Abstract
Schizophrenia patients exhibit increased hippocampal activity that is correlated with positive symptoms. While the cause of this hippocampal hyperactivity has not been demonstrated, it likely involves a decrease in GABAergic signaling. Thus, we posit that restoring GABAergic function may provide a novel therapeutic approach for the treatment of schizophrenia. It has been demonstrated that transplanted GABAergic precursor cells from the medial ganglionic eminence (MGE) can migrate and differentiate into mature interneurons. Here, we demonstrate that ventral hippocampal (vHipp) MGE transplants can restore hippocampal function and normalize downstream dopamine neuron activity in a rodent model of schizophrenia. Furthermore, MGE transplants also reverse the hyper-responsive locomotor response to amphetamine. Taken together, these data demonstrate that restoring interneuron function reverses neurophysiological and behavioral deficits in a rodent model of schizophrenia and moreover, demonstrate the feasibility of a neuronal transplant procedure as a potential novel therapeutic approach for the treatment of schizophrenia.
Keywords: Schizophrenia, interneuron, cell transplant, dopamine, hippocampus
Introduction
One of the oldest hypotheses of schizophrenia, the dopamine hypothesis, suggests that hyperactivity within the mesolimbic dopamine system underlies positive symptoms associated with the disease (1, 2). As there is no apparent pathology within the dopamine neurons themselves, it is likely the regulation of the dopamine system is altered in the disease. The hippocampus is one such region that is consistently implicated in schizophrenia. Specifically, hyperactivity within hippocampal subfields at rest is a consistent observation in both human imaging studies and preclinical rodent models (3-7). Furthermore, this augmented hippocampal activity has been demonstrated to drive a dopamine system hyperfunction (3, 8, 9) that is correlated with positive symptoms in schizophrenia patients (7) and aberrant behavior in rodent models (3, 8). The exact cause of the hippocampal hyperactivity is not currently known, however we posit it may be explained by a loss of GABAergic function, particularly in those fast-spiking, peri-somatic targeting interneurons identified by the calcium binding protein parvalbumin (PV). Thus, post-mortem studies in schizophrenia patients (10), as well as in rodent models (11, 12), display decreases in PV expression that correlate with alterations in coordinated neuronal activity (11).
Consequently, a more effective therapy may lie in restoring aberrant hippocampal function. We have demonstrated that pharmacological (13) or surgical (deep brain stimulation) (8) attenuation of vHipp activity effectively normalizes augmented dopamine neuron activity and reverses the increased locomotor response to amphetamine in the MAM rodent model of schizophrenia (for review see (14)). In addition, deep brain stimulation of the vHipp was also effective in reversing cognitive deficits in the MAM model (8). Given that the increase in hippocampal activity is likely secondary to a pathological decrease in interneuron function, we posit that a more effective and potentially long term, therapy may be to replace dysfunctional interneurons. Cell-based therapies are now being studied for the treatment of neurological conditions that require an enhancement or modulation of GABAergic inhibition. Recent studies have employed the use of MGE-derived GABAergic precursor neurons as a means to increase inhibition in models of epilepsy and Parkinson's disease (15-18). When transplanted into an adult rodent brain, MGE-derived cells are able to migrate, differentiate into functional interneurons, and make functional synapses with existing neurons to rescue deficits in GABAergic signaling (15-20). Taken together, we propose that transplantation of MGE-derived cells into the vHipp of MAM-treated rats will increase GABAergic signaling, thereby restoring aberrant hippocampal activity and normalizing augmented dopamine system function and behavior.
Materials and Methods
All experiments were performed in accordance with the guidelines outlined in the USPH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center.
Animals
Methylazoxymethanol acetate (MAM) treatments were performed as previously described (21). Briefly, timed pregnant female Sprague Dawley rats were obtained from Harlan Laboratories on gestational day (GD) 16 and MAM (22 mg/kg, i.p.) was administered on GD17. Control dams received injections of saline (1 ml/kg, i.p.). Male pups were weaned on post-natal day 21 and housed with littermates in groups of 2-3 until adulthood (>PD 60). All experiments were performed on multiple litters of MAM- and saline-treated rats.
Cell Transplantation Surgeries
Survival surgeries were performed in a semi-sterile environment under general anesthesia. Male MAM- and saline-treated rats (40-45 days old; 150-250 g) were anesthetized with pentobarbital (60 mg/kg, i.p.) and placed in a stereotaxic apparatus using blunt atraumatic ear bars. MGE cell transplants were performed by manual dissection of embryonic (GD14.5) MGE-derived tissue from GFP+ transgenic rats (SD-Tg(GFP)2BalRrrc). MGE tissue was dissociated in Leibovitz L-15 Medium (Sigma-Aldrich, St. Louis, MO) and bilaterally injected into the vHipp (A/P ±4.8; M/L ±4.8; D/V -7.0 mm from bregma). A portion of the adjacent fetal brain tissue was repeatedly frozen and thawed to produce dead neurons for control transplants. Rats were housed for a minimum of two months following transplantation before any behavior or electrophysiology experiments were performed.
Extracellular Recordings
MAM- and saline-treated rats (350-500 g) previously transplanted with MGE-derived cells were anesthetized with 8% chloral hydrate (400 mg/kg, i.p.). Rats were placed in a stereotaxic apparatus and a core body temperature of 37°C was maintained. Anesthesia was supplemented as required to maintain suppression of limb compression withdrawal reflex. Extracellular glass microelectrodes (impedance 6-14 MΩ) were lowered into the vHipp (A/P ±5.0; M/L ±4.5; D/V -4.0 to -8.0 mm from bregma) or VTA (A/P ±5.3; M/L ±0.6; D/V -6.5 to -9.0 mm from bregma). Putative pyramidal neurons were defined as those with firing <2 Hz (9, 22, 23). Spontaneously active dopamine neurons were identified using previously established electrophysiological criteria (24). Three parameters of dopamine neuron activity were measured: 1) population activity, 2) basal firing rate, and 3) the proportion of action potentials occurring in bursts. Electrophysiological recordings were analyzed by a two-way ANOVA (Strain and Treatment as factors), followed by a Holm-Sidak post hoc test.
Amphetamine-Induced Hyper-locomotion
MAM- and saline-treated transplanted rats were placed in an open field arena (Med Associates, USA) and spontaneous locomotor activity in the x-y plane was determined for 45 minutes by beam breaks and recorded with Open Field Activity software (Med Associates). Immediately following the baseline recording, all rats were injected with increasing doses of D-amphetamine sulfate (0.5 mg/kg followed by 2.0 mg/kg, i.p.) and locomotor activity was recorded for an additional 45 minutes following each dose. Locomotor data were analyzed by three separate three-way ANOVA's (Strain, Treatment, and time as factors), one for each of the relevant time periods (Baseline, 0.5 mg/kg, 2.0 mg/kg) followed by a Holm-Sidak post hoc test.
Immunohistochemistry
At the cessation of all experiments rats were transcardially perfused with saline (100 mL), followed by formaldehyde (150 mL; 4% v/v in PBS). Rats were decapitated and brains extracted, post-fixed for at least 24 hours, and cryoprotected (25% w/v sucrose in PBS) until saturated. Brains were coronally sectioned (50 µm) using a cryostat (Leica). Ventral hippocampal slices were used to detect the expression of PV and GAD65/67 within transplanted (GFP positive) neurons. Slices were washed three times (10 minutes) in PBS then blocked (2% normal goat serum & 0.3% Triton Tx100) for 30 minutes at room temperature. Primary antibodies [anti-PV 1:1000 (Abcam; ab11427) or anti-GAD65/67 1:1000 (Abcam; ab49832)] were applied (in PBS containing 1% normal goat serum and 0.3% Triton Tx100) overnight at 4°C followed by incubation with AlexaFluor® 594 goat anti-rabbit IgG (H+L) for 1 hour at room temperature. Slices were then mounted and cover slipped with ProLong gold anti-fade reagent.
Analysis
Electrophysiological analysis of dopamine neuron activity was performed with commercially available computer software (LabChart version 7.1; ADInstruments Ltd., Chalgrove, Oxfordshire, UK) and analyzed by using Prism software (GraphPad Software Inc., San Diego, CA), while locomotor activity was collected with Activity Monitor (MED Associates). To confirm the phenotype of the transplanted neurons, cell counts of GFP+ cells or cells stained for PV or GAD65/67 were performed from images obtained from an AxioCam ICc 1 (Zeiss) camera on an Axio Lab.A1 microscope (Zeiss). GFP+ cells were counted from 4-8 vHipp coronal sections per animal. Representative images for Figure 1 were acquired using a confocal microscope (Zeiss 510 NLO) excited by an Argon (458/488/514 nm; 25 mW) or Green HeNe (543nm, 1mW) laser. Data are represented as the mean ± S.E.M. unless stated otherwise, with n-values representing the number of animals per experimental group or number of neurons per group where indicated. Statistics were calculated using SigmaPlot (Systat Software Inc., USA).
Figure 1.
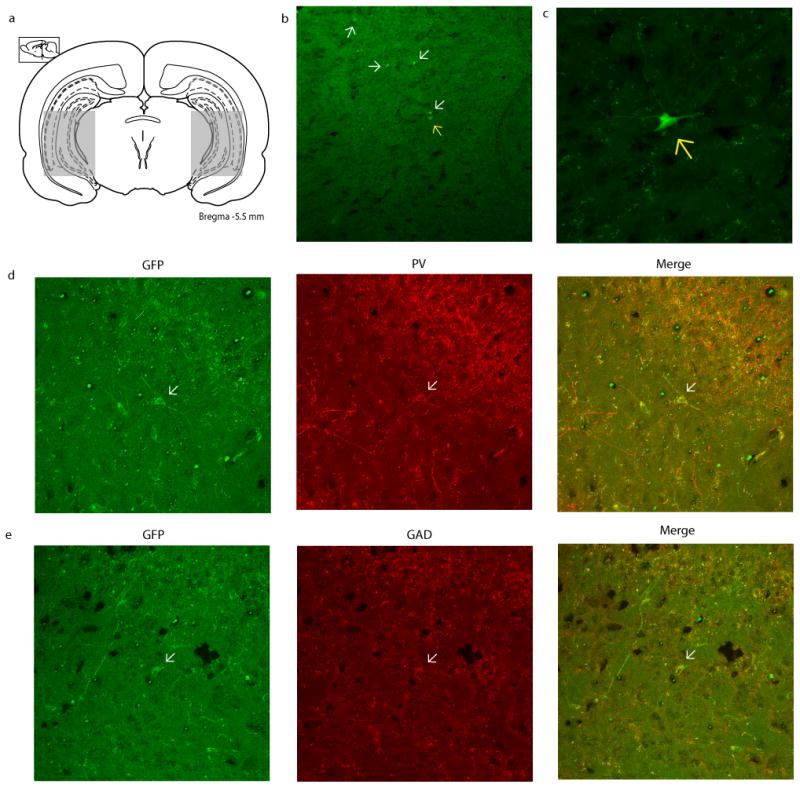
GFP+ MGE-derived cell transplants migrate throughout the hippocampus and adjacent regions. (a) Schematic depicting the approximate span of transplanted cell migration. (b) A representative hippocampal section displaying transplanted GFP+ MGE-derived cells (arrows) at a minimum of 2 months post-transplantation (10× magnification). The cell represented by a yellow arrow is displayed at a higher magnification (40×) in (c). Representative GFP+ MGE-derived transplanted cells stained for PV and GAD were imaged at 25× and are depicted in (d & e). Approximately 45.7% of the transplanted cells are positive for GAD, while 18.1% are PV positive.
Materials
MAM was purchased from Midwest Research Institute (Kansas City, MO, USA). Chloral hydrate, sodium pentobarbital sodium, Dulbecco's phosphate buffered saline, Leibovitz L-15 Medium, D-amphetamine sulfate, and anti-GABA antibody (A2052) were all from Sigma (USA). GFP+ transgenic rats (SD-Tg(GFP)2BalRrrc) were purchased from the Rat & Research Resource Center (donated by Carlos Lois - California Institute of Technology). The anti-PV (ab11427) and anti-GAD65/67 (ab49832) antibodies were from ABCAM. AlexaFluor 596-conjugated anti-rabbit (A11012) and ProLong Gold antifade mountant were from Invitrogen (Grand Island, NY, USA). All other chemicals and reagents were of either analytical or laboratory grade and purchased from various suppliers.
Results
Electrophysiology
MAM-treated rats exhibit elevated vHipp firing rates which we posit is due to a decrease in GABAergic signaling (3, 9). To verify that MGE cell transplants alter vHipp activity, we recorded putative pyramidal cell activity throughout the vHipp. Consistent with previous studies (3, 8), MAM-treated control rats (bilaterally implanted with dead cells) display significantly higher firing rates (0.88Hz ± 0.07, n=56) when compared to saline-treated controls (n=48; 0.55Hz ± 0.08; two-way ANOVA; FStrain=6.33; FTreatment=11.11; FStrain × Treatment=4.47; Holm-Sidak; t= 3.10; p=0.002). Saline-treated rats transplanted with live cells did not display a significant change in firing rate (0.46Hz ± 0.06, n=74) when compared to saline-treated control rats (two-way ANOVA; Holm-Sidak; t= 0.86; p=0.388). However, vHipp firing rates were normalized in MAM-treated rats transplanted with live MGE cells (n=59; 0.49Hz ± 0.07) causing a significant decrease in the frequency when compared to MAM-treated controls (two-way ANOVA; Holm-Sidak; t= 3.84; p<0.001).
In addition, MGE cell transplants caused downstream changes in dopamine neuron activity within the ventral tegmental area (VTA) of MAM-treated rats. Saline-treated control rats (n=8) displayed an average of 0.96 ± 0.09 spontaneously active dopamine neurons per track consistent with previous findings in untreated rats (3, 8, 9). MAM-treated control rats (n=7) had a significantly higher number of spontaneously active dopamine neurons (1.89 ± 0.10) when compared to saline-treated control rats (two-way ANOVA; FStrain= 19.44; FTreatment=13.71; FStrain × Treatment=26.62; Holm Sidak; t= 6.65; p<0.001), again consistent with previous observations (3, 8). Consistent with the lack of effect on vHipp pyramidal cell activity, MGE cell transplants had no significant effect on dopamine neuron activity in saline-treated rats (n=8; 1.10 ± 0.09; two-way ANOVA; Holm-Sidak; t=0.87, p=0.388), but were able to normalize the aberrant VTA dopamine neuron population activity observed in MAM-treated rats (n=8; 1.03 ± 0.09; two-way ANOVA; Holm Sidak; t= 6.16; p<0.001). MGE cell transplants also caused subtle changes in firing rates and bursting activity, not believed to be physiologically relevant, as those parameters are not dependent on, or altered by, inputs from the vHipp (3).
Amphetamine-induced Hyper-locomotion
MAM-treated control rats (n=12) display a significantly enhanced locomotor response to low dose (0.5 mg/kg, i.p.) amphetamine administration when compared to saline-treated rats (n=12; three-way ANOVA of 0.5 mg/kg dose; FStrain × Treatment= 30.94; Holm Sidak; t=3.84; p<0.001), as previously demonstrated (3, 8). Consistent with the effect on dopamine neuron activity, the augmented locomotor response to low dose amphetamine was reversed by vHipp MGE cell transplants (n=12; t=3.41; p<0.001). It should also be noted that the live cell transplants had an effect on the locomotor response in saline-treated rats (n=17; Holm Sidak; t=4.51; p<0.001), causing an enhanced locomotor response to low dose amphetamine when compared to saline-treated controls. A similar pattern was observed at the high dose of amphetamine (2.0 mg/kg, i.p.) whereby MAM-treated control rats exhibited an enhanced locomotor response when compared to saline-treated controls, that was also reversed by the MGE cell transplants (three-way ANOVA of 2.0 mg/kg dose; FStrain=4.80; FTreatment=10.98; FStrain × Treatment=18.95; Holm Sidak; t=4.48; p<0.001).
Immunohistochemistry
Immunohistochemistry was performed postmortem to confirm that the phenotype of the transplanted neurons was consistent with previous characterizations (20). Thus, a significant proportion of the transplanted cells were positive for GAD65/67 (n=204 out of 446 GFP+ cells; 45.7%), while a much smaller number expressed the calcium binding protein PV (n=92 out of 508 GFP+ cells; 18.1%) consistent with previous studies (18-20). Taken together, these data indicate that the transplanted cells possess a GABAergic phenotype and thus posses the ability to increase inhibitory tone within the vHipp.
Discussion
Recent evidence suggests that aberrant hippocampal activity underlies the dysfunction present in the dopamine system of both schizophrenia patients (5, 7, 25) and rodent models of the disorder (3, 8, 9). We posit that this augmented hippocampal activity is secondary to a decrease in GABAergic neurotransmission, as suggested by both preclinical (9, 11) and clinical studies (10, 26). Thus, we propose that transplantation of GABAergic precursor cells will restore hippocampal function and normalize downstream alterations in dopamine neuron activity and associated behavior. To examine this hypothesis we used a rodent model of schizophrenia, the MAM GD17 model. MAM-treated rats consistently display anatomical, physiological, and behavioral deficits commonly associated with schizophrenia, thus providing a useful model of the disease (14). Here we demonstrate that by transplanting GABAergic precursor cells into the vHipp of MAM-treated rats we can normalize hyperactivity present within that region, as well as, restore downstream dopamine system function and a behavioral correlate for the positive symptoms of schizophrenia.
MGE cell transplants have been utilized in various rodent models of disease to increase GABAergic inhibition (15, 18, 27). Transplanted cells are able to survive, migrate, possess a GABAergic phenotype, and integrate into the existing brain circuitry (18, 20). Here we demonstrate that transplanted MGE cells are able to migrate within the hippocampus of both MAM- and saline-treated rats. Furthermore, the transplanted cells were observed at a minimum of two months post transplantation and displayed characteristics of mature GABAergic cells. Pertinent to our study, we used immunohistochemical techniques to stain hippocampal brain sections for GAD65/67, and PV. Indeed, a large percentage of the transplants cells were GAD positive with a smaller percentage positive for PV, consistent with previous studies that characterized the use of MGE tissue grafts in rodent models (15, 19, 20). It should be noted that while some cells were observed in the pyramidal cell layer, this does not indicate that these were excitatory neurons. Transplanted cells injected into mature rats do not follow the same migratory routes as they would in the developing brain and it has been previously demonstrated that even cells clearly located in principle cell layers of the hippocampus are GABAergic (19).
As demonstrated previously, vHipp activity is significantly greater in MAM-treated rats (3, 9). This is consistent with imaging studies in human schizophrenia patients demonstrating greater activity within hippocampal subfields (7, 28). We believe that the increase in hippocampal activity is attributable to a decrease in interneuron function, particularly those expressing the calcium binding protein PV. PV interneurons are fast firing, perisomatic targeting neurons that regulate the firing activity of pyramidal neurons in the hippocampus. Moreover, a wealth of clinical (10, 29) and preclinical studies (11, 30) demonstrate reductions in PV expression throughout the cortex and hippocampus. We posit that decreased interneuron function is the cause of the aberrant hippocampal activity in schizophrenia. Indeed, we have previously demonstrated that the enzymatic degradation of the extracellular matrix surrounding vHipp PV interneurons is sufficient to augment hippocampal activity, and produce aberrant dopamine system function and behavior (9). We now demonstrate that transplantation of MGE-derived GABAergic precursor cells into the vHipp normalizes the augmented activity of pyramidal neurons in MAM-treated rats.
Previous studies have demonstrated that the hippocampus regulates dopaminergic transmission via a multi-synaptic pathway involving the nucleus accumbens and ventral pallidum (31, 32). In addition, we have previously demonstrated that the aberrant hippocampal activity observed in MAM-treated rats is the cause of the augmented dopamine system function (3, 8). Given that the transplantation of GABAergic precursor cells decreased activity within the vHipp of MAM-treated rats, we predicted that this would also normalize the aberrant VTA dopamine neuron activity observed in MAM-treated rats (3, 8, 13). Indeed, while having no effect in control animals, MGE transplants decreased the number of spontaneously active dopamine neurons in MAM-treated rats to a level consistent with that of control rats, thus demonstrating the feasibility of this approach to normalize aberrant dopamine system function in a model of schizophrenia.
To assess whether this normalization of aberrant dopamine neuron activity translates into alterations in behavior, we performed an amphetamine induced locomotion study. This behavioral assay is commonly used as a correlate for the positive symptoms associated with schizophrenia. Psychomotor stimulants can induce psychosis in humans, while schizophrenia patients are more sensitive to these effects (1, 33). Consistent with the enhanced sensitivity observed in human patients, animal models of schizophrenia display an enhanced locomotor response to a low-dose of amphetamine when compared to controls (3, 8, 9, 34). We now demonstrate that this behavioral hyper-excitability can be completely normalized by transplantation of GABAergic precursor cells into the vHipp. It should be noted that MGE transplants produced the opposite effect in saline-treated control animals, i.e. an enhanced locomotor response to amphetamine, a finding which is similar to that observed in our recent study utilizing deep brain stimulation to decrease hippocampal function (8). The reasons underlying this effect are not currently known; however as we did not observe any changes in dopamine neuron population activity, and only subtle changes in firing rate (Fig 3), this is likely due to changes in presynaptic dopamine release either from a direct vHipp projection or via a polysynaptic pathway. The exact mechanisms contributing to the augmented locomotor activity in control rats remain to be elucidated.
Figure 3.
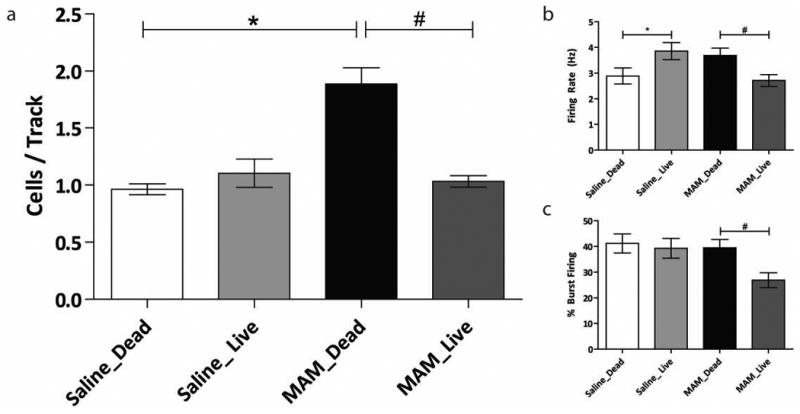
(a) MGE-derived cell transplants restore aberrant dopamine neuron population activity in MAM-treated rats (n=7-8 rats per group; Two-way ANOVA). (b and c) MGE-derived cell transplants had subtle effects on average firing rate and burst firing, respectively (* represents a significant difference from saline control rats; whereas # represents significant difference from MAM control rats)
Taken together we demonstrate that MGE-derived cells, when transplanted into the vHipp of MAM-treated rats, are able to normalize aberrant pyramidal neuron activity and downstream dopamine system function. In addition, by normalizing vHipp and dopamine neuron activity, the hyper-responsivity to psychomotor stimulants was reversed demonstrating a potential therapeutic effect on positive symptoms. It should be noted that we are not advocating that fetal precursor cells be used for the treatment of schizophrenia; however, recent advances in cell based therapies have enabled investigators to differentiate stem cells into distinct lineages. Such advances will ultimately lead to the ability to generate GABAergic neurons that may be effective in treating schizophrenia.
Figure 2.
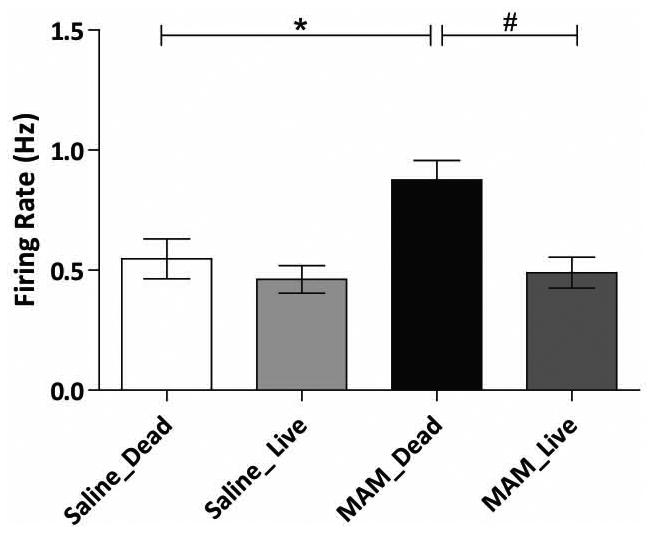
Transplantation of MGE-derived GABAergic precursor cells normalizes enhanced pyramidal neuron vHipp firing rates in MAM-treated rats (n=48-74 cells per group; Two-way ANOVA). *represents significant difference from saline-treated control rats; whereas # represents significant difference from MAM control rats.
Figure 4.
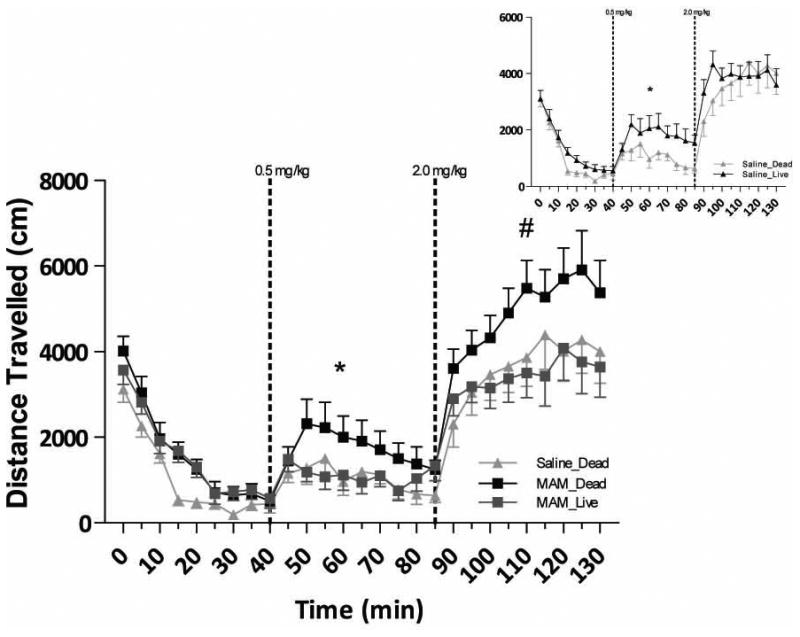
MGE-cell transplants reverse the enhanced locomotor activity observed in MAM-treated rats with low and high dose (0.5, 2.0 mg/kg) amphetamine (n=12-17 rats per group; *#represents a mean effect of treatment; Three-way ANOVA). Inset demonstrates that MGE transplants produce the opposite response (i.e. augment the locomotor activity) to low-dose amphetamine in control, saline-treated rats.
Acknowledgments
This work was supported by a mental health research grant from the Hogg Foundation and an R01 (MH090067) and F31 (MH098564) from the NIH. Representative images were generated in the Core Optical Imaging Facility which is supported by UTHSCSA, NIH-NCI P30 CA54174 (CTRC at UTHSCSA) and NIH-NIA P01AG19316.
Footnotes
Conflict of Interest: Dr. Lodge reports receiving consulting fees from Dey Pharmaceuticals while Ms. Perez does not have any disclosures or conflicts of interest.
References
- 1.Laruelle M, Abi-Dargham A. Dopamine as the wind of psychotic fire: new evidence from brain imaging studies. Journal of Psychopharmacology. 1999;13(4):358–371. doi: 10.1177/026988119901300405. [DOI] [PubMed] [Google Scholar]
- 2.Abi-Dargham A. Do we still believe in the dopamine hypothesis? New data bring new evidence. Int J Neuropsychopharmacol. 2004 Mar;7(Suppl 1):S1–5. doi: 10.1017/S1461145704004110. [DOI] [PubMed] [Google Scholar]
- 3.Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27:11424–11430. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Carpenter WT, McMahon R. Correlations between rCBF and symptoms in two independent cohorts of drug-free patients with schizophrenia. Neuropsychopharmacology. 2006 Jan;31(1):221–230. doi: 10.1038/sj.npp.1300837. [DOI] [PubMed] [Google Scholar]
- 5.Medoff DR, Holcomb HH, Lahti AC, Tamminga CA. Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus. 2001;11(5):543–550. doi: 10.1002/hipo.1070. [DOI] [PubMed] [Google Scholar]
- 6.Harrison PJ. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl) 2004 Jun;174(1):151–162. doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- 7.Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D, et al. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Archives of general psychiatry. 2009;66(9):938–946. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez SM, Shah A, Asher A, Lodge DJ. Hippocampal deep brain stimulation reverses physiological and behavioral deficits in a rodent model of schizophrenia. The International Journal of Neuropsychopharmacology. 2012 doi: 10.1017/S1461145712001344. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah A, Lodge DJ. A loss of hippocampal perineuronal nets produces deficits in dopamine system function: relevance to the positive symptoms of schizophrenia. Transl Psychiatry. 2013;3:e215. doi: 10.1038/tp.2012.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nature Reviews Neuroscience. 2005;6(4):312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 11.Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(8):2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ducharme G, Lowe GC, Goutagny R, Williams S. Early alterations in hippocampal circuitry and theta rhythm generation in a mouse model of prenatal infection: implications for schizophrenia. PLoS ONE. 2012;7(1):e29754. doi: 10.1371/journal.pone.0029754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gill KM, Lodge DJ, Cook JM, Aras S, Grace AA. A novel α5GABAAR-positive allosteric modulator reverses hyperactivation of the dopamine system in the MAM model of schizophrenia. Neuropsychopharmacology. 2011;36(9):1903–1911. doi: 10.1038/npp.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lodge DJ, Grace AA. Gestational methylazoxymethanol acetate administration: A developmental disruption model of schizophrenia. Behav Brain Res. 2009;7(204(2)):306–312. doi: 10.1016/j.bbr.2009.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baraban SC, Southwell DG, Estrada RC, Jones DL, Sebe JY, Alfaro-Cervello C, et al. Reduction of seizures by transplantation of cortical GABAergic interneuron precursors into Kv1.1 mutant mice. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(36):15472–15477. doi: 10.1073/pnas.0900141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez-Cerdeno V, Noctor SC, Espinosa A, Ariza J, Parker P, Orasji S, et al. Embryonic MGE Precursor Cells Grafted into Adult Rat Striatum Integrate and Ameliorate Motor Symptoms in 6-OHDA-Lesioned Rats. Cell Stem Cell. 2010;6(3):238–250. doi: 10.1016/j.stem.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka DH, Toriumi K, Kubo K, Nabeshima T, Nakajima K. GABAergic precursor transplantation into the prefrontal cortex prevents phencyclidine-induced cognitive deficits. J Neurosci. 2011 Oct 5;31(40):14116–14125. doi: 10.1523/JNEUROSCI.2786-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calcagnotto ME, Zipancic I, Piquer-Gil M, Mello LE, Ãlvarez-Dolado M. Grafting of GABAergic precursors rescues deficits in hippocampal inhibition. Epilepsia. 2010;51(SUPPL. 3):66–70. doi: 10.1111/j.1528-1167.2010.02613.x. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez-Dolado M, Calcagnotto ME, Karkar KM, Southwell DG, Jones-Davis DM, Estrada RC, et al. Cortical inhibition modified by embryonic neural precursors grafted into the postnatal brain. J Neurosci. 2006 Jul 12;26(28):7380–7389. doi: 10.1523/JNEUROSCI.1540-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wichterle H, Garcia-Verdugo JM, Herrera DG, Alvarez-Buylla A. Young neurons from medial ganglionic eminence disperse in adult and embryonic brain. Nature neuroscience. 1999 May;2(5):461–466. doi: 10.1038/8131. [DOI] [PubMed] [Google Scholar]
- 21.Moore H, Jentsch JD, Ghajarnia M, Geyer MA, Grace AA. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biol Psychiatry. 2006 Aug 1;60(3):253–264. doi: 10.1016/j.biopsych.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ranck JB., Jr Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats. I. Behavioral correlates and firing repertoires. Experimental Neurology. 1973;41(2):461–531. doi: 10.1016/0014-4886(73)90290-2. [DOI] [PubMed] [Google Scholar]
- 23.Van Der Meer MAA, Redish AD. Theta phase precession in rat ventral striatum links place and reward information. Journal of Neuroscience. 2011;31(8):2843–2854. doi: 10.1523/JNEUROSCI.4869-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--1. Identification and characterization. Neuroscience. 1983 Oct;10(2):301–315. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- 25.Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ, et al. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci. 1998 Aug;1(4):318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez-Burgos G, Hashimoto T, Lewis DA. Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr Psychiatry Rep. 2010 Aug;12(4):335–344. doi: 10.1007/s11920-010-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perry TL, Kish SJ, Buchanan J, Hansen S. Gamma-aminobutyric-acid deficiency in brain of schizophrenic patients. Lancet. 1979 Feb 3;1(8110):237–239. doi: 10.1016/s0140-6736(79)90767-0. [DOI] [PubMed] [Google Scholar]
- 28.Schobel SA, Kelly MA, Corcoran CM, Van Heertum K, Seckinger R, Goetz R, et al. Anterior hippocampal and orbitofrontal cortical structural brain abnormalities in association with cognitive deficits in schizophrenia. Schizophrenia research. 2009;114(1-3):110–118. doi: 10.1016/j.schres.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. Journal of Neuroscience. 2003;23(15):6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curley AA, Eggan SM, Lazarus MS, Huang ZJ, Volk DW, Lewis DA. Role of glutamic acid decarboxylase 67 in regulating cortical parvalbumin and GABA membrane transporter 1 expression: Implications for schizophrenia. Neurobiol Dis. 2013 Feb;50:179–186. doi: 10.1016/j.nbd.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lodge DJ, Grace AA. Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends in Pharmacological Sciences. 2011;32(9):507–513. doi: 10.1016/j.tips.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends in Neurosciences. 2007;30(5):220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci U S A. 1997 Mar 18;94(6):2569–2574. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swerdlow NR, Halim N, Hanlon FM, Platten A, Auerbach PP. Lesion size and amphetamine hyperlocomotion after neonatal ventral hippocampal lesions: more is less. Brain Res Bull. 2001 May 1;55(1):71–77. doi: 10.1016/s0361-9230(01)00492-0. [DOI] [PubMed] [Google Scholar]


