporadic diseases. Eur J Endocrinol. 2005;153:477–87. doi: 10.1530/eje.1.02004. [DOI] [PubMed] [Google Scholar]
- 2.Burgess JR, Nord B, David R, et al. Phenotype and phenocopy: the relationship between genotype and clinical phenotype in a single large family with multiple endocrine neoplasia type 1 (MEN 1) Clin Endocrinol (Oxf) 2000;53:205–11. doi: 10.1046/j.1365-2265.2000.01032.x. [DOI] [PubMed] [Google Scholar]
- 3.Marx SJ. Molecular genetics of multiple endocrine neoplasia types 1 and 2. Nat Rev Cancer. 2005;5:367–75. doi: 10.1038/nrc1610. [DOI] [PubMed] [Google Scholar]
- 4.Bertolino P, Radovanovic I, Casse H, Aguzzi A, Wang ZQ, Zhang CX. Genetic ablation of the tumor suppressor menin causes lethality at mid-gestation with defects in multiple organs. Mech Dev. 2003;120:549–60. doi: 10.1016/s0925-4773(03)00039-x. [DOI] [PubMed] [Google Scholar]
- 5.Chandrasekharappa SC, Guru SC, Manickam P, et al. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science. 1997;276:404–7. doi: 10.1126/science.276.5311.404. [DOI] [PubMed] [Google Scholar]
- 6.Lemmens I, Van de Ven WJ, Kas K, et al. Identification of the multiple endocrine neoplasia type 1 (MEN1) gene. The European Consortium on MEN1 Hum Mol Genet. 1997;6:1177–83. doi: 10.1093/hmg/6.7.1177. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal SK, Guru SC, Heppner C, et al. Menin interacts with the AP1 transcription factor JunD and represses JunD-activated transcription. Cell. 1999;96:143–52. doi: 10.1016/s0092-8674(00)80967-8. [DOI] [PubMed] [Google Scholar]
- 8.Mensah-Osman EJ, Veniaminova NA, Merchant JL. Menin and JunD regulate gastrin gene expression through proximal DNA elements. Am J Physiol Gastrointest Liver Physiol. 2011;301:G783–90. doi: 10.1152/ajpgi.00160.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang J, Gurung B, Wan B, et al. The same pocket in menin binds both MLL and JUND but has opposite effects on transcription. Nature. 2012;482:542–6. doi: 10.1038/nature10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heppner C, Bilimoria KY, Agarwal SK, et al. The tumor suppressor protein menin interacts with NF-kappaB proteins and inhibits NF-kappaB-mediated transactivation. Oncogene. 2001;20:4917–25. doi: 10.1038/sj.onc.1204529. [DOI] [PubMed] [Google Scholar]
- 11.Kaji H, Canaff L, Lebrun JJ, Goltzman D, Hendy GN. Inactivation of menin, a Smad3-interacting protein, blocks transforming growth factor type beta signaling. Proc Natl Acad Sci U S A. 2001;98:3837–42. doi: 10.1073/pnas.061358098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas MK, Rastalsky N, Lee JH, Habener JF. Hedgehog signaling regulation of insulin production by pancreatic beta-cells. Diabetes. 2000;49:2039–47. doi: 10.2337/diabetes.49.12.2039. [DOI] [PubMed] [Google Scholar]
- 13.Lau J, Hebrok M. Hedgehog signaling in pancreas epithelium regulates embryonic organ formation and adult beta-cell function. Diabetes. 2010;59:1211–21. doi: 10.2337/db09-0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang J, Hui CC. Hedgehog signaling in development and cancer. Dev Cell. 2008;15:801–12. doi: 10.1016/j.devcel.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasca di Magliano M, Hebrok M. Hedgehog signalling in cancer formation and maintenance. Nat Rev Cancer. 2003;3:903–11. doi: 10.1038/nrc1229. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, McMahon AP, Allen BL. Shifting paradigms in Hedgehog signaling. Curr Opin Cell Biol. 2007;19:159–65. doi: 10.1016/j.ceb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Hahn H, Wicking C, Zaphiropoulous PG, et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–51. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- 18.Taipale J, Chen JK, Cooper MK, et al. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–9. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 19.Nolan-Stevaux O, Lau J, Truitt ML, et al. GLI1 is regulated through Smoothened-independent mechanisms in neoplastic pancreatic ducts and mediates PDAC cell survival and transformation. Genes Dev. 2009;23:24–36. doi: 10.1101/gad.1753809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dennler S, Andre J, Alexaki I, et al. Induction of sonic hedgehog mediators by transforming growth factor-beta: Smad3-dependent activation of Gli2 and Gli1 expression in vitro and in vivo. Cancer Res. 2007;67:6981–6. doi: 10.1158/0008-5472.CAN-07-0491. [DOI] [PubMed] [Google Scholar]
- 21.Beauchamp E, Bulut G, Abaan O, et al. GLI1 is a direct transcriptional target of EWS-FLI1 oncoprotein. J Biol Chem. 2009;284:9074–82. doi: 10.1074/jbc.M806233200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kouzarides T. Acetylation: a regulatory modification to rival phosphorylation? EMBO J. 2000;19:1176–9. doi: 10.1093/emboj/19.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zupkovitz G, Tischler J, Posch M, et al. Negative and positive regulation of gene expression by mouse histone deacetylase 1. Mol Cell Biol. 2006;26:7913–28. doi: 10.1128/MCB.01220-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canettieri G, Di Marcotullio L, Greco A, et al. Histone deacetylase and Cullin3-REN(KCTD11) ubiquitin ligase interplay regulates Hedgehog signalling through Gli acetylation. Nat Cell Biol. 2010;12:132–42. doi: 10.1038/ncb2013. [DOI] [PubMed] [Google Scholar]
- 25.Gurung B, Feng Z, Iwamoto DV, et al. Menin epigenetically represses Hedgehog signaling in MEN1 tumor syndrome. Cancer Res. 2013;73:2650–8. doi: 10.1158/0008-5472.CAN-12-3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinelli DC, Fan CM. Gas1 extends the range of Hedgehog action by facilitating its signaling. Genes Dev. 2007;21:1231–43. doi: 10.1101/gad.1546307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinelli DC, Fan CM. A sonic hedgehog missense mutation associated with holoprosencephaly causes defective binding to GAS1. J Biol Chem. 2009;284:19169–72. doi: 10.1074/jbc.C109.011957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izzi Luisa CF. Boc and Gas1 Each Form Distinct Shh Receptor Complexes with Ptch1 and Are Required for Shh-Mediated Cell Proliferation. Developmental Cell. 2011;20:788–801. doi: 10.1016/j.devcel.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schnepp RW, Chen YX, Wang H, et al. Mutation of tumor suppressor gene Men1 acutely enhances proliferation of pancreatic islet cells. Cancer Res. 2006;66:5707–15. doi: 10.1158/0008-5472.CAN-05-4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen YX, Yan J, Keeshan K, et al. The tumor suppressor menin regulates hematopoiesis and myeloid transformation by influencing Hox gene expression. Proc Natl Acad Sci U S A. 2006;103:1018–23. doi: 10.1073/pnas.0510347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sasaki H, Hui C, Nakafuku M, Kondoh H. A binding site for Gli proteins is essential for HNF-3beta floor plate enhancer activity in transgenics and can respond to Shh in vitro. Development. 1997;124:1313–22. doi: 10.1242/dev.124.7.1313. [DOI] [PubMed] [Google Scholar]
- 32.Allen BL, Song JY, Izzi L, et al. Overlapping roles and collective requirement for the coreceptors GAS1, CDO, and BOC in SHH pathway function. Dev Cell. 2011;20:775–87. doi: 10.1016/j.devcel.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Y, Gurung B, Wu T, Wang H, Stoffers DA, Hua X. Reversal of preexisting hyperglycemia in diabetic mice by acute deletion of the Men1 gene. Proc Natl Acad Sci U S A. 2010;107:20358–63. doi: 10.1073/pnas.1012257107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yauch RL, Gould SE, Scales SJ, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–10. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 35.Stalberg P, Grimfjard P, Santesson M, et al. Transfection of the multiple endocrine neoplasia type 1 gene to a human endocrine pancreatic tumor cell line inhibits cell growth and affects expression of JunD, delta-like protein 1/preadipocyte factor-1, proliferating cell nuclear antigen, and QM/Jif-1. J Clin Endocrinol Metab. 2004;89:2326–37. doi: 10.1210/jc.2003-031228. [DOI] [PubMed] [Google Scholar]
- 36.Dahmane N, Lee J, Robins P, Heller P, Ruiz i Altaba A. Activation of the transcription factor Gli1 and the Sonic hedgehog signalling pathway in skin tumours. Nature. 1997;389:876–81. doi: 10.1038/39918. [DOI] [PubMed] [Google Scholar]
- 37.Regl G, Neill GW, Eichberger T, et al. Human GLI2 and GLI1 are part of a positive feedback mechanism in Basal Cell Carcinoma. Oncogene. 2002;21:5529–39. doi: 10.1038/sj.onc.1205748. [DOI] [PubMed] [Google Scholar]
- 38.Pollack BP, Kotenko SV, He W, Izotova LS, Barnoski BL, Pestka S. The human homologue of the yeast proteins Skb1 and Hsl7p interacts with Jak kinases and contains protein methyltransferase activity. J Biol Chem. 1999;274:31531–42. doi: 10.1074/jbc.274.44.31531. [DOI] [PubMed] [Google Scholar]
- 39.Kim H, Lee JE, Cho EJ, Liu JO, Youn HD. Menin, a tumor suppressor, represses JunD-mediated transcriptional activity by association with an mSin3A-histone deacetylase complex. Cancer Res. 2003;63:6135–9. [PubMed] [Google Scholar]
- 40.Lauth M, Bergstrom A, Shimokawa T, Toftgard R. Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists. Proc Natl Acad Sci U S A. 2007;104:8455–60. doi: 10.1073/pnas.0609699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wickstrom M, Dyberg C, Shimokawa T, et al. Targeting the hedgehog signal transduction pathway at the level of GLI inhibits neuroblastoma cell growth in vitro and in vivo. Int J Cancer. 2012 doi: 10.1002/ijc.27820. [DOI] [PubMed] [Google Scholar]
- 42.Cretnik M, Musani V, Oreskovic S, Leovic D, Levanat S. The Patched gene is epigenetically regulated in ovarian dermoids and fibromas, but not in basocellular carcinomas. Int J Mol Med. 2007;19:875–83. [PubMed] [Google Scholar]
- 43.Jagani Z, Mora-Blanco EL, Sansam CG, et al. Loss of the tumor suppressor Snf5 leads to aberrant activation of the Hedgehog-Gli pathway. Nat Med. 2010;16:1429–33. doi: 10.1038/nm.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sowa H, Kaji H, Kitazawa R, et al. Menin inactivation leads to loss of transforming growth factor beta inhibition of parathyroid cell proliferation and parathyroid hormone secretion. Cancer Res. 2004;64:2222–8. doi: 10.1158/0008-5472.can-03-3334. [DOI] [PubMed] [Google Scholar]
- 45.Park KS, Martelotto LG, Peifer M, et al. A crucial requirement for Hedgehog signaling in small cell lung cancer. Nat Med. 2011 doi: 10.1038/nm.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shida T, Furuya M, Nikaido T, et al. Sonic Hedgehog-Gli1 signaling pathway might become an effective therapeutic target in gastrointestinal neuroendocrine carcinomas. Cancer Biol Ther. 2006;5:1530–8. doi: 10.4161/cbt.5.11.3458. [DOI] [PubMed] [Google Scholar]
- 47.Sif S, Saurin AJ, Imbalzano AN, Kingston RE. Purification and characterization of mSin3A-containing Brg1 and hBrm chromatin remodeling complexes. Genes Dev. 2001;15:603–18. doi: 10.1101/gad.872801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pal S, Yun R, Datta A, et al. mSin3A/histone deacetylase 2- and PRMT5-containing Brg1 complex is involved in transcriptional repression of the Myc target gene cad. Mol Cell Biol. 2003;23:7475–87. doi: 10.1128/MCB.23.21.7475-7487.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.