Abstract
Mammalian embryos produce several waves of hematopoietic cells before the establishment of the hematopoietic stem cell (HSC) hierarchy. These early waves of embryonic hematopoiesis present a reversed hierarchy in which hematopoietic potential is first displayed by highly specialized cells that are derived from transient uni- and bipotent progenitor cells. Hematopoiesis progresses through multilineage erythro-myeloid progenitor cells that lack self-renewal potential and, subsequently, to make distinct lymphoid progenitor cells before culminating in detectable definitive HSC. This review provides an overview of the stepwise development of embryonic hematopoiesis. We focus on recent progress in demonstrating that lymphoid lineages emerge from hemogenic endothelial cells before the presence of definitive HSC activity and discuss the implications of these findings.
Introduction
In adult mammals, the hematopoietic system is maintained by hematopoietic stem cells (HSC) that reside in the bone marrow. As the ultimate precursor of the adult hematopoietic hierarchy, HSCs can self-renew and give rise to multipotent progenitors, which then differentiate to oligopotent and unipotent progenitors and, eventually, produce mature platelet, erythroid, dendritic, myeloid, lymphoid, and natural killer cells. In this process, the hematopoietic potential (self-renewal ability and/or lineage potential) of each cell type decreases along the hierarchal tree [1–4]. However, in the developing embryo, the metabolic and growth-promoting processes dictate the generation of a series of unique hematopoietic cells that are only transiently produced from distinct progenitor cells long before the generation of a definitive HSC. While much of our understanding of the molecular regulation of the earliest events of embryonic hematopoiesis has been learned from frog and zebrafish systems, the greatest number of reagents and functional assays to study this developmental hematopoiesis reside in the murine system. In this review, we will provide an overview of the spatiotemporal emergence of hematopoietic cells in the mouse embryo (Fig. 1). We will attempt to identify the most recent progress and identify residual questions on the origin of each wave of murine hematopoiesis. Reviews on developmental hematopoiesis in frogs and zebrafish can be found elsewhere [5–8].
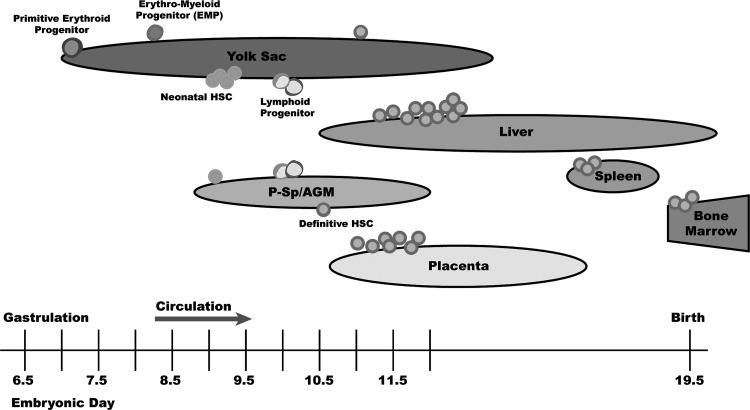
FIG. 1.
Murine hematopoiesis during embryonic development. Progenitors that can give rise to the primitive erythroid lineage emerge in the yolk sac at embryonic day 7.25 (E7.25). At E8.25, definitive erythro-myeloid progenitors (EMP) can be detected in the yolk sac. At E9.0, both yolk sac and para-aortic splanchnopleure (P-Sp) contain neonatal hematopoietic stem cells (HSC) that can reconstitute sublethally myeloablated newborn animals. Before the first definitive HSC can be detected, lymphoid progenitors that can differentiate into B or T lymphocytes arise in the yolk sac and P-Sp at E9.5. Finally, definitive HSC that can reconstitute lethally irradiated adult mice can be detected in the aorto-gonad-mesonephros (AGM) region at E10.5 and later in the yolk sac and placenta at E11. Definitive HSC expand in the placenta and fetal liver and migrate to the spleen and bone marrow before birth.
Primitive Hematopoiesis: The First Blood Cells
More than one hundred years ago, Alexander Maximow recognized that the first red blood cells emerging in the mouse embryo appeared “primitive” by displaying a number of distinct features that differed from adult “definitive” erythrocytes [9]. The primitive erythrocytes were exceptionally large and nucleated, more similar to the red blood cells of birds, reptiles, and fish than the small enucleated mature erythrocytes in adult mammals [10–12]. The first “primitive” red blood cells emerge in the mouse on embryonic day 7.5 (E7.5). These primitive erythroblasts divide rapidly and accumulate along with endothelial cells in the proximal yolk sac that eventually appear to form “blood islands” [13–15]. The primitive erythroblasts are six times larger and contain six times more hemoglobin compared with the adult-type definitive red blood cells [10,16–18]. At 4–8 somite pair (sp) stage (E8.25), when the embryonic heart starts beating, primitive erythroid cells enter the embryonic body through the nascent circulation [19–21] and go through a series of maturation steps, including cell division (until E13.5) [12,22], hemoglobin switching (E8.5–15.5) [23], and enucleation (E12.5–E16.5) [12,24,25]. At least some of the fully matured primitive erythrocytes persist in the bloodstream for the remainder of development but are progressively outnumbered by adult-type definitive red blood cells that are produced from E12 onward in the fetal liver [24,25].
While primitive erythroblasts may persist in the circulation throughout development, the primitive erythroid progenitor colony-forming cells (EryP-CFC) are only produced in a very transient developmental window. In the mouse, EryP-CFCs emerge as early as the mid-primitive streak stage (∼E7.25) exclusively in the yolk sac [12] and express low levels of the cell surface marker CD41 [14]. The number of EryP-CFC increases extensively in the late-primitive streak stage/early somite stage and decline hastily soon afterward. After E9.0 (∼20sp stage), no EryP-CFC can be identified in the mouse embryo [12]. Just as EryP-CFC mature simultaneously after 4–5 days of in vitro culture [12,26], the maturation of primitive erythroid cells in vivo occurs in a synchronized fashion as the cells migrate through the circulation [12,19,21–25].
Originally, the term “primitive” was only used to describe this first wave of primitive erythroblasts generated in the yolk sac based on their large, nucleated morphology [9]. However, accumulating evidence has shown that primitive erythroid cells are not the only product of this wave of hematopoiesis. Moore and Metcalf also described the presence of mature myeloid cells in the E7.5 yolk sac [27]. It is now known that some “primitive” megakaryocytes and macrophages, which exhibit unique features in terms of proliferation, differentiation, and maturation, are also produced in this first wave of mammalian hematopoiesis from E7.5 to E8.5 [12,27–39]. Thus, the nature of “primitive” hematopoiesis is more extensive than a single lineage of blood cells. Indeed, the recent report that embryonic macrophages emerging in the yolk sac at E8 give rise to long-term tissue macrophage populations in adult mice without ongoing contributions from HSCs indicates that some of the first emerging blood cells during embryogenesis should possess self-renewal properties [40–43].
Erythro-Myeloid Progenitor: Definitive Hematopoiesis Emerges as a Second Wave of Yolk Sac Hematopoiesis
Just as the term “primitive” was originally used to define a stage of erythroid development, the term “definitive” was originally used to describe the morphology of mature red blood cells in adult subjects [9]. However, over time, the term definitive was applied to other lineages of cells (macrophages, megakaryocytes, and mast cells) that emerged in development at the same time as the definitive adult-type erythroid cells. The term has also been applied to describe the HSC emerging during development, which display the capacity to give rise to multilineage hematopoiesis on transplantation into myeloablated adult mice [44]. However, the determination that erythro-myeloid progenitor (EMP) cells emerge in the yolk sac with properties similar to adult-type EMP cells has caused some confusion about use of the term “definitive” to describe these cells. Thus, this wave of hematopoiesis in the yolk sac has been referred to as the “second wave,” the EMP phase, or the transient definitive stage [45].
From E8.25, various EMPs, including definitive erythroid colony-forming cells (BFU-E and CFU-E), macrophage colony-forming cells (CFU-M), granulocyte-macrophage colony-forming cells (CFU-GM), and multipotent high proliferative potential colony-forming cells (HPP-CFC), can be detected in the mouse yolk sac [12,14,27,46]. These progenitors enter the circulation and colonize the fetal liver at E10 [12,45–48]. Once matured in the fetal liver, the EMP progeny re-enter the circulation as mature cells at E12–E13 [21]. In contrast to primitive erythroblasts that mature intravascularly, definitive erythroid cells are enucleated and fully matured in the extravascular fetal liver tissue before entering the blood stream [12,25,45].
In wild-type mice, EMPs are first detectable in the yolk sac and then in the circulation, embryo proper, and placenta before liver colonization [12,28,46–48], indicating the possibility that this wave of EMPs is exclusively produced in the yolk sac and then distributed to other tissues through systemic blood flow. However, after the discovery of the first definitive HSC, which could reconstitute adult myeloablated recipients, in the aorto-gonad-mesonephros (AGM) region [49,50], the term “definitive” became frequently used to depict HSC-derived hematopoiesis. This raised the possibility that “definitive EMP” may be derived from the para-aortic splanchnopleure (P-Sp)/AGM region [51,52], and caused some controversy with regard to a yolk sac versus P-Sp/AGM origin for EMP [53–55]. Indeed, the establishment of the systemic circulation at E8.25, which overlaps with the timing of EMP emergence, makes it difficult to determine when and where the EMPs first emerge. Thus, transgenic mice lacking expression of the sodium-calcium exchanger protein NCX1, which are unable to establish a systemic circulation due to the lack of a heartbeat [56], were employed to examine EMP origin independent of circulating blood. In Ncx1−/− (Ncx1-null) mice, essentially all the hematopoietic progenitor cells emerge and are retained in the yolk sac, before the embryos die at E10–E11 [20,57]. In addition, the number of yolk sac hematopoietic progenitors in the Ncx1-null mice was unchanged from the total number of progenitors within the whole wild-type mouse concepts at the same developmental stage [20,57]. These data suggest that EMPs in the embryo proper of wild-type mice originate in the yolk sac (Fig. 1). However, it is also known that the biomechanical force generated by flowing blood is crucial to the production of hematopoietic cells in zebrafish [58] and mouse [59] embryos and may be critical for EMP emergence in the P-Sp of the embryo proper. Indeed, after being exposed to sheer stress ex vivo, the colony-forming unit (CFU) forming activity of P-Sp cells from Ncx1-null mice was significantly elevated [59]. Precirculation allantois explants and placental cells from Ncx1-null embryos also possess the potential to produce myeloid and definitive erythroid progenitors in vitro, although at greatly diminished concentrations compared with the yolk sac [60–63]. Likewise, a subset of the heart tube endocardium possesses EMP potential at a precirculation stage [64].
Unlike primitive hematopoietic progenitors that either derive directly from the mesoderm or emerge from a hemogenic endothelial intermediate [65–69], the direct precursor of the EMP wave of progenitor cells is less controversial. EMPs are considered the direct derivatives from the hemogenic endothelium [70–72]. VE-cadherin+CD45−Ter119− endothelial cells from E8.5 yolk sac and P-Sp can produce erythroid and myeloid cells in vitro [73]. In murine embryonic stem (ES) cell differentiation culture, EMPs are also derived from hemogenic endothelial cells [69,74]. In vivo, CD41+ clusters, which contain EMPs, are attached to endothelial cells forming the yolk sac capillary bed at E8.25 [14]. At E8.5, about 2% of the endothelial cells in the yolk sac are estimated to be the hemogenic endothelium that can produce multipotent EMPs [75,76].
CFU-Spleen, Lymphoid Progenitors, and Neonatal Repopulating HSCs: Precursors to the Dawn of HSCs
From E8.5 to the onset of definitive HSCs at E10.5, another three classes of hematopoietic progenitor/stem cells are produced in the mouse conceptus. The spatiotemporal origin of these progenitor cells largely overlaps, and a few markers have been developed to adequately probe their similarities or differences. Each unique cell type will be briefly introduced, and any comparisons that have been conducted between these cells and definitive HSC will be discussed.
CFU-Spleen
CFU-spleen (CFU-S), which are considered as representing short-term multilineage precursors [46,77], can be found in the mouse yolk sac and P-Sp/AGM as early as E9.5 by transplantation into adult hosts [27,50,78–80]. Although it is believed that CFU-S originate from the AGM because this region contains more CFU-S than the yolk sac at E10.0 [78,80,81], both E8.0–E8.5 yolk sac and P-Sp cells can give rise to CFU-S after being cocultured with AGM-S3 stromal cells (an E10.5 AGM-derived stromal cell line) [82]. CFU-S have not been detected in progenitor cells that are derived from differentiated murine ES cells [83]. Thus, the spatial origin of these cells in the murine conceptus remains unclear.
Lymphoid Progenitors
In the search for the site of definitive HSC emergence in the mouse embryo, lymphoid potential in each hematopoietic organ (yolk sac, P-Sp, and placenta) had been extensively examined, as it had been believed that lymphoid cells could be produced only by HSCs, and that detection of lymphoid cells would predict the presence of an HSC. Most of these studies were done by coculturing embryonic tissues with stromal cell lines such as OP9 and S-17 that support B lymphopoiesis in vitro. Godin et al. cocultured yolk sac and P-Sp cells with S17 stromal cells and detected lymphocyte potential in both tissues from 10-25sp embryos [84]. In addition, through a single cell assay, they confirmed that these progenitors were multipotent and could differentiate into B and T cells and myeloid cells in vitro [84]. Through an in vitro culture, Liu and Auerbach detected T-lymphoid potential from E8 yolk sac [85]; while Huang et al. detected B-lymphoid potential from both E9 yolk sac and embryo proper [86]. Cumano et al. separated yolk sac and P-Sp tissues before the establishment of the systemic circulation and cultured these tissue explants in vitro. Cells from P-Sp, but not yolk sac explants showed the potential to differentiate into B, T, and myeloid cell lineages in vitro [51,52]. Furthermore, P-Sp explant-derived cells could also provide long-term multilineage reconstitution (including B and T lymphocytes) to sublethally irradiated Rag2γc−/− recipient mice [52]. These results were interpreted as evidence that the P-Sp/AGM is the only site of emergence for definitive HSC and lymphoid cells. Yokota et al. also showed, using RAG1-GFP mice, that the majority of the earliest (E10.5) RAG1/GFPlowCD45+ primitive lymphoid cells were distributed in the embryo body. When examining tissues that were cocultured with OP9 stromal cells, 8-12sp (E8.5–E9.0) P-Sp cells, but not yolk sac cells, gave rise to RAG1/GFP+CD45+ lymphoid cells [87]. In contrast, using the same OP9 coculture system, Nishikawa et al. identified B and T lymphocyte potential in VE-cadherin+CD45−Ter119− endothelial cells from E9.5 yolk sac and embryo proper [73]. Thus, some controversy has emerged with regard to the spatiotemporal origin of lymphoid cells in the developing embryo. Since the embryonic heartbeat starts at E8.25 and the systemic circulation may cause a mixture of the lymphoid progenitor cells derived from different organs, a novel approach to resolve the controversy was required.
To ask the origin of B- and T-lymphoid cells in the mouse embryo, we have utilized Ncx1-null mice that display normal cardiac development, but lack the onset of cardiac contractions and fail to generate systemic blood flow. We also asked what kind of B cells would be generated from the yolk sac and P-Sp before HSC emergence. Murine B lymphocytes are generally segregated into 3 populations: B-1, B-2, and marginal zone (MZ) B cells. B-2 cells develop in postnatal bone marrow from HSCs and mature into follicular B cells in the spleen. B-1 cells, which are subdivided into CD5+B-1a cells and CD5− B-1b cells, are a part of innate immunity and secrete natural IgM antibodies in a T-cell-independent manner [88,89]. B-1 cells reside in the peritoneal cavity and are considered of fetal origin, because fetal liver reconstitutes B-1 cells much more efficiently than adult HSCs [89,90], and transplantation of a single purified HSC failed to reconstitute B-1a cells in recipient mice [88]. Recent reports that B-1-specific progenitor cells found in the fetal liver and neonatal bone marrow are diminished in the adult bone marrow also support this idea [91,92]. MZ B cells, which have similar functions with B-1 cells, are considered as belonging to B-2 cells, but it is also suggested that a part of them are embryonic derived [93]. Thus, we hypothesized that the B cells produced in the yolk sac and P-Sp may belong to a B-1 cell subset. We have demonstrated that E9.5 Ncx1-null yolk sac and P-Sp cells as well as wild-type cells from these tissues gave rise to lin−AA4.1+CD19+B220lo−neg B-1 progenitor cells in coculture with OP9 stromal cells [94]. These B cells were derived from sorted CD41− VE-cadherin+ hemogenic endothelial cells from E9.5 yolk sac and P-Sp, but not CD41+ VE-cadherin− hematopoietic cells. Of interest, B cells could be generated in vitro using CD41+ VE-cadherin− hematopoietic cells, but only after the 28 somite pair stage [94]. Importantly, CD19+ cells were detected in VE-cadherin+ endothelial cells in the yolk sac by confocal microscopy. Direct intraperitoneal transplantation of freshly isolated E9.5 yolk sac cells engrafted in NOD/SCID/IL2γcnull (NSG) neonates as mature B-1 (both B-1a and B-1b) cells and MZ B cells, but no other lineages of hematopoietic cells, indicating that restricted B-1 and MZ B cell progenitors have emerged in the embryo at E9.5 [94]. E9.5 yolk sac and P-Sp derived lin−AA4.1+CD19+B220lo-neg B-1 specific progenitor cells engrafted in NSG neonates as mature B-1 cells (IgM+IgDloMac1+) for approximately 9 months in primary hosts and for at least 4 months in secondary recipient hosts [94]. In addition, these yolk sac and P-Sp-derived IgM+ B cells produced anti-phosphorylchorine IgM on stimulation without T-cell support. Thus, autonomous yolk sac and P-Sp lymphoid potential was confirmed using the Ncx1-null mouse model, and the B cells produced from these sites at a predefinitive HSC stage were identified as B-1 cells. In contrast, conventional B-2 cells are not derived from either the yolk sac or P-Sp at this predefinitive HSC stage [94].
Similarly, T-cell potential was also confirmed in wild-type and Ncx1-null yolk sac and P-Sp tissues before E10 when cells were cocultured on Delta-like ligand 1 (Dll1) over-expressing OP9 (OP9-DL1) cells [95]. Using this system, CD4+CD8+ double-positive T cells as well as TCRβ and TCRγδ T cells were produced from E9.5 wild-type and Ncx1-null yolk sac and P-Sp cells. These yolk sac and P-Sp derived T cells were transplantable into NSG neonates, reconstituted the neonatal thymus, and differentiated into numerous T-cell subsets, including naïve, memory, and regulatory T cells [95]. More importantly, freshly isolated E9.5 wild-type and Ncx1-null yolk sac cells reconstituted only T cells, but not cells of other lineages, in NSG mice on transplantation; while freshly isolated cells from E9.5 P-Sp showed no such reconstitution capability. These results provide compelling evidence of the existence of T-cell-restricted progenitors emerging within the E9.5 yolk sac and maturing to a thymic engrafting state, 1 day before the emergence of definitive HSCs [95].
Taken together, these studies provide evidence that both yolk sac and P-Sp tissues contain B and T progenitor cells at 1 or 2 days before definitive HSCs are detectable within the AGM region (Fig. 1). In agreement with our results, Boiers et al. have recently detected restricted lymphomyeloid lineage progenitors that emerged before definitive HSCs during murine development [96]. These results are important for several reasons and raise several issues: (1) Since definitive HSC-independent lymphopoiesis arises in the developing embryo, lymphoid potential displayed by a tissue does not predict the existence of an HSC. (2) Definitive HSC-independent lymphoid cells may contribute to innate immunity in postnatal life. (3) Definitive HSC-independent lymphoid cells may be depleted in a bone marrow transplant setting and will not be reconstituted by HSC transplantation. For example, in the mouse, the B-1 cell potential of HSC and common lymphoid progenitors has been proved to decrease with advancing age. Single cell transplantation of adult long-term HSC failed to reconstitute B-1a cells in recipient mice though B-2, MZ, and B-1b cells were donor derived [88,89,91]. More work will be required to fully understand the implications of these new findings in both mouse and human subjects.
Neonatal Repopulating HSCs
Although definitive HSCs, which can provide long-term, multilineage reconstitution in adult recipient mice, do not exist in the mouse embryo until E10.5 [47,49,50,97–100], cells with long-term multilineage repopulating ability that can reconstitute sublethally myeloablated newborn animals can be detected in the E9.0–E10.0 yolk sac and P-Sp (Fig. 1) [14,101–103]. Neonatal HSCs are enriched in CD34+ckit+ cells [104] and are also positive for CD41 [14] and VE-cadherin [103]. On injecting into the blood, peritoneal cavity, or liver of myeloablated neonatal mice, these cells can provide long-term, multilineage engraftment in primary recipients for 9 months and in secondary adult recipients for at least 6 months [102]. In another report, E9.0 yolk sac cells were micro injected into E11–E15 anemic fetal hosts, and gave rise to long-term reconstitution in the recipient animals after birth [105]. Likewise, an injection of donor yolk sac cells into host yolk sac tissues in utero gave rise to donor hematopoietic reconstitution postnatally [106]. Neonatal repopulating HSC display many of the features of the latter appearing AGM-derived definitive HSC but lack the expression of stem cell antigen-1 (Sca-1) and CD45 [81,107,108] and are noted to express a different transcriptome than the AGM-derived definitive HSC [109].
Many questions regarding neonatal repopulating HSCs are yet to be answered: Do they differentiate in the fetus to produce mature blood cells before the establishment of the definitive HSC hierarchy? Are they the precursors of definitive HSCs that emerge in the embryo proper at E10.5 or do they diminish after the emergence of AGM-derived definitive HSCs? Rybtsov S et al. have defined a hierarchical pathway of VE-cadherin+CD45−CD41+ pre-HSC isolated from the E9.5 AGM that may subsequently mature into definitive HSC via in vitro coaggregation with OP9 stromal cells for 4 days before transplantation [110]. Whether these pre-HSC cells represent the sublethally ablated newborn engrafting CD34+CD41+CD45− cells present in the E9.0 yolk sac and P-Sp/AGM remains to be determined. Finally, the origin of neonatal HSCs within the yolk sac and P-Sp has not been defined. Finding the specific cell surface markers or gene expression patterns of neonatal repopulating HSCs would be a reasonable next step to answer these questions [109].
Definitive HSCs: The Final Product of Embryonic Hematopoiesis
At E10.5, definitive HSCs that can reconstitute myeloablated adult recipient mice start emerging from the ventral wall of the aorta in the AGM region (Fig. 1) [47,49,50, 70,97,98,111,112]. They can also be found in vitelline/umbilical arteries [97] at the same stage and slightly later in the placenta [47,100,113,114] and yolk sac [47,49,98]. At E11.5–E12, HSCs start being detectable in the fetal liver and circulation [47,115]. Afterward, they colonize the fetal thymus and spleen and eventually, seed the bone marrow right before birth [116,117].
The Endothelial Origin of HSCs
The concept of a “hemogenic endothelial cell” may have been first described in chick embryos [118]. The endothelial cells were labeled with DiI-labeled acetylated low-density lipoprotein (AcLDL) in the dorsal aorta of chick embryos, and CD45+ hematopoietic cells labeled with the Dil-AcLDL were observed as budding off from the endothelial intima [118]. Similarly, in mouse embryos, hematopoietic budding from the ventral wall of the dorsal aorta has been observed at E10.5–E11.5 [70,107,112,119–121]. These hematopoietic clusters are known to express Runx1, and deletion of this gene causes loss of all EMP and HSCs [107,121–123]. Embryos that lack GATA2 [124–127], Scl [128,129], and Notch1 [120], as well as Runx1 [107,121–123], lose hematopoietic clusters and HSC potential in the dorsal aorta. Thus, the hematopoietic clusters budding from the dorsal aorta are considered an indicator of definitive HSC emergence. Recent lineage tracing experiments revealed that most adult hematopoietic cells resident in the bone marrow were the descendants of VE-cadherin-expressing endothelial cells [72]. When Runx1 was deleted in the VE-cadherin expressing cells, the intra-aortic clusters and definitive HSCs were totally abrogated [122]. In contrast, when Runx1 expression was deleted in hematopoietic cells, definitive HSC emergence from the AGM region was unaffected, indicating that Runx1 is indispensable for the endothelial to HSC transition, but not thereafter [122]. Finally, the actual endothelial-hematopoietic transition events in the mouse [70] and zebrafish [71,130] dorsal aorta have been unveiled by live imaging confirming that this lineage fate change occurs as a normal developmental event.
Seeking the HSC Origin: Potential Yolk Sac Contribution to HSC Emergence
The first HSCs that display the ability to reconstitute an adult hematopoietic system arise in the AGM region [49,50,97,98,111]; however, definitive HSCs are also detectable in the yolk sac and placenta at 1 day later [47,49,98,100,113,114]. Thus, while the AGM may be the first, it may not be the only tissue that has the potential to generate definitive HSC. For example, E8.5 yolk sac cells reconstituted lethally irradiated adult mice after being cocultured with AGM-derived stromal cells [82]. Yolk sac and P-Sp-derived neonatal repopulating HSCs converted into definitive HSCs at a time of secondary transplantation [101,102,104]. Pre-HSCs identified in the murine embryo are capable of engrafting in myeloablated adult recipient mice if first coaggregated with OP9 stromal cells in vitro [110]. These data suggest that there are particular maturational mechanisms which instruct or permit cells that lack adult repopulating ability to acquire that capacity.
Samokhvalov et al. have developed an inducible lineage tracing system in which GFP or lacZ could be coexpressed in Runx1 expressing cells by a single tamoxifen injection [131]. Using this system, they demonstrated that Runx1+ cells in the E7.5 yolk sac (no Runx1 expression reported in P-Sp at this time) contributed to multiple lineages of hematopoietic cells and the HSC compartment in fetal liver and adult bone marrow cells [131]. Interestingly, E7.5 tagged yolk sac Runx1+ progeny were detected within the endothelial cell intima of the umbilical vein, artery, and dorsal aorta at E10.5–E11.5 [131]. This observation implied that some of the endothelial cells in these HSC-producing regions were derived from E7.5-labeled yolk sac cells. In a subsequent study, Tanaka et al. developed an inducible Runx1 rescue system by a single tamoxifen injection in Runx1 knockout embryos [132]. Definitive hematopoiesis and HSC generation were rescued by a single injection of tamoxifen at E6.5 to E7.5, but not at any other time point [132]. This result suggested that Runx1 expression in the E7.5 yolk sac was indispensable for HSC production that persisted into the adult bone marrow compartment. However, for these two reports, some investigators have questioned whether some Runx1+ cells could also exist in the P-Sp at E7.5, or whether the half life of injected tamoxifen in the embryo could have been longer than the authors anticipated, raising the possibility that certain intraembryonic Runx1-expressing cells may have also contributed to the HSC marking. Further studies may yield new insights into the role of the very early E7.5 yolk sac endothelium or mesoderm, in contributing to HSC formation.
Placenta
The placenta has been considered a hematopoietic organ for decades [133]. Emergence of definitive HSCs in the placenta has been reported to occur around the same time as they appear in the yolk sac and AGM region [47,100]. At E11.5–E12.5, HSCs expand 15 times more than in the AGM region, and, thus, the placenta is recognized as an important niche for HSCs in the mouse embryo. In Ncx1-null embryos (no circulation embryos), CD41+ hematopoietic cell clusters can be found in the placenta vasculature at E10.5 [63]. In addition, E8.5–E9.5 placental cells from Ncx1-null embryos displayed lymphoid and myeloid potential in coculture with OP9 or OP9-Dll1 stromal cells [63]. These studies suggested the autonomous emergence of hematopoietic cells within the placental vasculature. Direct evidence for the production of engrafting HSC from this site in the Ncx1-null embryo remains elusive.
Summary
The emergence of definitive HSC is a relatively late event in embryonic hematopoiesis. Before the establishment of adult-type hematopoiesis, several waves of hematopoiesis are developed to support the survival and growth of the embryo. Based on current data, it seems that the yolk sac contributes to the early waves of progenitor cells (primitive hematopoietic progenitors, EMPs, and lymphoid progenitors); while the P-Sp (later becomes AGM) sequentially displays B- and T-lymphoid potential before producing the first definitive HSC. To date, it is not clear whether these waves of embryonic hematopoiesis are independently developed from different hemogenic endothelial cells or depend on the maturation of hemogenic endothelial cells possessing multilineage hematopoietic potential. It is important to acknowledge that attempts to generate definitive HSCs from ES cells and induced pluripotent stem (IPS) cells have recapitulated the derivation of all of the waves of hematopoiesis seen in the yolk sac, but have yet to prove definitive HSC emergence [36,37,134,135]. Many investigators are attempting to better understand how hematopoiesis is initiated in the AGM region, as an important first step in identifying candidate pathways to modulate in differentiating ES or IPS cells. Such a search is tedious given the only reliable assay is to transplant cells and demonstrate multilineage engraftment for proof of a definitive HSC (as it is now clear that lymphoid cell presence does not predict the presence of an HSC). A better understanding of the mechanisms of definitive HSC expansion in the fetal liver would also provide new directions for ex vivo HSC expansion. The number of definitive HSCs in the fetal liver is increased 38-fold from E12 to E16 of development [115]. These and numerous other questions raised earlier highlight developmental hematopoiesis as a field that continues to hold great promise for yielding new insights toward understanding how the hematopoietic system is established and the contributions of many different embryonic precursors throughout adult life.
Acknowledgments
The work was supported in part by the Riley Children's Foundation and NIH AI080759.
Author Disclosure Statement
There is nothing to disclose other than the funding sources cited earlier.
References
- 1.Akashi K, Traver D, Miyamoto T. and Weissman IL. (2000). A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 404:193–197 [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharya D, Bryder D, Rossi DJ. and Weissman IL. (2006). Rapid lymphocyte reconstitution of unconditioned immunodeficient mice with non-self-renewing multipotent hematopoietic progenitors. Cell Cycle 5:1135–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kondo M, Weissman IL. and Akashi K. (1997). Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell 91:661–672 [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto R, Morita Y, Ooehara J, Hamanaka S, Onodera M, Rudolph KL, Ema H. and Nakauchi H. (2013). Clonal analysis unveils self-renewing lineage-restricted progenitors generated directly from hematopoietic stem cells. Cell 154:1112–1126 [DOI] [PubMed] [Google Scholar]
- 5.Ciau-Uitz A, Liu F. and Patient R. (2010). Genetic control of hematopoietic development in Xenopus and zebrafish. Int J Dev Biol 54:1139–1149 [DOI] [PubMed] [Google Scholar]
- 6.Clements WK. and Traver D. (2013). Signalling pathways that control vertebrate haematopoietic stem cell specification. Nat Rev Immunol 13:336–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stachura DL. and Traver D. (2011). Cellular dissection of zebrafish hematopoiesis. Methods Cell Biol 101:75–110 [DOI] [PubMed] [Google Scholar]
- 8.Zhang C, Patient R. and Liu F. (2013). Hematopoietic stem cell development and regulatory signaling in zebrafish. Biochim Biophys Acta 1830:2370–2374 [DOI] [PubMed] [Google Scholar]
- 9.Maximow AA. (1909). Untersuchungen uber blut und bindegewebe. Die fruhesten entwicklungsstadien der blut- und binde- gewebszellan bein saugetierembryo, bis zum anfang der blutbilding unden leber. Arch Mikroskop Anat 73:444–561 [Google Scholar]
- 10.Brotherton TW, Chui DH, Gauldie J. and Patterson M. (1979). Hemoglobin ontogeny during normal mouse fetal development. Proc Natl Acad Sci U S A 76:2853–2857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kovach JS, Marks PA, Russell ES. and Epler H. (1967). Erythroid cell development in fetal mice: ultrastructural characteristics and hemoglobin synthesis. J Mol Biol 25:131–142 [DOI] [PubMed] [Google Scholar]
- 12.Palis J, Robertson S, Kennedy M, Wall C. and Keller G. (1999). Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development 126:5073–5084 [DOI] [PubMed] [Google Scholar]
- 13.Silver L. and Palis J. (1997). Initiation of murine embryonic erythropoiesis: a spatial analysis. Blood 89:1154–1164 [PubMed] [Google Scholar]
- 14.Ferkowicz MJ, Starr M, Xie X, Li W, Johnson SA, Shelley WC, Morrison PR. and Yoder MC. (2003). CD41 expression defines the onset of primitive and definitive hematopoiesis in the murine embryo. Development 130:4393–4403 [DOI] [PubMed] [Google Scholar]
- 15.Haar JL. and Ackerman GA. (1971). A phase and electron microscopic study of vasculogenesis and erythropoiesis in the yolk sac of the mouse. Anat Rec 170:199–223 [DOI] [PubMed] [Google Scholar]
- 16.Barker JE. (1968). Development of the mouse hematopoietic system. I. Types of hemoglobin produced in embryonic yolk sac and liver. Dev Biol 18:14–29 [DOI] [PubMed] [Google Scholar]
- 17.Fantoni A, De la Chapelle A. and Marks PA. (1969). Synthesis of embryonic hemoglobins during erythroid cell development in fetal mice. J Biol Chem 244:675–681 [PubMed] [Google Scholar]
- 18.Steiner R. and Vogel H. (1973). On the kinetics of erythroid cell differentiation in fetal mice. I. Microspectrophotometric determination of the hemoglobin content in erythroid cells during gestation. J Cell Physiol 81:323–338 [DOI] [PubMed] [Google Scholar]
- 19.Ji RP, Phoon CK, Aristizabal O, McGrath KE, Palis J. and Turnbull DH. (2003). Onset of cardiac function during early mouse embryogenesis coincides with entry of primitive erythroblasts into the embryo proper. Circ Res 92:133–135 [DOI] [PubMed] [Google Scholar]
- 20.Lux CT. and Yoder MC. (2010). Novel methods for determining hematopoietic stem and progenitor cell emergence in the murine yolk sac. Int J Dev Biol 54:1003–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGrath KE, Koniski AD, Malik J. and Palis J. (2003). Circulation is established in a stepwise pattern in the mammalian embryo. Blood 101:1669–1676 [DOI] [PubMed] [Google Scholar]
- 22.De la Chapelle A, Fantoni A. and Marks PA. (1969). Differentiation of mammalian somatic cells: DNA and hemoglobin synthesis in fetal mouse yolk sac erythroid cells. Proc Natl Acad Sci U S A 63:812–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kingsley PD, Malik J, Emerson RL, Bushnell TP, McGrath KE, Bloedorn LA, Bulger M. and Palis J. (2006). “Maturational”. globin switching in primary primitive erythroid cells. Blood 107:1665–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fraser ST, Isern J. and Baron MH. (2007). Maturation and enucleation of primitive erythroblasts during mouse embryogenesis is accompanied by changes in cell-surface antigen expression. Blood 109:343–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kingsley PD, Malik J, Fantauzzo KA. and Palis J. (2004). Yolk sac-derived primitive erythroblasts enucleate during mammalian embryogenesis. Blood 104:19–25 [DOI] [PubMed] [Google Scholar]
- 26.Palis J, Malik J, McGrath KE. and Kingsley PD. (2010). Primitive erythropoiesis in the mammalian embryo. Int J Dev Biol 54:1011–1018 [DOI] [PubMed] [Google Scholar]
- 27.Moore MA. and Metcalf D. (1970). Ontogeny of the haemopoietic system: yolk sac origin of in vivo and in vitro colony forming cells in the developing mouse embryo. Br J Haematol 18:279–296 [DOI] [PubMed] [Google Scholar]
- 28.Wong PM, Chung SW, Chui DH. and Eaves CJ. (1986). Properties of the earliest clonogenic hemopoietic precursors to appear in the developing murine yolk sac. Proc Natl Acad Sci U S A 83:3851–3854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naito M, Yamamura F, Nishikawa S. and Takahashi K. (1989). Development, differentiation, and maturation of fetal mouse yolk sac macrophages in cultures. J Leukoc Biol 46:1–10 [DOI] [PubMed] [Google Scholar]
- 30.Takahashi K, Yamamura F. and Naito M. (1989). Differentiation, maturation, and proliferation of macrophages in the mouse yolk sac: a light-microscopic, enzyme-cytochemical, immunohistochemical, and ultrastructural study. J Leukoc Biol 45:87–96 [DOI] [PubMed] [Google Scholar]
- 31.MJ Xu, Matsuoka S, Yang FC, Ebihara Y, Manabe A, Tanaka R, Eguchi M, Asano S, Nakahata T. and Tsuji K. (2001). Evidence for the presence of murine primitive megakaryocytopoiesis in the early yolk sac. Blood 97:2016–2022 [DOI] [PubMed] [Google Scholar]
- 32.Xie X, Chan RJ, Johnson SA, Starr M, McCarthy J, Kapur R. and Yoder MC. (2003). Thrombopoietin promotes mixed lineage and megakaryocytic colony-forming cell growth but inhibits primitive and definitive erythropoiesis in cells isolated from early murine yolk sacs. Blood 101:1329–1335 [DOI] [PubMed] [Google Scholar]
- 33.Tober J, Koniski A, McGrath KE, Vemishetti R, Emerson R, de Mesy-Bentley KK, Waugh R. and Palis J. (2007). The megakaryocyte lineage originates from hemangioblast precursors and is an integral component both of primitive and of definitive hematopoiesis. Blood 109:1433–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tober J, McGrath KE. and Palis J. (2008). Primitive erythropoiesis and megakaryopoiesis in the yolk sac are independent of c-myb. Blood 111:2636–2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klimchenko O, Mori M, Distefano A, Langlois T, Larbret F, Lecluse Y, Feraud O, Vainchenker W, Norol F. and Debili N. (2009). A common bipotent progenitor generates the erythroid and megakaryocyte lineages in embryonic stem cell-derived primitive hematopoiesis. Blood 114:1506–1517 [DOI] [PubMed] [Google Scholar]
- 36.Fujimoto TT, Kohata S, Suzuki H, Miyazaki H. and Fujimura K. (2003). Production of functional platelets by differentiated embryonic stem (ES) cells in vitro. Blood 102:4044–4051 [DOI] [PubMed] [Google Scholar]
- 37.Zambidis ET, Peault B, Park TS, Bunz F. and Civin CI. (2005). Hematopoietic differentiation of human embryonic stem cells progresses through sequential hematoendothelial, primitive, and definitive stages resembling human yolk sac development. Blood 106:860–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bertrand JY, Jalil A, Klaine M, Jung S, Cumano A. and Godin I. (2005). Three pathways to mature macrophages in the early mouse yolk sac. Blood 106:3004–3011 [DOI] [PubMed] [Google Scholar]
- 39.Naito M, Umeda S, Yamamoto T, Moriyama H, Umezu H, Hasegawa G, Usuda H, Shultz LD. and Takahashi K. (1996). Development, differentiation, and phenotypic heterogeneity of murine tissue macrophages. J Leukoc Biol 59:133–138 [DOI] [PubMed] [Google Scholar]
- 40.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, et al. (2012). A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336:86–90 [DOI] [PubMed] [Google Scholar]
- 41.Psaltis PJ, Harbuzariu A, Delacroix S, Witt TA, Holroyd EW, Spoon DB, Hoffman SJ, Pan S, Kleppe LS, et al. (2012). Identification of a monocyte-predisposed hierarchy of hematopoietic progenitor cells in the adventitia of postnatal murine aorta. Circulation 125:592–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS. and Allen JE. (2011). Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 332:1284–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, LG Ng, et al. (2010). Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330:841–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dzierzak E. (1999). Embryonic beginnings of definitive hematopoietic stem cells. Ann N Y Acad Sci 872:256–262; discussion 262–254. [DOI] [PubMed] [Google Scholar]
- 45.McGrath KE, Frame JM, Fromm GJ, Koniski AD, Kingsley PD, Little J, Bulger M. and Palis J. (2011). A transient definitive erythroid lineage with unique regulation of the beta-globin locus in the mammalian embryo. Blood 117:4600–4608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palis J, Chan RJ, Koniski A, Patel R, Starr M. and Yoder MC. (2001). Spatial and temporal emergence of high proliferative potential hematopoietic precursors during murine embryogenesis. Proc Natl Acad Sci U S A 98:4528–4533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gekas C, Dieterlen-Lievre F, Orkin SH. and Mikkola HK. (2005). The placenta is a niche for hematopoietic stem cells. Dev Cell 8:365–375 [DOI] [PubMed] [Google Scholar]
- 48.Alvarez-Silva M, Belo-Diabangouaya P, Salaun J. and Dieterlen-Lievre F. (2003). Mouse placenta is a major hematopoietic organ. Development 130:5437–5444 [DOI] [PubMed] [Google Scholar]
- 49.Muller AM, Medvinsky A, Strouboulis J, Grosveld F. and Dzierzak E. (1994). Development of hematopoietic stem cell activity in the mouse embryo. Immunity 1:291–301 [DOI] [PubMed] [Google Scholar]
- 50.Medvinsky A. and Dzierzak E. (1996). Definitive hematopoiesis is autonomously initiated by the AGM region. Cell 86:897–906 [DOI] [PubMed] [Google Scholar]
- 51.Cumano A, Dieterlen-Lievre F. and Godin I. (1996). Lymphoid potential, probed before circulation in mouse, is restricted to caudal intraembryonic splanchnopleura. Cell 86:907–916 [DOI] [PubMed] [Google Scholar]
- 52.Cumano A, Ferraz JC, Klaine M, Di Santo JP. and Godin I. (2001). Intraembryonic, but not yolk sac hematopoietic precursors, isolated before circulation, provide long-term multilineage reconstitution. Immunity 15:477–485 [DOI] [PubMed] [Google Scholar]
- 53.Cumano A. and Godin I. (2007). Ontogeny of the hematopoietic system. Annu Rev Immunol 25:745–785 [DOI] [PubMed] [Google Scholar]
- 54.Godin I. and Cumano A. (2002). The hare and the tortoise: an embryonic haematopoietic race. Nat Rev Immunol 2:593–604 [DOI] [PubMed] [Google Scholar]
- 55.Gordon-Keylock S, Sobiesiak M, Rybtsov S, Moore K. and Medvinsky A. (2013). Mouse extraembryonic arterial vessels harbor precursors capable of maturing into definitive HSCs. Blood 122:2338–2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koushik SV, Wang J, Rogers R, Moskophidis D, Lambert NA, Creazzo TL. and Conway SJ. (2001). Targeted inactivation of the sodium-calcium exchanger (Ncx1) results in the lack of a heartbeat and abnormal myofibrillar organization. FASEB J 15:1209–1211 [DOI] [PubMed] [Google Scholar]
- 57.Lux CT, Yoshimoto M, McGrath K, Conway SJ, Palis J. and Yoder MC. (2008). All primitive and definitive hematopoietic progenitor cells emerging before E10 in the mouse embryo are products of the yolk sac. Blood 111:3435–3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.North TE, Goessling W, Peeters M, Li P, Ceol C, Lord AM, Weber GJ, Harris J, Cutting CC, et al. (2009). Hematopoietic stem cell development is dependent on blood flow. Cell 137:736–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adamo L, Naveiras O, Wenzel PL, McKinney-Freeman S, Mack PJ, Gracia-Sancho J, Suchy-Dicey A, Yoshimoto M, Lensch MW, et al. (2009). Biomechanical forces promote embryonic haematopoiesis. Nature 459:1131–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Corbel C, Salaun J, Belo-Diabangouaya P. and Dieterlen-Lievre F. (2007). Hematopoietic potential of the pre-fusion allantois. Dev Biol 301:478–488 [DOI] [PubMed] [Google Scholar]
- 61.Downs KM, Gifford S, Blahnik M. and Gardner RL. (1998). Vascularization in the murine allantois occurs by vasculogenesis without accompanying erythropoiesis. Development 125:4507–4520 [DOI] [PubMed] [Google Scholar]
- 62.Zeigler BM, Sugiyama D, Chen M, Guo Y, Downs KM. and Speck NA. (2006). The allantois and chorion, when isolated before circulation or chorio-allantoic fusion, have hematopoietic potential. Development 133:4183–4192 [DOI] [PubMed] [Google Scholar]
- 63.Rhodes KE, Gekas C, Wang Y, Lux CT, Francis CS, Chan DN, Conway S, Orkin SH, Yoder MC. and Mikkola HK. (2008). The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell 2:252–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nakano H, Liu X, Arshi A, Nakashima Y, van Handel B, Sasidharan R, Harmon AW, Shin JH, Schwartz RJ, et al. (2013). Haemogenic endocardium contributes to transient definitive haematopoiesis. Nat Commun 4:1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choi K, Kennedy M, Kazarov A, Papadimitriou JC. and Keller G. (1998). A common precursor for hematopoietic and endothelial cells. Development 125:725–732 [DOI] [PubMed] [Google Scholar]
- 66.Fehling HJ, Lacaud G, Kubo A, Kennedy M, Robertson S, Keller G. and Kouskoff V. (2003). Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development 130:4217–4227 [DOI] [PubMed] [Google Scholar]
- 67.Huber TL, Kouskoff V, Fehling HJ, Palis J. and Keller G. x(2004). Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature 432:625–630 [DOI] [PubMed] [Google Scholar]
- 68.Kinder SJ, Tsang TE, Quinlan GA, Hadjantonakis AK, Nagy A. and Tam PP. (1999). The orderly allocation of mesodermal cells to the extraembryonic structures and the anteroposterior axis during gastrulation of the mouse embryo. Development 126:4691–4701 [DOI] [PubMed] [Google Scholar]
- 69.Lancrin C, Sroczynska P, Stephenson C, Allen T, Kouskoff V. and Lacaud G. (2009). The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature 457:892–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boisset JC, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E. and Robin C. (2010). In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature 464:116–120 [DOI] [PubMed] [Google Scholar]
- 71.Kissa K. and Herbomel P. (2010). Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 464:112–115 [DOI] [PubMed] [Google Scholar]
- 72.Zovein AC, Hofmann JJ, Lynch M, French WJ, Turlo KA, Yang Y, Becker MS, Zanetta L, Dejana E, et al. (2008). Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell 3:625–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nishikawa SI, Nishikawa S, Kawamoto H, Yoshida H, Kizumoto M, Kataoka H. and Katsura Y. (1998). In vitro generation of lymphohematopoietic cells from endothelial cells purified from murine embryos. Immunity 8:761–769 [DOI] [PubMed] [Google Scholar]
- 74.Eilken HM, Nishikawa S. and Schroeder T. (2009). Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature 457:896–900 [DOI] [PubMed] [Google Scholar]
- 75.Goldie LC, Lucitti JL, Dickinson ME. and Hirschi KK. (2008). Cell signaling directing the formation and function of hemogenic endothelium during murine embryogenesis. Blood 112:3194–3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nadin BM, Goodell MA. and Hirschi KK. (2003). Phenotype and hematopoietic potential of side population cells throughout embryonic development. Blood 102:2436–2443 [DOI] [PubMed] [Google Scholar]
- 77.Osawa M, Hanada K, Hamada H. and Nakauchi H. (1996). Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science 273:242–245 [DOI] [PubMed] [Google Scholar]
- 78.Medvinsky AL, Samoylina NL, Muller AM. and Dzierzak EA. (1993). An early pre-liver intraembryonic source of CFU-S in the developing mouse. Nature 364:64–67 [DOI] [PubMed] [Google Scholar]
- 79.Medvinsky AL, Gan OI, Semenova ML. and Samoylina NL. (1996). Development of day-8 colony-forming unit-spleen hematopoietic progenitors during early murine embryogenesis: spatial and temporal mapping. Blood 87:557–566 [PubMed] [Google Scholar]
- 80.de Bruijn MF, Peeters MC, Luteijn T, Visser P, Speck NA. and Dzierzak E. (2000). CFU-S(11) activity does not localize solely with the aorta in the aorta-gonad-mesonephros region. Blood 96:2902–2904 [PubMed] [Google Scholar]
- 81.Dzierzak E. and Speck NA. (2008). Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nat Immunol 9:129–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matsuoka S, Tsuji K, Hisakawa H, Xu M, Ebihara Y, Ishii T, Sugiyama D, Manabe A, Tanaka R, et al. (2001). Generation of definitive hematopoietic stem cells from murine early yolk sac and paraaortic splanchnopleures by aorta-gonad-mesonephros region-derived stromal cells. Blood 98:6–12 [DOI] [PubMed] [Google Scholar]
- 83.Muller AM. and Dzierzak EA. (1993). ES cells have only a limited lymphopoietic potential after adoptive transfer into mouse recipients. Development 118:1343–1351 [DOI] [PubMed] [Google Scholar]
- 84.Godin I, Dieterlen-Lievre F. and Cumano A. (1995). Emergence of multipotent hemopoietic cells in the yolk sac and paraaortic splanchnopleura in mouse embryos, beginning at 8.5 days postcoitus. Proc Natl Acad Sci U S A 92:773–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu CP. and Auerbach R. (1991). In vitro development of murine T cells from prethymic and preliver embryonic yolk sac hematopoietic stem cells. Development 113:1315–1323 [DOI] [PubMed] [Google Scholar]
- 86.Huang H, Zettergren LD. and Auerbach R. (1994). In vitro differentiation of B cells and myeloid cells from the early mouse embryo and its extraembryonic yolk sac. Exp Hematol 22:19–25 [PubMed] [Google Scholar]
- 87.Yokota T, Huang J, Tavian M, Nagai Y, Hirose J, Zuniga-Pflucker JC, Peault B. and Kincade PW. (2006). Tracing the first waves of lymphopoiesis in mice. Development 133:2041–2051 [DOI] [PubMed] [Google Scholar]
- 88.Ghosn EE, Yamamoto R, Hamanaka S, Yang Y, Herzenberg LA, Nakauchi H. and Herzenberg LA. (2012). Distinct B-cell lineage commitment distinguishes adult bone marrow hematopoietic stem cells. Proc Natl Acad Sci U S A 109:5394–5398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kantor AB, Stall AM, Adams S, Herzenberg LA. and Herzenberg LA. (1992). Differential development of progenitor activity for three B-cell lineages. Proc Natl Acad Sci U S A 89:3320–3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hayakawa K, Hardy RR, Herzenberg LA. and Herzenberg LA. (1985). Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med 161:1554–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barber CL, Montecino-Rodriguez E. and Dorshkind K. (2011). Reduced production of B-1-specified common lymphoid progenitors results in diminished potential of adult marrow to generate B-1 cells. Proc Natl Acad Sci U S A 108:13700–13704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Montecino-Rodriguez E, Leathers H. and Dorshkind K. (2006). Identification of a B-1 B cell-specified progenitor. Nat Immunol 7:293–301 [DOI] [PubMed] [Google Scholar]
- 93.Carey JB, Moffatt-Blue CS, Watson LC, Gavin AL. and Feeney AJ. (2008). Repertoire-based selection into the marginal zone compartment during B cell development. J Exp Med 205:2043–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yoshimoto M, Montecino-Rodriguez E, Ferkowicz MJ, Porayette P, Shelley WC, Conway SJ, Dorshkind K. and Yoder MC. (2011). Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential. Proc Natl Acad Sci U S A 108:1468–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yoshimoto M, Porayette P, Glosson NL, Conway SJ, Carlesso N, Cardoso AA, Kaplan MH. and Yoder MC. (2012). Autonomous murine T-cell progenitor production in the extra-embryonic yolk sac before HSC emergence. Blood 119:5706–5714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Boiers C, Carrelha J, Lutteropp M, Luc S, Green JC, Azzoni E, Woll PS, Mead AJ, Hultquist A, et al. (2013). Lymphomyeloid contribution of an immune-restricted progenitor emerging prior to definitive hematopoietic stem cells. Cell Stem Cell 13:535–548 [DOI] [PubMed] [Google Scholar]
- 97.de Bruijn MF, Speck NA, Peeters MC. and Dzierzak E. (2000). Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. EMBO J 19:2465–2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kumaravelu P, Hook L, Morrison AM, Ure J, Zhao S, Zuyev S, Ansell J. and Medvinsky A. (2002). Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development 129:4891–4899 [DOI] [PubMed] [Google Scholar]
- 99.Mikkola HK, Gekas C, Orkin SH. and Dieterlen-Lievre F. (2005). Placenta as a site for hematopoietic stem cell development. Exp Hematol 33:1048–1054 [DOI] [PubMed] [Google Scholar]
- 100.Ottersbach K. and Dzierzak E. (2005). The murine placenta contains hematopoietic stem cells within the vascular labyrinth region. Dev Cell 8:377–387 [DOI] [PubMed] [Google Scholar]
- 101.Yoder MC. and Hiatt K. (1997). Engraftment of embryonic hematopoietic cells in conditioned newborn recipients. Blood 89:2176–2183 [PubMed] [Google Scholar]
- 102.Yoder MC, Hiatt K. and Mukherjee P. (1997). In vivo repopulating hematopoietic stem cells are present in the murine yolk sac at day 9.0 postcoitus. Proc Natl Acad Sci U S A 94:6776–6780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fraser ST, Ogawa M, RT Yu, Nishikawa S, Yoder MC. and Nishikawa S. (2002). Definitive hematopoietic commitment within the embryonic vascular endothelial-cadherin(+) population. Exp Hematol 30:1070–1078 [DOI] [PubMed] [Google Scholar]
- 104.Yoder MC, Hiatt K, Dutt P, Mukherjee P, Bodine DM. and Orlic D. (1997). Characterization of definitive lymphohematopoietic stem cells in the day 9 murine yolk sac. Immunity 7:335–344 [DOI] [PubMed] [Google Scholar]
- 105.Toles JF, Chui DH, Belbeck LW, Starr E. and Barker JE. (1989). Hemopoietic stem cells in murine embryonic yolk sac and peripheral blood. Proc Natl Acad Sci U S A 86:7456–7459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Weissman I, Papaioannou V. and Gardner R. (1978). Fetal hematopoietic orgins of the adult hematolymphoid system. In: Cold Spring Harbor Conferences on Cell Proliferation Vol 5, Differentiation of Normal and Neoplastic Hematopoietic Cells. Clarkson B, Mark R, Till J, eds. Cold Spring Harbor Lab, New York, pp. 33–47 [Google Scholar]
- 107.North TE, de Bruijn MF, Stacy T, Talebian L, Lind E, Robin C, Binder M, Dzierzak E. and Speck NA. (2002). Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity 16:661–672 [DOI] [PubMed] [Google Scholar]
- 108.Bertrand JY, Giroux S, Golub R, Klaine M, Jalil A, Boucontet L, Godin I. and Cumano A. (2005). Characterization of purified intraembryonic hematopoietic stem cells as a tool to define their site of origin. Proc Natl Acad Sci U S A 102:134–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McKinney-Freeman S, Cahan P, Li H, Lacadie SA, Huang HT, Curran M, Loewer S, Naveiras O, Kathrein KL, et al. (2012). The transcriptional landscape of hematopoietic stem cell ontogeny. Cell Stem Cell 11:701–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rybtsov S, Sobiesiak M, Taoudi S, Souilhol C, Senserrich J, Liakhovitskaia A, Ivanovs A, Frampton J, Zhao S. and Medvinsky A. (2011). Hierarchical organization and early hematopoietic specification of the developing HSC lineage in the AGM region. J Exp Med 208:1305–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Taoudi S. and Medvinsky A. (2007). Functional identification of the hematopoietic stem cell niche in the ventral domain of the embryonic dorsal aorta. Proc Natl Acad Sci U S A 104:9399–9403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.de Bruijn MF, Ma X, Robin C, Ottersbach K, Sanchez MJ. and Dzierzak E. (2002). Hematopoietic stem cells localize to the endothelial cell layer in the midgestation mouse aorta. Immunity 16:673–683 [DOI] [PubMed] [Google Scholar]
- 113.Gekas C, Rhodes KE, Van Handel B, Chhabra A, Ueno M. and Mikkola HK. (2010). Hematopoietic stem cell development in the placenta. Int J Dev Biol 54:1089–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee LK, Ueno M, Van Handel B. and Mikkola HK. (2010). Placenta as a newly identified source of hematopoietic stem cells. Curr Opin Hematol 17:313–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ema H. and Nakauchi H. (2000). Expansion of hematopoietic stem cells in the developing liver of a mouse embryo. Blood 95:2284–2288 [PubMed] [Google Scholar]
- 116.Christensen JL, Wright DE, Wagers AJ. and Weissman IL. (2004). Circulation and chemotaxis of fetal hematopoietic stem cells. PLoS Biol 2:E75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jenkinson EJ, Jenkinson WE, Rossi SW. and Anderson G. (2006). The thymus and T-cell commitment: the right niche for Notch? Nat Rev Immunol 6:551–555 [DOI] [PubMed] [Google Scholar]
- 118.Jaffredo T, Gautier R, Eichmann A. and Dieterlen-Lievre F. (1998). Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development 125:4575–4583 [DOI] [PubMed] [Google Scholar]
- 119.Garcia-Porrero JA, Godin IE. and Dieterlen-Lievre F. (1995). Potential intraembryonic hemogenic sites at pre-liver stages in the mouse. Anat Embryol (Berl) 192:425–435 [DOI] [PubMed] [Google Scholar]
- 120.Kumano K, Chiba S, Kunisato A, Sata M, Saito T, Nakagami-Yamaguchi E, Yamaguchi T, Masuda S, Shimizu K, et al. (2003). Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity 18:699–711 [DOI] [PubMed] [Google Scholar]
- 121.North T, Gu TL, Stacy T, Wang Q, Howard L, Binder M, Marin-Padilla M. and Speck NA. (1999). Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development 126:2563–2575 [DOI] [PubMed] [Google Scholar]
- 122.Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E. and Speck NA. (2009). Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature 457:887–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Okuda T, van Deursen J, Hiebert SW, Grosveld G. and Downing JR. (1996). AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell 84:321–330 [DOI] [PubMed] [Google Scholar]
- 124.Ling KW, Ottersbach K, van Hamburg JP, Oziemlak A, Tsai FY, Orkin SH, Ploemacher R, Hendriks RW. and Dzierzak E. (2004). GATA-2 plays two functionally distinct roles during the ontogeny of hematopoietic stem cells. J Exp Med 200:871–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lim KC, Hosoya T, Brandt W, Ku CJ, Hosoya-Ohmura S, Camper SA, Yamamoto M. and Engel JD. (2012). Conditional Gata2 inactivation results in HSC loss and lymphatic mispatterning. J Clin Invest 122:3705–3717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.de Pater E, Kaimakis P, Vink CS, Yokomizo T, Yamada-Inagawa T, van der Linden R, Kartalaei PS, Camper SA, Speck N. and Dzierzak E. (2013). Gata2 is required for HSC generation and survival. J Exp Med 210:2843–2850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gao X, Johnson KD, Chang YI, Boyer ME, Dewey CN, Zhang J. and Bresnick EH. (2013). Gata2 cis-element is required for hematopoietic stem cell generation in the mammalian embryo. J Exp Med 210:2833–2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Robb L, Lyons I, Li R, Hartley L, Kontgen F, Harvey RP, Metcalf D. and Begley CG. (1995). Absence of yolk sac hematopoiesis from mice with a targeted disruption of the scl gene. Proc Natl Acad Sci U S A 92:7075–7079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Porcher C, Swat W, Rockwell K, Fujiwara Y, Alt FW. and Orkin SH. (1996). The T cell leukemia oncoprotein SCL/tal-1 is essential for development of all hematopoietic lineages. Cell 86:47–57 [DOI] [PubMed] [Google Scholar]
- 130.Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DY. and Traver D. (2010). Haematopoietic stem cells derive directly from aortic endothelium during development. Nature 464:108–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Samokhvalov IM, Samokhvalova NI. and Nishikawa S. (2007). Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature 446:1056–1061 [DOI] [PubMed] [Google Scholar]
- 132.Tanaka Y, Hayashi M, Kubota Y, Nagai H, Sheng G, Nishikawa S. and Samokhvalov IM. (2012). Early ontogenic origin of the hematopoietic stem cell lineage. Proc Natl Acad Sci U S A 109:4515–4520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dancis J, Jansen V, Gorstein F. and Douglas GW. (1968). Hematopoietic cells in mouse placenta. Am J Obstet Gynecol 100:1110–1121 [DOI] [PubMed] [Google Scholar]
- 134.Irion S, Clarke RL, Luche H, Kim I, Morrison SJ, Fehling HJ. and Keller GM. (2010). Temporal specification of blood progenitors from mouse embryonic stem cells and induced pluripotent stem cells. Development 137:2829–2839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.SK Cho, Webber TD, Carlyle JR, Nakano T, Lewis SM. and Zuniga-Pflucker JC. (1999). Functional characterization of B lymphocytes generated in vitro from embryonic stem cells. Proc Natl Acad Sci U S A 96:9797–9802 [DOI] [PMC free article] [PubMed] [Google Scholar]