Abstract
PURPOSE
We hypothesized that transection of the pubo-urethral ligament (PUL) in the female rat would cause stress urinary incontinence (SUI) indicated by decreased leak point pressures (LPP). Our aim was to create a novel model of PUL-deficiency in the rat, and validate our model through comparison with an established model of SUI.
MATERIALS AND METHODS
A total of 22 female age-matched Sprague-Dawley rats were randomly assigned into 5 groups: PUL-transection (PULT) or sham-PULT with LPP measured at 4 days (group 1 and 2), or 10 days (Group 3 and 4) post-operatively, and bilateral pudendal nerve transection (PNT) with LPP measured at 4 days post-operatively (Group 5). LPP was measured in all groups via suprapubic catheter. Wilcoxon signed rank tests were used to evaluate differences between groups.
RESULTS
LPP was significantly decreased in PULT groups compared to sham groups after four days (16.3 cm ± 2.74 vs. 36.6 ± 8.39 cm H2O, p<0.00001), but was no different from the PNT group (14.5 ± 1.06 cm H2O p<0.44). Ten days after surgery, LPP remained significantly lower in PULT groups compared to sham groups (17.6 ± 6.36 vs 31.2 ± 5.14 cm H2O, p<0.00001), indicating durability of PULT on inducing SUI in female rat.
CONCLUSIONS
Our results demonstrate that deficiency of the PUL in the female rat induces SUI comparable to a previously established model of PNT-induced SUI. This novel rat model could be used to investigate mechanisms of urethral hypermobility in female SUI, or potential therapeutic interventions for SUI.
Keywords: Stress Urinary Incontinence, Pubo-urethral ligament, Animal Model, Rat
INTRODUCTION
Urinary incontinence affects 40% of women in the United States, and stress urinary incontinence (SUI) accounts for a large portion of these women 1. In the US, over 160,000 surgical procedures are performed for SUI, and mid-urethral slings have become the most commonly performed procedure for SUI 2, 3. Mid-urethral sling procedures are based on the studies of the female urethra by Petros and Ulmsten which showed that a deficient pubo-urethral ligament (PUL), with attachments between the ventral surface of the urethra and the pubis, may lead to urethral mobility and stress urinary incontinence (SUI) or mixed UI in women 4, 5. These authors describe the role of the PUL within an “integral theory” of the pathophysiology of urinary incontinence. In a search for an animal model to elucidate the pathophysiology of PUL-deficiency, we investigated the role of PUL deficiency in altering leak point pressures (LPP) in the female rat. Our aim was to create an animal model of PUL deficiency in the female rat as a potential model of SUI, and validate this model through comparison with an established animal model of SUI produced via pudendal nerve transection (PNT) 6.
MATERIALS AND METHODS
Following protocol approval by our Institutional Animal Care and Use Committee, a total of 22 female age-matched Sprague-Dawley rats (Harlan, Indianapolis, Indiana) weighing 220 to 280 grams were randomly assigned to 1 of 5 groups: PUL-transection (PULT) and sham-PULT with LPP measured 4 days post op (Groups 1 and 2), a separate group of PULT and sham-PULT with LPP measured 10 days post-op (Group 3 and 4), and bilateral pudendal nerve transection (PNT) with LPP measured 4 days post-op (Group 5) serving as a positive control. PULT or sham surgery was performed as described below. For each group, LPP was determined in each anaesthetized animal via a previously implanted suprapubic catheter. Following LPP measurement, animals were sacrificed in an anesthetized state via inhalation of 100% CO2. Details of surgical and urodynamic procedures are as follows.
Pubo-Urethral Ligament Transection
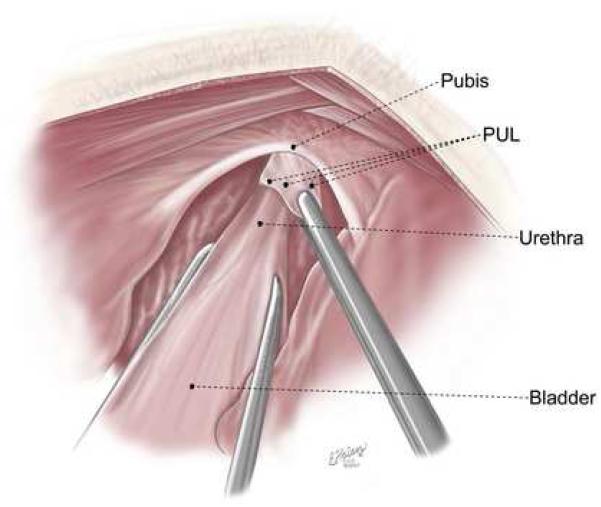
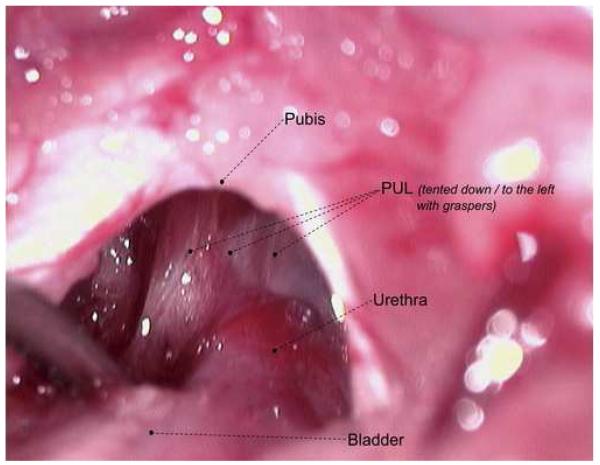
The rats were prepared for aseptic surgery. From a midline suprapubic incision, the midurethra was identified. Reverse trendelenburg positioning was used to adequately visualize the retropubic space. The pubo-urethral ligament was identified (Figure 1A and B) and sharply incised. For sham surgery, an identical suprapubic incision was made, mid-urethral identification was performed, but the PUL was left intact. Incisions were closed in layers with 5-zero Vicryl for the abdominal muscle and 4-zero Vicryl for the skin.
Figure 1A and B.
Anatomy of the Pubo-urethral ligament
Pudendal Nerve Transection (PNT)
The methods of PNT have been previously reported by our laboratory 6, 7. The rats were prepared for aseptic surgery. Using a dorsal longitudinal incision, the ischiorectal fossa was opened bilaterally. Under high magnification, the pudendal nerve was isolated and transected. The incision was then closed in layers using 5-zero Vicryl (Ethicon) for the muscle and 3-zero Vicryl for the skin.
Suprapubic Tube Implantation
Suprapubic tube insertion was performed as modified from the method of Malmgren et al 8. Briefly, rats were anaesthetized by a single intraperitoneal injection of ketamine hydrochloride 100 mg/kg (Ketaset; Fort Dodge Laboratories, Fort Dodge, IA) and of xylazine 10 mg/kg (Rompun; Miles, Shawnee Mission, KS). A purse-string suture with 6-zero chromic was placed on the bladder dome. A 1mm incision was made in the bladder in the center of the purse string suture, through which PE-50 tubing with a flared tip was inserted in the bladder, and the purse-string suture was secured around the tube. The tube was then tunneled through the abdominal wall and subcutaneous tissue to the nape of the neck and secured into place with 5-zero Vicryl (Ethicon) until LPP testing.
LPP Testing
the LPP testing was performed as described by Damaser et al 9. Investigators performing LPP were blinded to the procedure and group of each animal. Two days after implantation of SPT and while under anesthesia with urethane (1.2 g/ kg; intraperitoneal injection); the rats were placed supine at the level of zero pressure and the bladder emptied manually. Subsequently, the bladder was filled with saline at room temperature (1 ml per minute) through the suprapubic catheter, while bladder pressure was recorded. The suprapubic catheter was connected to a syringe pump (Kent Scientific Corp., Torrington, Connecticut) and a pressure transducer (Grass Instrument Division, Astro-med, Inc., West Warwick, Rhode Island). All bladder pressures were referenced to air pressure at the level of the bladder. Pressure and force transducer signals were amplified (Grass Instrument Division, Astro-med, Inc.) and digitalized for computer data collection using PolyView software (BioBench, version 1.2, National Instruments Corp., Austin, Texas) at 10 samples per second.
Peak bladder pressure was calculated for each LPP measurement at half-bladder capacity by slowly and manually increasing abdominal pressure until a leak occurred, and external pressure was immediately withdrawn 9. LPP was performed nine times per rat. The bladder was emptied using the Credé maneuver and refilled between LPP measurements.
Data Analysis
The mean of nine measurements of LPP from each of four animals per group was collected and used for further statistical analysis. Data are presented as the mean ± SEM of 4 animals from each group. Pairwise differences in LPP between all five groups were calculated. The difference between LPP at 4 or 10 day time points after PUL transection, sham PUL-transection, or PNT transection was tested using the Wilcoxon rank sum test with a p-value <0.5 considered significant (JMP 6.0 software, SAS, Cary, NC).
RESULTS
Of a total of 22 rats in all five groups, one rat in the PULT group died at anesthesia induction. Necropsy revealed injury to the liver during intraperitoneal injection, and subsequent care was taken to avoid such injury. Data on the remaining 21 rats was analyzed.
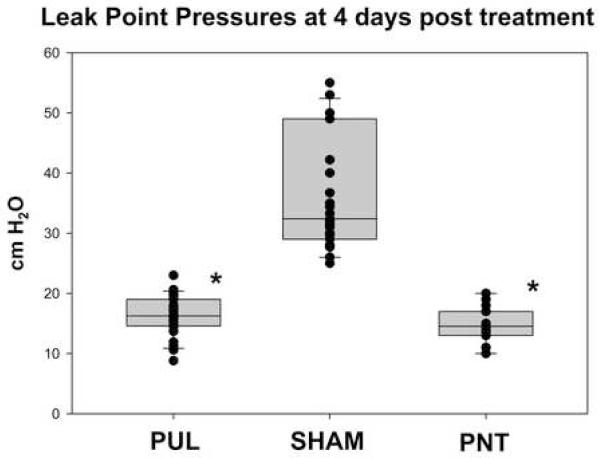
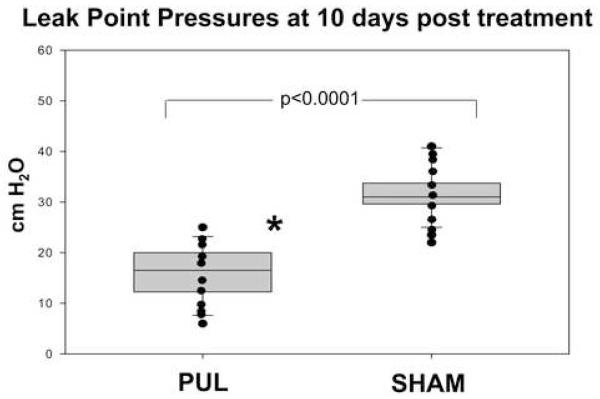
As demonstrated in previous studies, significant variability in LPP could be noted between rats, with little difference in the LPP of each rat for a given time point 7. At four days post-procedure (Groups 1, 2 and 5), the LPP in the PULT group was significantly decreased compared to the sham group (16.3 cm ± 2.74 vs. 36.6 ± 8.39 cm H2O, p<0.00001) (Figure 2), but was not significantly different from the LPP of the PNT group (16.3±2.74 vs. 14.5 ± 1.06 cm H2O p<0.44). At ten days post procedure (Group 3 and 4), the LPP in the PULT group remained significantly lower that the sham PULT (17.6 ± 6.36 vs 31.2 ± 5.14 cm H2O, p<0.00001) (Figure 3), indicating durability of the effect of PULT in inducing SUI in the female rat.
Figure 2.
Leak Point Pressure in 4 days after PULT, sham or PNT (n=4 in each group)
Figure 3.
Leak Point Pressure in 10 days after PULT, or sham (n=4 in each group)
These data support the hypothesis that the PUL is involved in urethral support during intra-abdominal stress, and surgical disruption of this ligament results in significantly decreased LPP.
DISCUSSION
Urinary incontinence is one of the most prevalent conditions of the lower urinary tract, affecting approximately 40% of women in the United States 1. In order to study the mechanisms of SUI in the female, investigators have recently developed and tested animal models in the female rat that mimic the symptoms of SUI. These investigators have used either the vaginal distension model (VD), which causes injury to the tissues of the pelvic floor similar to birth trauma9, 10, or the model of direct injury to the pudendal or sciatic nerve, inducing manifestations of SUI 7, 9, 11. The symptoms and signs of SUI in these animal models have been assessed by in-vivo measures such as LPP 7, 9, 12 which is similar to the clinical measure of bladder outlet competency, as well as by quantitative morphometry measuring post-partum damage to the external sphincter and pudendal nerve 10, 13. These models demonstrate tissue injury similar to that following birth trauma 14. Both the VD and the nerve injury models have been accepted as the surrogates of post-partum SUI in women.
It is also known, however, that nulliparous women can also develop SUI 15. Animal models of nulliparous SUI should focus on alternative anatomic targets for controlling continence during intra-abdominal stress. In 1961, Zacharin described the attachments of the PUL and the vaginal insertion of the anterior portion of the levator ani, and was the first to suggest the role of these structures in continence 16. In 1998, Petros analyzed the structure and insertions of the PUL in female patients during the intravaginal slingplasty procedure. He described the PUL as descending like a fan from the lower pubic bone, and appearing as a continuous sheet of connective tissue, with the ventral aspect inserting into the midpart of the urethra 4. This urethral attachment has been hypothesized to provide ventral tethering of the urethra during intra-abdominal strain, preventing urethral mobility and subsequent leak.
Based on the postulated role of PUL in SUI, recent surgical treatments for SUI based on the ‘integral theory’ have led to the development of mid-urethral slings, pioneered by transvaginal tape (TVT) procedure. This procedure, introduced by Ulmsten and Petros, has enjoyed worldwide popularity with excellent long term efficacy 17. It is estimated that over one million TVT’s have been performed worldwide.
The observed clinical efficacy of mid-urethral slings, which is based on the concept of PUL deficiency in the human, prompted our investigation into the effects of PUL deficiency in a small animal model. The PUL in the female rat is similar in structure, insertion and origin to that described by Petros in the human female. This structure could easily be identified in the animal and we were able to transect it sharply without damage to other peri-urethral structures. Subsequently, we followed the design of experiments described for other models of SUI with measurement of LPP at 4 and 10 days—the time points when subsequent recovery of LPP in the VD or pudendal nerve crush models have been reported 9.
Our initial data suggest that PUL transection causes SUI in female rats as measured by decreased LPP for at least 10 days. This decrease in LPP following PULT was similar to our positive control PNT group at 4 days, and significantly lower than sham PULT at 4 and 10 days. These findings encourage us to pursue further investigations into this model to examine the effect of PULT on LPP beyond 10 days; the natural history of SUI following PULT-- does recovery occur, and if yes, when and how; and studies of other potential mechanisms such as peri-urethral nerve injury that could potentially be responsible for observed effects on LPP.
The results of our study should be interpreted with respect to its limitations. Although a rat model is desirable due to cost and ease of handling, a quadruped with an abdominal bladder may not be considered ideal for modeling SUI in humans. Nonetheless, SUI has been shown to be easily and reproducibly induced in the rat, and this model may serve as an excellent surrogate in place of larger animal studies. Also, LPP produced by abdominal Credé maneuver may not fully reproduce the mechanism of SUI in humans. However, it has been shown to serve as a reproducible and reliable indicator of bladder and urethral outlet function in several other studies 7, 9, 12, 13, 18-20. Our PULT model provides the potential for further investigation of SUI in the female. This model may be useful for future studies of the pathophysiology of SUI in nulliparous women.
CONCLUSION
Our results demonstrate that deficiency of the PUL in the female rat causes SUI compared to the established model of PNT. This novel PUL-deficient rat model could be used for investigation of mechanisms and potential therapeutic interventions of SUI. Longer term effects of PULT on LPP will require future investigations.
Acknowledgments
Supported by grant NIH-NIDDK- DK070004-01
Standard abbreviations
- SUI
stress urinary incontinence
- PULT
pubo-urethral ligament transection
- PNT
pudendal nerve transection
- PUL
pubo-urethral ligament
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Diokno AC, Burgio K, Fultz H, Kinchen KH, Obenchain R, Bump RC. Prevalence and outcomes of continence surgery in community dwelling women. J Urol. 2003;170:507. doi: 10.1097/01.ju.0000070404.24755.35. [DOI] [PubMed] [Google Scholar]
- 2.Kim HL, Gerber GS, Patel RV, Hollowell CM, Bales GT. Practice patterns in the treatment of female urinary incontinence: a postal and internet survey. Urology. 2001;57:45. doi: 10.1016/s0090-4295(00)00885-2. [DOI] [PubMed] [Google Scholar]
- 3.Leach GE, Dmochowski RR, Appell RA, Blaivas JG, Hadley HR, Luber KM, et al. Female Stress Urinary Incontinence Clinical Guidelines Panel summary report on surgical management of female stress urinary incontinence. Journal of Urology. 1997;158:875. doi: 10.1097/00005392-199709000-00054. [DOI] [PubMed] [Google Scholar]
- 4.Petros PE. The pubourethral ligaments--an anatomical and histological study in the live patient. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:154. doi: 10.1007/BF02001085. [DOI] [PubMed] [Google Scholar]
- 5.Petros PE, Ulmsten UI. An integral theory of female urinary incontinence. Experimental and clinical considerations. Acta Obstet Gynecol Scand Suppl. 1990;153:7. doi: 10.1111/j.1600-0412.1990.tb08027.x. [DOI] [PubMed] [Google Scholar]
- 6.Hijaz A, Daneshgari F, Huang X, Bena J, Liu G, Saffore L, et al. Role of sling integrity in the restoration of leak point pressure in the rat vaginal sling model. J Urol. 2005;174:771. doi: 10.1097/01.ju.0000164721.52278.29. [DOI] [PubMed] [Google Scholar]
- 7.Hijaz A, Daneshgari F, Cannon T, Damaser M. Efficacy of a vaginal sling procedure in a rat model of stress urinary incontinence. J Urol. 2004;172:2065. doi: 10.1097/01.ju.0000138476.42556.b8. [DOI] [PubMed] [Google Scholar]
- 8.Malmgren A, Uvelius B, Andersson KE, Andersson PO. On the reversibility of functional bladder changes induced by infravesical outflow obstruction in the rat. Journal of Urology. 1990;143:1026. doi: 10.1016/s0022-5347(17)40176-5. [DOI] [PubMed] [Google Scholar]
- 9.Damaser MS, Broxton-King C, Ferguson C, Kim FJ, Kerns JM. Functional and neuroanatomical effects of vaginal distention and pudendal nerve crush in the female rat. J Urol. 2003;170:1027. doi: 10.1097/01.ju.0000079492.09716.43. [DOI] [PubMed] [Google Scholar]
- 10.Cannon TW, Wojcik EM, Ferguson CL, Saraga S, Thomas C, Damaser MS. Effects of vaginal distension on urethral anatomy and function. BJU Int. 2002;90:403. doi: 10.1046/j.1464-410x.2002.02918.x. [DOI] [PubMed] [Google Scholar]
- 11.Cannon TW, Sweeney DD, Conway DA, Kamo I, Yoshimura N, Sacks M, et al. A tissue-engineered suburethral sling in an animal model of stress urinary incontinence. BJU Int. 2005;96:664. doi: 10.1111/j.1464-410X.2005.05702.x. [DOI] [PubMed] [Google Scholar]
- 12.Conway DA, Kamo I, Yoshimura N, Chancellor MB, Cannon TW. Comparison of leak point pressure methods in an animal model of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:359. doi: 10.1007/s00192-004-1263-4. [DOI] [PubMed] [Google Scholar]
- 13.Lin AS, Carrier S, Morgan DM, Lue TF. Effect of simulated birth trauma on the urinary continence mechanism in the rat. Urology. 1998;52:143. doi: 10.1016/s0090-4295(98)00136-8. [DOI] [PubMed] [Google Scholar]
- 14.Allen RE, Hosker GL, Smith AR, Warrell DW. Pelvic floor damage and childbirth: a neurophysiological study. Br J Obstet Gynaecol. 1990;97:770. doi: 10.1111/j.1471-0528.1990.tb02570.x. [DOI] [PubMed] [Google Scholar]
- 15.Lukacz ES, Lawrence JM, Contreras R, Nager CW, Luber KM. Parity, mode of delivery, and pelvic floor disorders. Obstet Gynecol. 2006;107:1253. doi: 10.1097/01.AOG.0000218096.54169.34. [DOI] [PubMed] [Google Scholar]
- 16.Zacharin RF. The anatomic supports of the female urethra. Obstetrics & Gynecology. 1968;32:754. [PubMed] [Google Scholar]
- 17.Nilsson CG, Kuuva N, Falconer C, Rezapour M, Ulmsten U. Long-term results of the tension-free vaginal tape (TVT) procedure for surgical treatment of female stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(Suppl 2):S5. doi: 10.1007/s001920170003. [DOI] [PubMed] [Google Scholar]
- 18.Heidkamp MC, Leong FC, Brubaker L, Russell B. Pudendal denervation affects the structure and function of the striated, urethral sphincter in female rats. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:88. doi: 10.1007/BF01982215. [DOI] [PubMed] [Google Scholar]
- 19.Hijaz A, Daneshgari F, Huang X, Bena J, Liu G, Saffore L, et al. Role of sling integrity in the restoration of leak point pressure in the rat vaginal sling model. J Urol. 2005;174:771. doi: 10.1097/01.ju.0000164721.52278.29. [DOI] [PubMed] [Google Scholar]
- 20.Kerns JM, Damaser MS, Kane JM, Sakamoto K, Benson JT, Shott S, et al. Effects of pudendal nerve injury in the female rat. Neurourol Urodyn. 2000;19:53. doi: 10.1002/(sici)1520-6777(2000)19:1<53::aid-nau7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]