Abstract
In this event-related fMRI study, we investigated age-related differences in brain activity associated with conceptual repetition priming in young and older adults. Participants performed a speeded “living/non-living” classification task with three repetitions of familiar objects. Both young and older adults showed a similar magnitude of behavioral priming to repeated objects and evidencing repetition-related activation reductions in fusiform gyrus, superior occipital, middle and inferior temporal cortex, as well as inferior frontal and insula regions. The neural priming effect in young adults was extensive and continued through both the second and third stimulus repetitions, whereas neural priming in older adults was markedly attenuated and reached floor at the second repetition. In young adults, greater neural priming in multiple brain regions correlated with greater behavioral facilitation whereas in older adults, only activation reduction in the left inferior frontal correlated with faster behavioral responses. These findings provide evidence for altered neural priming in older adults despite preserved behavioral priming, and suggest the possibility that age-invariant behavioral priming is observed as a result of more sustained neural processing of stimuli in older adults which may be a form of compensatory neural activity.
Keywords: Aging, fMRI, repetition priming, priming, implicit memory, recognition
1. INTRODUCTION
Normal aging is associated with declines in many cognitive functions including episodic memory, working memory, processing speed and executive functions (Baltes and Lindenberger, 1997; Nilsson, 2003; Park, et al., 2001; Salthouse, 1996), but there is preservation in select cognitive domains such as verbal abilities and world knowledge (Park et al., 2002; Park and Reuter-Lorenz, 2009; Goh et al., 2011; for reviews, see Hedden and Gabrieli, 2004). A key cognitive function that is purported to remain relatively unaffected by age is implicit memory, which involves changes in behavioral performance,--usually facilitation--due to prior stimulus exposure and priming that does not require conscious awareness. The facilitation typically takes the form of faster responses to a previously-presented stimulus or “prime,” or recognition or production of the prime to a probe without awareness that the item had been processed earlier. Several have reported that priming is preserved in normal older adults (Ballesteros et al., 2008; Ballesteros et al., 2009; Caggiano et al., 2006; Mitchell and Bruss, 2003; Wiggs et al., 2006), and even in Alzheimer’s disease patients (Ballesteros and Reales, 2004; Ballesteros et al., 2007; for reviews, Fleischman, 2007; Fleischman and Gabrieli, 1998). Despite such age-invariant behavioral priming effects, it is still not clear whether the neural mechanisms supporting such implicit memory processes also show age-invariance between young and older adults. In at least one behavioral study, young adults showed stable priming effects for a week after initial testing, but older adults showed significant reduction in priming after only a day (Wiggs et al., 2006). Moreover, there is a great deal of evidence that both episodic memory and working memory are accompanied by increased neural activity with age that is typically viewed as compensatory (see Park and Reuter-Lorenz, 2009 for a review). In the present study, we investigated whether equivalent behavioral priming in young and older adults was associated with differences in neural activity, using a repetition priming paradigm involving conceptual judgments of visual objects.
Neuroimaging studies of repetition priming in young adults have shown that activity is reduced in several brain regions when processing previously-encountered stimuli. This repetition-related suppression of neural activity or neural priming effect has been attributed to improved efficiency and decreased cognitive demands associated with repeated stimulus presentation (e.g., Buckner et al., 1998; Grill-Spector et al., 2006; Wig et al., 2009; for reviews see, Henson, 2003; Schacter and Buckner, 1998; Schacter et al., 2007). In particular, neuroimaging studies of conceptual object processing in young adults have shown that repeated meaning-based classifications of visual objects reduced both processing time and neural activity for repeated compared to new stimuli in several occipital/temporal regions, including the fusiform and middle occipital gyri, and left frontal cortices such as the lateral inferior prefrontal cortex (e.g., Buckner et al., 1998; Grill-Spector et al., 1999; Henson, 2003; Koutstaal et al., 2001; Schacter and Buckner, 1998; van Turennout et al., 2000; Vuilleumier at al., 2002; Wig et al., 2005; Zago et al., 2005). The frontal brain regions involved in these studies have been shown to mediate retrieval of semantic knowledge necessary to perform the conceptual tasks, whereas the occipital/posterior brain regions are involved in coding the perceptual representation of the stimuli (Bunzeck et al., 2006; Daselaar et al., 2005; Maccotta and Buckner 2004; for a review see Schacter et al., 2007).
In contrast to young adult studies, there have been relatively few neuroimaging studies on repetition priming in older adults, and these studies have reported mixed effects of age on neural priming (Bäckman et al., 1997; Bergerbest et al., 2009; Daselaar et al., 2005; Gold et al., 2009; Lawson et al., 2007; Lustig and Buckner, 2004; Olichney et al., 2010). In an early positron emission tomography (PET) study using a word-stem completion task, Bäckman et al. (1997) reported similar repetition-related blood flow reductions in the right extrastriate cortex as well as similar behavioral priming effects in both young and older adults. Using a living/nonliving word classification task, Lustig and Buckner (2004) found that both young and older adults showed equivalently faster response times for repeated compared to novel words along with decreases in activation in the left inferior frontal gyrus. In a perceptual repetition-priming task involving abstract shapes, Soldan et al. (2008) also found that older adults show reduced activation to repeated stimuli to the same extent as young adults in occipital regions. Gold et al. (2009) also reported age invariant behavioral and neural priming on a verbal lexical-semantic facilitation task. In contrast to these studies, Daselaar et al. (2005) used a word-stem completion task and found less behavioral priming as well as less repetition-related activation reductions in older compared to younger adults in the left anterior superior temporal and right occipital regions. Similarly, Bergerbest et al. (2009) employed an abstract/concrete word judgment task and found similar behavioral priming in both age groups but smaller repetition-related activation reductions in older compared to younger adults in left prefrontal regions with additional repetition-related reductions in right prefrontal regions. Importantly, activation reduction in the right frontal regions correlated positively with a vocabulary measure in older adults suggesting a compensatory role for the additional right hemisphere recruitment in their study. Finally, given these mixed reports of age effects on neural priming across these studies that involve different types of tasks and stimuli, it is unclear if the neural system supporting priming across the brain is preserved in older adults.
In the present study, we investigated the effects of aging on neural priming in the whole brain, using an event-related fMRI experiment that involved an implicit conceptual priming task with repeated pictures of familiar objects. We reasoned that, repetition of meaningful objects accompanied with a conceptual judgment task would engage semantic retrieval processes in the frontal regions, as word stimuli do, as well as additional perceptual processing in posterior regions, which may be less pronounced when processing word stimuli. Thus, we were able to examine age-related effects of neural priming in both anterior and posterior parts of the brain and their role in behavioral priming within the same study. In addition, we chose pictures of familiar objects to reduce possible age-related variation related to differences in task difficulty (e.g., Soldan et al., 2008).
Because several studies have shown that younger and older adults exhibit equivalent behavioral priming effects, we expected age invariant behavioral priming, measured as response time benefits, with object repetitions. At the neural level, however, we considered that posterior attentional and perceptual processing of visual stimuli might operate with less specialization and efficiency in older compared to younger adults, a finding that has been reported in several previous studies (Chee et al., 2006; Goh et al., 2010; Huang et al., 2011; Park et al, 2004; Carp et al., 2010; Park et al., 2012). Because stimulus processing in these posterior regions occurs with less fidelity with age, more neural resources in other brain regions such as in the frontal lobe are required to maintain equivalent behavioral performance (Gutchess et al. 2005; Goh et al., 2010; Park et al. 2004). Therefore, we hypothesized that less efficient stimulus processing in older adults’ posterior brain regions as well as the accompanying greater frontal recruitment would be generally associated with less activation reduction to repeated stimuli compared to younger adults. Of note, to validate our sample’s performance with previous studies, we also included a recognition test to behaviorally assess participants’ implicit and explicit memory (e.g., Bergerbest, 2009; Lustig and Buckner, 2004; Soldan et al., 2008), expecting that relative to younger adults, older adults would show comparable implicit memory performance but worse explicit memory performance.
2. METHODS
2.1. Participants
There were 19 young (mean age 24.6 yrs, SD 3.0, range 20–32; 9 males and 10 females) and 18 older adults (mean age 66.3 yrs, SD 3.8, range 61–72; 10 males and 8 females) who participated in this study. All participants had an MMSE > 27 with means 29.2 (SD 1.1) and 28.3 (SD 1.0) for the young and older adults, respectively. Participants were screened for counter-indications for MRI scanning and health status, with the presence or history of clinical dementia, neurological disorders, stroke, depression, and cardiovascular disease as exclusionary criteria (although hypertension under medicated control was not exclusionary). Additionally, participants underwent a brief neuropsychological test battery. The results can be found in Supplementary Table 1 (Table S1) and are within range of performance associated with normal age-related cognitive differences as reported in numerous previous studies (e.g., Park et al., 2002).
Visual acuity in the scanner was corrected to 20/20 on the Snellen Scale and participants with cataracts and macular degeneration were not included in this experiment. All participants gave informed consent for participation in the study, which was approved by the University of Illinois at Urbana-Champaign Institutional Review Board, and were remunerated for their participation.
2.2. Stimuli
The stimulus set for the fMRI experiment consisted of 48 color photographs of familiar objects selected from different libraries. The pictures were selected so that 24 items depicted living objects (e.g. tree, bear) and 24 items depicted non-living objects (e.g. whistle, refrigerator). These living and non-living items were equally distributed into two lists of 24 stimuli each, one list for each functional imaging run. Within each list, 12 pictures were presented once and the other 12 were presented 3 times. Half of the items in each list were living objects and the other half were non-living objects. Items presented in the scanner were also used to evaluate recognition memory for objects after the scanning session (see below). Stimulus presentation and recording of responses were implemented using E-Prime software (Psychology Software Tools Inc. Pittsburg, PA). Stimuli were back projected onto a screen located at the rear of the scanner. Participants viewed the screen through a mirror placed above the eyes. Pictures were presented on a white background set at 6.77 × 4.9 cm with a fixed resolution of 600 × 800 pixels. The largest object subtended a visual angle of 6.8° × 19.1° and the smallest object subtended a visual angle of 2.0° × 3.9°.
2.3. fMRI experiment procedure
There were two functional imaging runs in this event-related fMRI experiment. In each run, items from one of the stimuli lists were presented such there were 48 trials of object stimuli within a run (12 presented once, 12 presented 3 times). The lag for the repetitions of items was between 1 to 48 objects with intervals of, on average, 55.5 s between repetition (Rep) 1 and Rep 2 (range: 3–215 s), 56.6 s between Rep 2 and Rep 3 (range: 3–231 s), and 112 s between Rep 1 and Rep 3 (range: 6–237 s). This repetition priming procedure is identical to previous reports, ensuring robust repetition effects (Xue et al., 2010, Soldan et al., 2008). Each object remained on the screen for one second. Trials were interleaved with periods of fixation that varied in duration between 2, 6, and 10 s. Stimulus presentation order was randomized across participants with the restriction that the same stimulus could not appear more than two times consecutively. The order of objects was completely counterbalanced and randomized across participants to minimize confounds of stimulus list, presentation order, repetition lag, or interactions between these variables. Participants indicated their responses via button press using the index (living object) and middle fingers (non-living object) of their right hand. Participants were instructed to respond as quickly and accurately as possible.
Brain imaging data were acquired on a 3.0 T Allegra scanner (Siemens, Erlangen, Germany), with a single-channel head coil. For each participant, 150 functional scans were acquired in each of two runs using a gradient-echo echo planar imaging sequence with repetition time of 2000 ms, and a field of view of 220 × 220 mm, 64 × 64 matrix. 32 axial slices parallel to the anterior-posterior commissure line and 4 mm thick (with 0.4 mm gap) were acquired. A quick high-resolution axial T2 anatomical image was also acquired that was co-planar to the functional images. Finally, a 3D MPRAGE T1 anatomical image was acquired to allow for co-registration of the T2 images (along with the aligned functional images) to the 3D anatomical image and subsequent normalization to MNI (Montreal Neurological Institute) space.
2.4. Recognition memory test
Following the fMRI experiment, participants also performed an object memory recognition test outside of the scanner to evaluate explicit memory. Participants were presented with a total of 96 objects, 48 studied and 48 additionally acquired novel items. Of the 48 studied items, 24 were previously presented once during the fMRI experiments and 24 were presented three times. Participants were asked to indicate whether each presented object was old or new. Objects remained on the screen until the participant responded or the duration exceeded 5000 ms. For the analysis of recognition data, the stimuli correctly recognized as “old” were hits while false-alarms were objects recognized as “old” which were in fact new objects. The sensitivity index d′ [Z(hit rate) - Z(false alarm rate] (Green and Swets, 1966; MacMillan and Creelman, 1991) was calculated for each participant using the TDS_EXPER program (Reales and Ballesteros, 1994; 2000).
2.5. First-level functional image analysis
Imaging data were analyzed and processed using SPM5 (Welcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm/). Motion and slice-time corrections were applied to the functional data, along with a Gaussian smoothing kernel with full-width at half maximum of 8 mm.
For the first-level voxel-wise analysis of each participant’s imaging data, we applied a multiple regression analysis, with delta function predictors coding the onsets of the first presentation, and second and third repetitions of object stimuli, individually for each subject. Delta functions had 0s durations in accordance with event-related specifications in SPM5.The onset predictors were then convolved with a canonical hemodynamic response function and the resulting individual brain response estimates from this model were used in subsequent group-level whole-brain analyses.
2.6. Group-level whole-brain contrast analysis
In this group-level image analysis, whole-brain contrasts were performed to evaluate differences in the pattern of repetition-related activation reduction across the whole brain between the two age groups. To identify regions that showed repetition-related reduction, we contrasted the first presentation condition to the second (Rep 1 > Rep 2) as well as the third repetition (Rep 1 > Rep 3), separately for young and older adults. To identify regions that showed age differences in repetition-related reduction, we directly compared these repetition-related contrasts between the young and older adults. All group-level whole-brain contrasts were conducted at a statistical threshold of p < 0.001 with a spatial extent of 10 contiguous voxels (473 mm3).
2.7. Group-level whole-brain conjunction analysis
In order to evaluate more specific age differences in neural priming during Rep 2 and Rep 3 conditions relative to the first presentation, we also examined the brain responses in regions-of-interest (ROIs) derived from areas that commonly showed repetition-related activation reduction in both young and older adults. To identify these common regions, we performed a conjunction of four contrasts that included the repetition-related reduction during the second and the third repetition for the young and older adults: (Rep 1 > Rep 2)Young ∩ (Rep 1 > Rep 3)Young ∩ (Rep 1 > Rep 2)Old ∩ (Rep 1 > Rep 3)Old. A statistical threshold of p < 0.005 was used due to the stricter nature of this conjunction analysis. Similar procedures have been used in previous studies (Goh et al., 2004). ROIs were defined as all contiguous voxels significantly activated within 10 mm radius of a peak conjunction location. Individual response estimates to each condition in these ROIs were extracted and then submitted to repeated-measures ANOVA with Age (Young, Old) and Repetition (Rep 1, Rep 2, Rep 3) as independent variables.
2.8. Correlation analysis of ROI neural and behavioral priming
We also performed correlation analyses on the response estimates extracted from the above ROIs and reaction time measures of behavioral priming. We computed Pearson’s correlation coefficient, examining reaction time (RT) difference and magnitude of repetition-related activation reductions (Rep 1 – Rep 2; Rep 1 – Rep 3) in the ROIs for younger adults, older adults, and all participants together.
3. RESULTS
3.1. Behavioral repetition priming
Table 1 (top) summarizes the performance measures (mean percent accuracy and RTs) corresponding to the living/non-living classification task. Both groups performed the task with a high level of accuracy (Mean percent correct: 91% and 85% for young and older adults, respectively). A two-way repeated measures ANOVA was conducted on the accuracy scores with Age (young vs. old adults) as a between-subjects factor and Repetitions (Rep 1, 2, and 3) as the within-subject factor. There was a marginal significant effect of Age on accuracy [F(1, 35) = 3.67, p = .06], with no significant effect of Repetition or interaction. We also performed the same analysis on the RT data, including only the correct trials. This analysis revealed a significant main effect of Repetition [F(2,70) = 93.42, p < 0.01] with participants responding faster with repeated presentation. The main effect of Age [F(1,35) = 11.41, p < 0.01] was significant because young adults were faster than older adults. Importantly, the interaction between Age and Repetition [F(1,70) = .72, n.s.] was not statistically significant, suggesting that the two age groups did not differ in the magnitude of behavioral priming. Both linear [(F(1,35) = 129.83, p < 0.001)] and quadratic [(F(1,35) = 29.78, p < 0.001] trends of the Repetition effect were significant, indicating that RTs decreased from Rep 1 to Rep 2, and from Rep 2 to Rep 3, with a greater decrease in the former compared to the latter case. Insert Table 1 about here
Table 1.
Behavioral performance of young and older adults during the living/non-living judgment fMRI task and the recognition task
| Young | Old | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Living/Non-Living Judgment | Rep 1 | Rep 2 | Rep 3 | Rep 1 | Rep 2 | Rep 3 |
| Proportion correct | 0.90 (0.02) | 0.91 (0.02) | 0.92 (0.02) | 0.84 (0.03) | 0.86 (0.03) | 0.85 (0.03) |
| RT (ms) | 765 (33) | 670 (27) | 651 (25) | 908 (31) | 799 (29) | 772 (27) |
|
| ||||||
| Recognition task | Rep 1 | Rep 3 | Rep 1 | Rep 3 | ||
|
| ||||||
| d′ | 3.59 (1.09) | 3.569 (0.95) | 2.73 (1.39) | 2.68 (1.03) | ||
Note: Accuracy and reaction time (RT) means for stimuli presented the first time (Rep 1), second time (Rep 2) and third time (Rep 3) in the living/non-living judgment task are shown on top. Means for d′ (discrimination) in the recognition task are shown at the bottom. S.E. are shown in parentheses.
3.2. Recognition task
Table 1 (bottom) summarizes the performance measures for each age group and repetition condition for the recognition task. Data from one older participant was excluded from the recognition behavioral analysis due to a large number of errors resulting in a final sample size for the recognition analysis of 19 younger and 17 older adults. An ANOVA was conducted for accuracy (d′), with Age (young and old) and Repetition (1 and 3) as factors. The analysis yielded a main effect of Age, [F(1,34) = 6.81, p < 0.01], with young adults having more accurate recognition memory than older adults. Neither the main effect of Repetition nor the Age x Repetition interaction approached significance (Fs < 1), suggesting that recognition memory in both groups did not differ whether stimuli were presented once or 3 times, possibly because performance was already at ceiling. The same analysis conducted on recognition RTs yielded a marginally significant main effect of Age [F(1,34) = 3.19, p = 0.08], suggesting that young adults were faster than older adults, with no significant main effect of Repetition or Age x Repetition interaction. Thus, our findings are consistent with previous studies showing an advantage in younger adults compared to older adults for recognition memory, despite similar implicit memory in both age groups.
3.3. Whole-brain neural priming in young and older adults
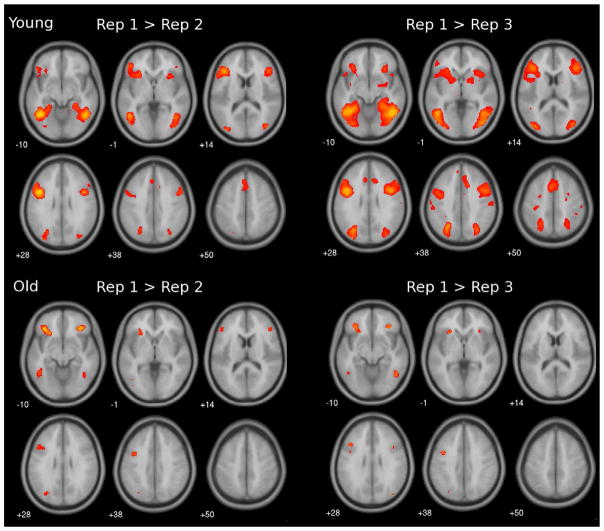
The whole brain analysis was first performed separately for each age group, contrasting first presentation with the second (Rep 1 > Rep 2) and third repetition (Rep 1 > Rep 3). Figure 1 shows that for the second repetition (Rep 1 > Rep 2), younger adults evidenced repetition-related activation reduction in brain responses in bilateral occipital, temporal, anterior cingulate, inferior frontal regions, and insula (see Table 2 for list of contrast peak locations). For the third repetition, additional brain areas showed repetition-related reduction that extended into the parahippocampal, hippocampal and parietal regions, bilaterally. Older adults showed similar brain regions with repetition-related activation reduction, but in fewer areas, and with a smaller spatial extent (Table 3).1 We did not find any regions where repeated items evoked greater responses than novel items in either group.
Figure 1.
Axial slices of whole-brain statistical maps showing repetition-related reduction in brain response for younger adults (top panel) and older adults (bottom panel), during the second (left) and third repetition (right), using a threshold of p < 0.001 (uncorrected), and a cluster size of at least 10 voxels.
Table 2.
Peak voxel locations, in MNI coordinates, of brain areas in young adults that showed repetition-related reduction in response during the 2nd and 3rd repetition relative to the first presentation.
| Contrast | Location | Brodmann’s Area | x | y | z | k | t |
|---|---|---|---|---|---|---|---|
| Young, Rep1 > Rep2 | L Inf. Frontal | 9 | −42 | 9 | 27 | 915* | 6.76 |
| L Inf. Frontal | 45 | −48 | 24 | 21 | 5.96 | ||
| L Inf. Frontal | 46 | −39 | 30 | 12 | 5.74 | ||
| L Insula | 13 | −27 | 21 | −3 | 4.65 | ||
| R Fusiform | 37 | 33 | −39 | −18 | 786* | 6.17 | |
| R Inf. Temporal | 37 | 45 | −60 | −6 | 6.94 | ||
| L Fusiform | 37 | −42 | −45 | −12 | 604* | 5.65 | |
| L Inf. Temporal | 19 | −45 | −57 | −9 | 6.7 | ||
| R Inf. Frontal | 9 | 45 | 9 | 30 | 324* | 5.48 | |
| R Inf. Frontal | 45 | 48 | 27 | 18 | 4.77 | ||
| R Mid. Frontal | 8 | 54 | 12 | 39 | 4.1 | ||
| L Sup. Occipital | 19 | −27 | −72 | 30 | 204* | 4.61 | |
| R Mid. Occipital | 19 | 39 | −81 | 18 | 4.93 | ||
| L Ant. Cingulate | 32 | −3 | 30 | 42 | 118* | 4.23 | |
| R Ant. Cingulate | 32 | 12 | 21 | 36 | 3.64 | ||
| R Insula | 13 | 30 | 18 | 0 | 56 | 4.04 | |
|
|
|||||||
| Young, Rep1 > Rep3 | Location | Brodmann’s Area | x | y | z | k | t |
| R Fusiform | 20 | 36 | −36 | −21 | 3022* | 8.39 | |
| R Fusiform | 37 | 42 | −48 | −15 | 7.08 | ||
| R Hippocampus | - | 27 | −15 | −21 | 3.7 | ||
| R Inf. Frontal | 9 | 45 | 6 | 33 | 7.19 | ||
| R Inf. Frontal | 46 | 45 | 33 | 12 | 5.87 | ||
| R Inf. Occipital | 18 | 45 | −78 | −3 | 6.78 | ||
| R Insula | 13 | 39 | 18 | −3 | 4.53 | ||
| R Mid. Frontal | 46 | 54 | 27 | 24 | 5.11 | ||
| R Mid. Occipital | 19 | 36 | −81 | 9 | 6.01 | ||
| R Mid. Temporal | 19 | 45 | −60 | −6 | 8.46 | ||
| R Parahippocampal | 28 | 36 | −18 | −21 | 3.91 | ||
| R Sup. Occipital | 19 | 33 | −75 | 21 | 4.95 | ||
| R Sup. Parietal | 7 | 30 | −69 | 36 | 5.7 | ||
| L Fusifom | 37 | −30 | −51 | −15 | 1770* | 8.93 | |
| L Mid. Occipital | 19 | −36 | −84 | 9 | 5.97 | ||
| L Mid. Temporal | 19 | −45 | −60 | −6 | 7.64 | ||
| L Parahippocampal | 36 | −27 | −36 | −12 | 4.8 | ||
| L Sup. Occipital | 19 | −30 | −72 | 27 | 6.61 | ||
| L Sup. Parietal | 7 | −24 | −63 | 39 | 6.59 | ||
| L Inf. Frontal | 9 | −45 | 6 | 30 | 1465* | 7.56 | |
| L Inf. Frontal | 45 | −48 | 24 | 21 | 6.31 | ||
| L Insula | 13 | −27 | 27 | −3 | 4.84 | ||
| L Mid. Frontal | 46 | −36 | 30 | 18 | 6.43 | ||
| L Ant. Cingulate | 32 | −6 | 6 | 54 | 472 | 5.42 | |
Supercluster
Table 3.
Peak voxel locations, in MNI coordinates, of brain areas in older adults that showed repetition-related reduction in response during the 2nd and 3rd repetition relative to the first presentation
| Contrast | Location | Brodmann’s Area | x | y | z | k | t |
|---|---|---|---|---|---|---|---|
| Old, Rep1 > Rep2 | L Mid. Occipital | 19 | −42 | −81 | 3 | 11 | 3.96 |
| R Fusiform | 20 | 36 | −36 | −21 | 19 | 3.64 | |
| R Mid. Temporal | 37 | 42 | −54 | −9 | 19 | 3.5 | |
| R Mid. Frontal | 46 | 45 | 33 | 15 | 20 | 3.9 | |
| L Sup. Occipital | 19 | −30 | −72 | 27 | 25 | 3.75 | |
| R Inf. Frontal | 47 | 36 | 33 | −12 | 65 | 5 | |
| 3 | L Fusiform | 37 | −39 | −45 | −15 | 121 | 4.56 |
| L Inf. Frontal | 47 | −36 | 33 | −12 | 128 | 5.19 | |
| L Inf. Frontal | 9 | −39 | 6 | 36 | 145 | 4.33 | |
| L Mid. Frontal | 46 | −48 | 30 | 21 | 3.92 | ||
|
|
|||||||
| Contrast | Location | Brodmann’s Area | x | y | z | k | t |
| Old, Rep1 > Rep3 | R Fusiform | 20 | 36 | −36 | −21 | 13 | 3.59 |
| L Fusiform | 37 | −42 | −45 | −18 | 18* | 3.59 | |
| L Inf. Temporal | 37 | −45 | −57 | −9 | 3.55 | ||
| R Inf. Temporal | 37 | 45 | −54 | −12 | 39* | 3.98 | |
| R Sup. Occipital | 19 | 39 | −81 | 27 | 3.72 | ||
| R Inf. Frontal | 47 | 33 | 33 | −9 | 46* | 4.18 | |
| R Inf. Frontal | 9 | 45 | 9 | 30 | 3.36 | ||
| R Insula | 13 | 27 | 30 | −3 | 3.79 | ||
| L Inf. Frontal | 47 | −36 | 36 | −15 | 122* | 4.29 | |
| L Inf. Frontal | 9 | −39 | 6 | 36 | 4.23 | ||
Supercluster
Revision of Ballesteros et al., 11–601, NBA 1
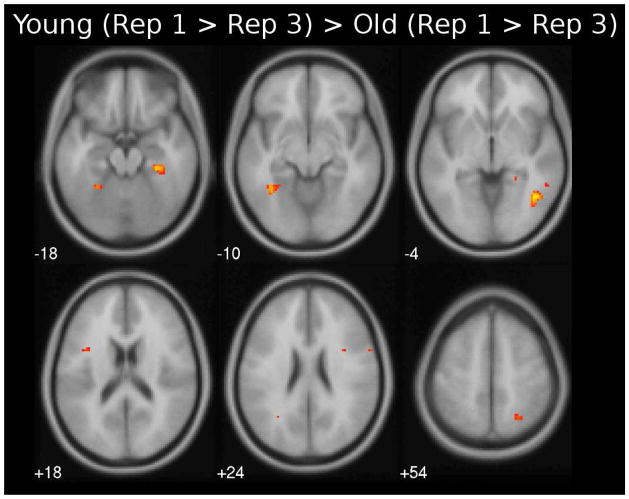
Specific areas showing direct differences in neural priming between young and older adults are listed in Table 4 with the age difference during the Rep 3 depicted in Figure 2. Overall, we found that young adults showed greater neural priming than older adults across several anterior and posterior brain regions. For the second repetition, young adults showed greater repetition-related reduction than older adults only in the right inferior temporal region (Table 4, top). During the third repetition, however, this age difference was apparent in multiple regions, including bilateral inferior frontal regions, left fusiform, and inferior parietal regions, and right middle frontal, inferior temporal, parahippocampal and superior parietal regions (Figure 2; Table 4, bottom)2. There were no significant regions that showed greater repetition-related reduction in older adults compared to young adults.
Table 4.
Peak voxel locations, in MNI coordinates, of brain areas that showed age differences in repetition- related reduction between young and older adults during the 2nd and 3rd repetition relative to the first presentation
| Contrast | Location | Brodmann’s Area | x | y | z | K | t |
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Rep1 > Rep2, Young > Old | R Inf. Temporal | 37 | 45 | −60 | −6 | 10 | 3.64 |
|
|
|||||||
| Rep1 > Rep3, Young > Old | L Fusiform | 37 | −30 | −51 | −15 | 21 | 4.3 |
| L Inf. Frontal | 9 | −42 | −6 | 30 | 11 | 4.1 | |
| L Inf. Frontal | 44 | −39 | 9 | 21 | 16 | 3.61 | |
| L Inf. Parietal | 39 | −24 | −60 | 39 | 15 | 3.78 | |
| L Med. Frontal | 8 | −3 | 45 | 39 | 11 | 3.56 | |
| L Precentral | 6 | −27 | −18 | 66 | 18 | 3.49 | |
| R Inf. Frontal | 9 | 27 | −9 | 21 | 21 | 3.8 | |
| R Inf. Frontal | 9 | 33 | 6 | 24 | 10 | 3.5 | |
| R Inf. Temporal | 37 | 45 | −57 | −3 | 90 | 4.34 | |
| R Mid. Frontal | 46 | 27 | 33 | 21 | 11 | 3.96 | |
| R Parahippocampal | 36 | 30 | −30 | −18 | 51 | 4.1 | |
| R Sup. Parietal | 7 | 24 | −57 | 45 | 31 | 3.71 | |
Figure 2.
Axial slices showing brain areas that have greater neural priming in younger than older adults for first presentation > third presentation using a threshold of p < 0.001 (uncorrected).
We additionally examined the repetition-related reduction between the second to third repetition for both age groups (Supplementary Figure 1). In young adults, we found significant further reduction from second to third repetition in right frontal areas, and precuneus, and bilateral parietal and fusiform areas. In contrast, older adults only showed significant reduction in the left temporal pole and supplementary motor areas. This finding is consistent with older adults having less repetition-related activation reduction compared to younger adults, and suggests that older adults have reached the floor in their neural priming response during the second repetition.
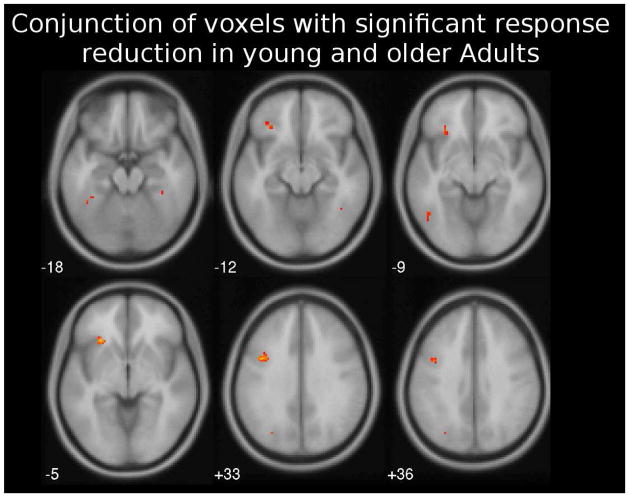
3.4. Common areas showing neural priming across age groups
Regions from a conjunction analysis showing repetition-related reduction common across both age groups and across the second and third repetitions are displayed in Figure 3 and listed in Table 5. Posterior regions observed in this analysis included bilateral fusiform gyrus, left inferior temporal gyrus, and superior occipital gyrus, and right middle temporal gyrus. In addition, common regions showing repetition-related reduction were also found in the left insula, and inferior frontal gyrus. Results of the ANOVA analysis performed on brain response estimates extracted from these common ROIs are shown in Table 5. All regions showed significant main effects of Repetition (all p < .05), which was expected since these regions were defined based on repetition-related activity. Age main effects were restricted to the left insula and the right fusiform and occurred because older adults showed greater overall activation in these regions compared to young adults. Significant Age x Repetition interactions occurred in the left inferior temporal (LIT) and the right middle temporal (RMT) regions (Figure 4). These interactions occurred because young adults showed continued reductions from the second to the third repetition (LIT: Rep 1 to Rep 2: t(18) = 4.923, p <. 001; Rep 2 to Rep 3: t(18) = 6.270, p <. 001; RMT: Rep 1 to Rep 2: t(18) = 7.359, p <. 001; Rep 2 to Rep 3: t(18) =1.986, p = .062), but in older adults, responses were reduced during the second repetition (LIT: Rep 1 to Rep 2: t(17) = 4.323, p <. 001; RMT: Rep 1 to Rep 2: t(17) = 4.34, p <. 001) but did not change during the third repetition (LIT: Rep 2 to Rep 3: t(17) = .451, n.s.; RMT: Rep 2 to Rep 3: t(17) =.862, n.s.). In summary, the ROI analysis revealed that older adults showed greater activation overall in the left insula and right fusiform, and showed less repetition-related reduction in temporal regions.
Figure 3.
Axial slices showing brain areas that show neural priming during both the second and third presentations, and across younger and older adults (conjunction analysis). All maps are shown at statistical threshold of p < 0.005 (uncorrected).
Table 5.
Peak voxel locations, in MNI coordinates, of common brain areas showing repetition-related reduction in response across the 2nd and 3rd repetitions, across young and older adults (conjunction analysis)
| Location | BA | X | Y | Z | T | Cluster Size | ANOVA (F-Ratio) | Correlation with RT- Reduction | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| Age | Rep | Age x Rep | Rep 1 – Rep 2 | Rep 1 – Rep 3 | |||||||||||
|
|
|||||||||||||||
| Y | O | All | Y | O | All | ||||||||||
|
|
|||||||||||||||
| L Insula | - | −27 | 24 | −3 | 4.41 | 88 | 6.45* | 32.36** | .07 | .58* | −.12 | .18 | .39 | .13 | .23 |
| L. Inf. Frontal | 45 | −39 | 6 | 33 | 4.03 | 137 | 3.85 | 20.73** | 1.30 | .45 | − .06 | .20 | .65* | .25 | .41* |
| R. Fusiform | 36 | 36 | −36 | 21 | 3.59 | 59 | 10.59** | 37.53** | 1.14 | .64* | − .06 | .17 | .57* | .24 | .36* |
| L. Fusiform | 37 | −42 | −45 | −18 | 3.59 | 116 | .08 | 34.21** | 1.35 | .60* | −.20 | .09 | .61* | .34 | .41* |
| L Inf. Temporal | 37 | −45 | −57 | −9 | 3.55 | -- | 2.99 | 40.85** | 4.42** | .40 | .17 | .20 | .59* | .20 | .30 |
| L. Sup. Occipital | 18 | −30 | −72 | 33 | 3.43 | 43 | 2.56 | 21.59* | .57 | .61* | .09 | .25 | .42 | .13 | .23 |
| R. Mid. Temporal | 21 | 45 | −63 | −12 | 3.38 | 59 | .42 | 56.22** | 7.61** | .25 | .21 | .15 | .45 | .27 | .23 |
| L. Inf. Frontal | 46 | −45 | 39 | 9 | 3.26 | -- | .68 | 42.39** | 3.08 | .26 | −.06 | .04 | .48* | .50* | .38* |
Note: Results of ROI analysis for these common brain areas are also shown. F-values of effects within each ROI are displayed. In addition, the right columns show results of the correlation analysis of repetition priming effects and reaction time differences (Y: Young, O: Old, All: Young and Old together). Note,
p < .05,
p < .01.
Figure 4.
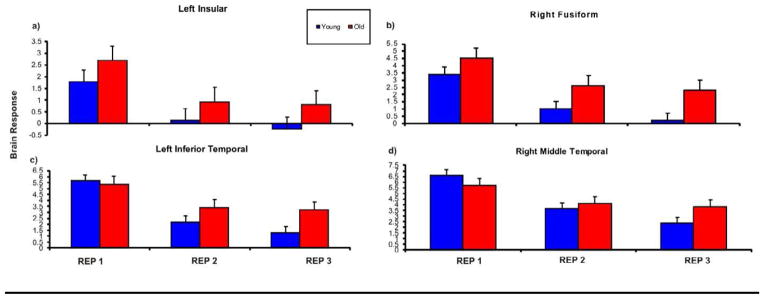
Brain responses to the repetition conditions for the young and older adults in four ROIs from the conjunction analysis. The left insula (a) and right fusiform gyrus (b) showed a significant main effect of age. The inferior temporal gyrus (a) and the right middle temporal gyrus (b) showed significant age x repetition interaction, p < .001. Error bars represent standard deviation.
3.5. Correlations between ROI neural priming and behavioral priming
Results of the correlation between behavioral priming (measured by reductions in reaction time) and neural priming (measured by repetition-related activation reduction in the ROIs from the conjunction analysis) are shown in Table 5. In young adults, greater neural priming was associated with greater behavioral priming in several brain areas for both the second repetition condition (in the left insula and superior occipital, and bilateral fusiform regions) and the third repetition condition (in the left inferior frontal and inferior temporal, and bilateral fusiform regions). In contrast, for older adults, the only significant correlation between behavioral priming and neural priming was in the left inferior frontal gyrus for the third repetition. Thus, whereas younger adults evidenced several functional-behavioral associations, this relationship was not as apparent in older adults, despite the comparable behavioral priming effects observed3.
4. DISCUSSION
This present study investigated age-related differences in the functional neural correlates of conceptual repetition priming for objects by comparing behavioral and neural priming in younger and older adults. The main findings were: (1) Both young and older adults showed similar behavioral repetition priming effects with both groups responding equally faster to repeated compared to unrepeated pictures; (2) Both age groups revealed significant repetition-related activation reductions in several anterior and posterior brain regions related to neural priming; (3) Whereas young adults showed a widespread network of brain regions sensitive to stimulus repetition, older adults showed a greatly reduced extent and magnitudes of neural priming to repeated visual objects; (4) In regions showing repetition-related reductions common across age groups, the degree of neural priming correlated positively with behavioral priming in several ROIs in young adults but only the left inferior frontal region in older adults.
Several posterior regions of the brain showed neural priming to stimulus repetition in our study that included the parietal, hippocampal and parahippocampal regions and the fusiform gyrus. These areas have been previously suggested to be involved in various cognitive processes related to visual object recognition (e.g., Seeck et al., 1995), such as attentional resource allocation (parietal cortex; Schacter et al., 1993; Mummery et al., 1999; Vuilleumier et al., 2002), visual categorization, and encoding of perceptual identity as well as episodic information (lateral and medial temporal regions; Goh et al., 2004; Goh et al., 2007; Rossel et al., 2001). Thus, repetition-related activation reduction in these regions indicates reduced engagement of these attentional, perceptual, and even memory processes, as a reflection of more efficient neural processing when encountering the same visual information again (Grill-Spector et al., 2006; Henson, 2003; Martin, 2007; Schacter et al., 2007; Wig et al., 2009). Indeed, in terms of behavior, repeated stimulus presentation was associated with reduced processing time in both young and older adults. Moreover, faster response times were also correlated with greater repetition-related reduction in anterior and posterior brain regions in young adults, and the left inferior frontal region for older adults.
Age-related differences in neural priming
Our study demonstrates, however, that despite the age-invariant behavioral priming, there are clear age differences in neural priming. The brain regions that displayed neural priming effects were less extensive in older adults compared to younger adults, even for stimuli repeated three times, with no regions that showed greater repetition-related reduction in older adults compared to young adults. Moreover, in regions that commonly displayed repetition-related activation reductions in both age groups, young adults still evidenced greater magnitudes of reduction than older adults. Specifically, activation reductions in young adults in these areas showed a linear reduction as a function of repetition but older adults showed activation reductions with the second repetition, but no further reduction with the third repetition. These findings are in agreement with Daselaar et al. (2005) and Bergerbest et al. (2009) who also reported less neural priming in older adults compared to young adults.
The decrease in neural activity that accompanies repetition is generally viewed as evidence for more efficient processing, particularly given that our results demonstrated it was coupled with decreased reaction time. Older adults do not show reductions in neural activity as pronounced as young, as this presumably reflects less elaborate processing of perceptual and semantic features of the stimulus as well as decreased availability of processing episodic information for the first presentation relative to young adults, so that further processing is required on the third presentation. In support of this, studies have found that aging is accompanied by declines in perceptual processing in posterior brain regions including the occipital, parietal, fusiform, and medial temporal regions (Chee et al., 2006; Goh et al., 2010; Huang et al., 2012; Park et al, 2004; Carp et al., 2010; Park et al., 2012). Importantly, these studies indicate that decreased neural selectivity in perceptual processing with age is a key neural mechanism where older adults’ visual and perceptual systems evidence less rapid tuning and adaptive learning from experiences, responding to stimuli in a more indiscriminant manner, with less affinity to reduce activity to repeated encounters. The present results extend this decreased selectivity of neural activity to object priming tasks.
Moreover, the finding that older adults had overall higher levels of activity than younger adults in the insula across stimulus repetitions, suggests that older adults may require greater neural effort in regions associated with conceptual or semantic processing task, even during the first stimulus presentation. In support of this explanation, studies have shown that the insula is involved in semantic retrieval processes which generally decline with age (Velanova et al., 2003), and older adults recruit greater levels of activity in this region even at lower levels of task difficulty than young adults (Reuter-Lorenz and Capell, 2008; Schneider-Garces et al., 2010). Our finding are consistent with these as well as several other functional neuroimaging and electrophysiological studies of aging that have also reported that older adults tend to show greater or bilateral frontal involvement during various cognitive task demands, whereas younger adults showed lower or more unilateral frontal responses (e.g., Cabeza et al., 2002; Grady, 2000; Osorio et al., 2010; Osorio et al., 2009). There is considerable evidence that this increased frontal activation with age reflects compensatory processing or neural scaffolding in older adults involving additional recruitment of neural circuitry to aid task performance (Park and Reuter-Lorenz, 2009). We suggest that part of the need for compensatory frontal engagement in older adults may be due to less efficient stimulus processing in posterior regions, as it is well-recognized that there is a shift to the engagement of anterior regions with age, due to decreased activity in posterior regions (Goh et al., 2010, Davis et al., 2008, Gutchess et al. 2005; Park et al., 2004). Specifically, greater frontal processing in older adults may be required to drive more elaborative stimulus processing that is lacking in the posterior regions. This may involve continued selection and disambiguation of conceptual/perceptual/episodic information that should have been encoded during the previous stimulus encounter, but were not completed because of less discriminant posterior activity in older adults.
Our findings are inconsistent with a recent study that showed age-invariant neural priming effects using word stimuli (Gold et al., 2009). We suggest two main reasons for these discrepancies: First, Gold et al. relied on word association for their priming response (e.g., “bread” was a prime for “butter”). This may have involved a less direct associative process than a veridical repetition, and a more blunted priming response. Second, Gold et al. used a short interval (250 ms) between novel and repeated items whereas our delays were much longer, averaging 50 s. Thus, the priming response in Gold et al. depended on maintenance of activation for a very brief moment with no episodic or working memory component, whereas the long delay in our task required both working memory and episodic maintenance. It would be important in future work to examine how lag from very short to long intervals affects age-associated neural activity. We also note that others have failed to find age differences in neural priming. Early studies had small sample sizes that likely limited power to detect differences (Bäckman et al., 1997; Lustig and Buckner, 2004)
Finally, Soldan et al., (2008) examined spatial patterns of repetition-related neural reductions during repeated presentation of possible and impossible objects and found age-invariance as a function of repetition. The authors note that the results reflect a null finding, which need to be interpreted with caution (Soldan et al., 2008). In fact, in all repetition contrasts Soldan et al., (2008) observed smaller repetition magnitudes in older adults as compared to younger adults, which failed to reach statistical significance. In our study, we have statistically shown that the null hypothesis can be rejected for age-related neural priming effects supporting our results that behavioral age-invariant priming effects are accompanied with age-related differences in repetition-related neural reduction effects.
Correlation between ROI, activation reductions and behavioral priming
Another important finding in the present study was the significant brain-behavior correlations we observed across several anterior and posterior regions in young adults, suggesting that activation reduction in these many areas contribute to processing efficiency with behavioral benefits. In contrast, activity reduction in the left inferior frontal area was sufficient to facilitate faster response times to repeated items in older adults. The lack of reliable associations between posterior brain responses and behavior in older adults may be due to decreased efficiency and less selective stimuli processing in these regions that decouples neural activity with subsequent behavior. It is plausible that this decline in posterior stimulus processing may underlie the overall slower responses in older relative to young adults when making living/non-living judgments in our task. Taking these together, we speculate that the left inferior frontal area and the insula, as highlighted above, may be critical regions determining behavioral priming during repeated conceptual classification performance in young and older adults, with processing in posterior regions adding overall timing advantage to young adults.
Spared behavioral repetition priming and impaired recognition in older adults
We note that the finding of spared behavioral conceptual priming coupled with compromised explicit recognition is consistent with age-related differences in implicit and explicit memory found in many other studies (Ballesteros and Reales, 2004; Bergerbest et al., 2009; Lustig and Buckner, 2004; Mitchell and Bruss, 2003; Osorio et al., 2009; Osorio et al., 2010; Sebastián and Ballesteros, 2012; Sebastián et al., 2011; Soldan et al., 2008; for reviews, see Fleischman, 2007; Fleischman and Gabrieli, 1998). Thus, whereas our sample of older showed behavioral benefits from repetition that were on par with young adults, they were representative of older adults in general, showing normal but poorer explicit memory. This is important in ensuring that our findings in implicit memory are not due to a select group of higher-performing older adults. Interestingly, this dissociation between these mnemonic functions in young adults (Cooper et al., 1992; Reales and Ballesteros, 1999) and older adults (Bergerbest et al., 2009; Soldan et al., 2008) supports the idea that implicit and explicit memory tasks are differentially sensitive to age effects (Tulving and Schacter, 1990; Squire, 2004), a topic for consideration in future studies.
Summary
To conclude, our results suggest that the relationship between brain function and behavioral priming found in young adults is altered in older adults. These age-related neural changes, however, do not affect behavioral priming in older adults, suggesting that neural priming in the brain regions observed in older adults are sufficient to facilitate behavioral performance due to repetition to the same degree as in young adults. Nevertheless, our findings challenge the notion that repetition-related priming, and implicit memory, are completely unaffected by aging.
Supplementary Material
Acknowledgments
This work was supported by grants from the Spanish Government (DGU PE-2007-0069; MINCINN PSI2010-21609-C02-01) and the Madrid Community (MULTIMAG: 2006/BIO-170; S2010/BMD-2349) awarded to Soledad Ballesteros and NIH grant 5R37 AG-006265-25 awarded to Denise Park.
Footnotes
We additionally performed a whole-brain ANOVA to examine the interaction effect between age and repetition. This analysis revealed regions showing repetition effects, which were dependent on age in the frontal, parietal, and temporal cortices similar to our t-test approach presented here (see Figure S2).
We performed these same whole-brain contrast analyses using unsmoothed functional imaging data to evaluate the possible contribution of brain atrophy in older adults to these age-related differences in neural priming. We found the same age differences such that young adults still showed greater neural priming than older adults in similar brain regions observed using the smoothed data. Thus, age differences in brain structure do not sufficiently account for the neural priming differences observed here.
An illustration of this effect in the insular cortex can be found in the supplementary material Figure S3.
DISCLOSURE ESTATEMENT
There are no actual or potential conflict of interest.
The data contained in the manuscript being submitted have not been previously published, have not been submitted elsewhere and will not be submitted elsewhere while under consideration at Neurobiology of Aging.
All authors have reviewed the contents of the manuscript being submitted, approve of its contents and validate the accuracy of the data.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bäckman L, Almkvist O, Andersson J, Nordberg A, Winblad B, Reineck R, Långstrom B. Brain activation in young and older adults during implicit and explicit retrieval. J Cogn Neurosci. 1997;9:378–391. doi: 10.1162/jocn.1997.9.3.378. [DOI] [PubMed] [Google Scholar]
- Ballesteros S, Reales JM. Intact haptic priming in normal aging and Alzheimer’s disease: Evidence for dissociable memory systems. Neuropsychologia. 2004;44:1063–1070. doi: 10.1016/j.neuropsychologia.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Ballesteros S, Reales JM, Mayas J. Picture priming in aging and dementia. Psicothema. 2007;19:239–244. [PubMed] [Google Scholar]
- Ballesteros S, Reales JM, Mayas J, Heller MA. Selective attention modulates visual and haptic repetition priming: Effects on ageing and Alzheimers’ disease. Exp Brain Res. 2008;189:473–483. doi: 10.1007/s00221-008-1441-6. [DOI] [PubMed] [Google Scholar]
- Ballesteros S, Gonzalez M, Mayas J, Reales JM, Garcia B. Crossmodal object priming in young and older adults: Multisensory processing in vision, touch, and audition. Eur J Cogn Psychol. 2009;21:366–387. [Google Scholar]
- Baltes PB, Lindengerber U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: A new window to the study of cognitive aging? Psychol Aging. 1997;12:12–21. doi: 10.1037//0882-7974.12.1.12. [DOI] [PubMed] [Google Scholar]
- Bergerbest D, Gabrieli JDE, Whitfield-Gabrieli S, Kim H, Stebbins GT. Age-associated reduction of asymmetry in prefrontal cortex function and preservation of conceptual repetition priming. Neuroimage. 2009;45:237–246. doi: 10.1016/j.neuroimage.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Goodman J, Burock M, Rotte M, Koutstaal W, Schacter DL, Rosen B, Dale A. Functional-anatomical correlates of object priming in humans revealed by rapid presentation event-related fMRI. Neuron. 1998;20:285–296. doi: 10.1016/s0896-6273(00)80456-0. [DOI] [PubMed] [Google Scholar]
- Bunzeck N, Schutze H, Duzel E. Category-specific organization of prefrontal response-facilitation during priming. Neuropsychologia. 2006;44:1765–1776. doi: 10.1016/j.neuropsychologia.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Caggiano DM, Jiang Y, Parasuraman R. Aging and repetition priming for targets and distracters in a working memory task. Aging, Neuropschol Cogn. 2006;13:552–573. doi: 10.1080/138255890969555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp J, Park J, Polk TA, Park DC. Age differences in neural distinctiveness revealed by multi-voxel pattern analysis. NeuroImage. 2010 May 15;56(2):736–43. doi: 10.1016/j.neuroimage.2010.04.267. Epub 2010 May 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MW, Goh JO, Venkatraman V, Tan JC, Gutchess A, Sutton B, Hebrank A, Leshikar E, Park DC. Age-related changes in object processing and contextual binding revealed using fMR adaptation. J Cogn Neurosci. 2006;18:495–507. doi: 10.1162/jocn.2006.18.4.495. [DOI] [PubMed] [Google Scholar]
- Cooper LA, Schacter DE, Ballesteros S, Moore C. Priming and recognition of transformed three-dimensional objects: Effects of size and reflection. J Exp Psychol – Learn Mem Cogn. 1992;18:43–57. doi: 10.1037//0278-7393.18.1.43. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Veltman DJ, Rombouts SARB, Raaijmakers JGW, Jonker C. Aging effects of perceptual and lexical/semantic components of word-stem priming: An event-related fMRI study. Neurobiol Learn Mem. 2005;83:251–262. doi: 10.1016/j.nlm.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cereb Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischman DA. Repetition priming in aging and Alzheimer’s disease: An integrative review and future directions. Cortex. 2007;43:889–897. doi: 10.1016/s0010-9452(08)70688-9. [DOI] [PubMed] [Google Scholar]
- Fleischman DA, Gabrieli JDE. Repetition priming in normal ageing and in Alzheimer’s disease. A review of findings and theories. Psychol Aging. 1998;13:88–119. doi: 10.1037//0882-7974.13.1.88. [DOI] [PubMed] [Google Scholar]
- Goh JO, Siong SC, Park DC, Gutchess A, Hebrank A, Chee MW. Cortical areas involved in object, background, and object-background processing revealed with functional magnetic resonance adaptation. J Neurosci. 2004;45:10223–10228. doi: 10.1523/JNEUROSCI.3373-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh JO, Chee MW, Tan JC, Venkatraman V, Hebrank A, Leshikar ED, Jenkins L, Sutton BP, Gutchess AH, Park DC. Age and culture modulate object processing and object-scene binding in the ventral visual area. Cogn Affect Behav Neurosci. 2007;7:44–52. doi: 10.3758/cabn.7.1.44. [DOI] [PubMed] [Google Scholar]
- Goh JO, Suzuki A, Park DC. Reduced neural selectivity increases fMRI adaptation with age during face discrimination. Neuroimage. 2010;51:336–344. doi: 10.1016/j.neuroimage.2010.01.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh JO, An Y, Resnick SM. Differential trajectories of age-related changes in components of executive and memory processes. Psychol Aging. 2011 Dec 26; doi: 10.1037/a0026715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BT, Andersen AH, Jicha GA, Smith CD. Aging influences the neural correlates of lexical decision but not automatic semantic priming. Cereb Cortex. 2009;19:2671–2679. doi: 10.1093/cercor/bhp018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Horwitz B, Rapoport SI. Age-related changes in the neural correlates of degraded and nondegraded face processing. Cogn Neuropsychol. 2000;217:165–186. doi: 10.1080/026432900380553. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal detection theory and psychophysics. Wiley; New York: 1966. [Google Scholar]
- Grill-Spector G, Henson RNA, Martin A. Repetition and the brain. Neural models of stimulus specific effects. Trends Cogn Sci. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Hedden T, Bangert A, Minear M, Liu LL, Park DC. Aging and the neural correlates of successful picture encoding: frontal activations compensate for decreased medial-temporal activity. J Cogn Neurosci. 2005;17:84–96. doi: 10.1162/0898929052880048. [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JDE. Insights into the ageing mind: A view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–97. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Henson RNA. Neuroimaging studies of priming. Prog Neurobiol. 2003;70:53–81. doi: 10.1016/s0301-0082(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Huang CM, Polk TA, Goh JO, Park DC. Both left and right posterior parietal activations contribute to compensatory processes in normal aging. Neuropsychologia. 2012;50:55–66. doi: 10.1016/j.neuropsychologia.2011.10.022. Epub 2011 Oct 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutstaal W, Wagner AD, Rotte M, Maril A, Buckner RL, Schacter DL. Perceptual specificity in visual object priming: Functional magnetic resonance imaging evidence for a laterality difference in fusiform cortex. Neuropsychologia. 2001;39:184–199. doi: 10.1016/s0028-3932(00)00087-7. [DOI] [PubMed] [Google Scholar]
- Lawson AL, Guo C, Jiang Y. Age effects on brain activity during repetition priming on target and distracters. Neuropsychologia. 2007;45:1223–1231. doi: 10.1016/j.neuropsychologia.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig C, Buckner RL. Preserved neural correlates of priming in old age and dementia. Neuron. 2004;42:865–875. doi: 10.1016/j.neuron.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Maccotta L, Buckner RL. Evidence for neural effects of repetition that directly correlate with behavioral priming. J Cogn Neurosci. 2004;16:1625–1632. doi: 10.1162/0898929042568451. [DOI] [PubMed] [Google Scholar]
- MacMillan NA, Creelman CD. Detection Theory: An user’s guide. Cambridge University Press; New York: 1991. [Google Scholar]
- Martin A. The representation of object concepts in the brain. Ann Rev Psychol. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- Mitchell DB, Bruss PJ. Age differences in implicit memory: Conceptual, perceptual or methodological? Psychol Aging. 2003;18:807–822. doi: 10.1037/0882-7974.18.4.807. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Shallice T, Price CJ. Dual-process model in semantic priming: A functional imaging perspective. Neuroimage. 1999;9:516–525. doi: 10.1006/nimg.1999.0434. [DOI] [PubMed] [Google Scholar]
- Nilsson LG. Memory function in normal ageing. Acta Neurol Scand. 2003;107:7–13. doi: 10.1034/j.1600-0404.107.s179.5.x. [DOI] [PubMed] [Google Scholar]
- Olichney JM, Jason R, Taylor JR, Hillert DG, Chan S-h, Salmon DP, Gatherwright J, Iragui VJ, Kutas M. fMRI congruous word repetition effects reflect memory variability in normal elderly. Neurob Aging. 2010;31:1975–1990. doi: 10.1016/j.neurobiolaging.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio A, Ballesteros S, Fay S, Pouthas V. The effect of age on word-stem cued recall: A behavioral and electrophysiological study. Brain Res. 2009;1289:56–68. doi: 10.1016/j.brainres.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Osorio A, Fay S, Pouthas V, Ballesteros S. Ageing affects brain activity in highly educated older adults: An ERP study using a word-stem priming task. Cortex. 2010;46:522–534. doi: 10.1016/j.cortex.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Park DC, Davidson L, Lautenschlager G, Smith AD, Smith P, Hedden T. Models of visuo-spatial and verbal memory across the adult lifespan. Psychol Aging. 2002;17:299–320. [PubMed] [Google Scholar]
- Park DC, Polk TA, Mikels JA, Taylor SF, Marshuetz C. Cerebral aging: Integration of brain and behavioral models of cognitive function. Dialogues Clin Neurosci. 2001;3:151–165. doi: 10.31887/DCNS.2001.3.3/dcpark. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Carp J, Kennedy KM, Rodrigue KM, Bischof GN, Huang CM, Rieck JR, Polk TA, Park DC. Neural broadening or neural attenuation? Investigating age-related dedifferentiation in the face network in a large lifespan sample. J Neurosci. 2012;32:2154–2158. doi: 10.1523/JNEUROSCI.4494-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR. Aging reduces neural specialization in ventral visual cortex. P Natl Acad Sci USA. 2004;101:13091–13095. doi: 10.1073/pnas.0405148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptative brain: Ageing and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reales JM, Ballesteros S. SDT_SP a program in Pascal for computing parameters and significance tests from several detection theory designs. Beh Res Meth, Inst Comp. 1994;26:151–155. [Google Scholar]
- Reales JM, Ballesteros S. A computer programme for signal detection theory. Universitas; Madrid: 2000. TDS_EXPER para Windows. Un programa informático para la teoría de detección de señales (TDS_EXPERT. [Google Scholar]
- Reales JM, Ballesteros S. Implicit and explicit representations of visual and haptic objects: A cross-modal study. J Exp Psychol: Learn Mem Cogn. 1999;20:1–25. [Google Scholar]
- Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Curr Dir Psychol Scienc. 2008;17:177–182. [Google Scholar]
- Rossell SL, Bullmore ET, Williams SCR, David AS. Brain activation during automatic and controlled word associations: A semantic priming experiment using lexical-decision task. Neuropsychologia. 2001;39:1167–1176. doi: 10.1016/s0028-3932(01)00049-5. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Buckner RL. Priming and the brain. Neuron. 1998;20:185–195. doi: 10.1016/s0896-6273(00)80448-1. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wig GS, Stevens WD. Reductions in cortical activity during priming. Curr Opin Neurobiol. 2007;17:171–176. doi: 10.1016/j.conb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Chiu CYP, Ochsner KN. Implicit memory: a selective review. Annu Rev Neurosci. 1993;16:159–182. doi: 10.1146/annurev.ne.16.030193.001111. [DOI] [PubMed] [Google Scholar]
- Schneider-Garces NJ, Gordon BA, Brumback-Peltz CR, Shin E, Lee Y, Sutton BP, Maclin EL, Gratton G, Fabiani M. Span, CRUNCH, and beyond: Working memory capacity and the aging brain. J Cog Neurosc. 2010;22:655–669. doi: 10.1162/jocn.2009.21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastián M, Ballesteros S. Effects of normal aging on event-related potentials and oscillatory brain activity during a haptic repetition priming task. Neuroimage. 2012;60:7–20. doi: 10.1016/j.neuroimage.2011.11.060. [DOI] [PubMed] [Google Scholar]
- Sebastián M, Reales JM, Ballesteros S. Ageing affects event-related potentials and brain oscillations: a behavioral and electrophysiological study using a haptic recognition memory task. Neuropsychologia. 2011;49:3967–3980. doi: 10.1016/j.neuropsychologia.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Seeck M, Schomer D, Mainwaring N, Ives J, Bubuisson D, Blume H, Cosgrove R, Ransil BJ, Mesulam MM. Selectively distributed processing of visual object recognition in the temporal and frontal lobes of the human brain. Ann Neurol. 1995;37:538–545. doi: 10.1002/ana.410370417. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory systems of the brain: a brief history and current perspective. Neurobiol Learn Mem. 2004;82:171–177. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Soldan A, Gazes E, Hilton HJ, Stern Y. Aging does not affect brain patterns of repetition priming of novel objects. J Cogn Neurosci. 2008;20:1762–1776. doi: 10.1162/jocn.2008.20130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E, Schacter DL. Priming and human memory systems. Science. 1990;247:301–306. doi: 10.1126/science.2296719. [DOI] [PubMed] [Google Scholar]
- Van Turennout M, Ellmore T, Martin A. Long-lasting cortical plasticity in the object naming system. Nat Neurosci. 2000;3:1329–1334. doi: 10.1038/81873. [DOI] [PubMed] [Google Scholar]
- Velanova K, Jacoby LL, Wheeler ME, McAvoy MP, Petersen SE, Buckner RL. Functional–anatomic correlates of sustained and transient processing components engaged during controlled retrieval. J Neurosci. 2003;17:8460–8470. doi: 10.1523/JNEUROSCI.23-24-08460.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Henson RN, Driver J, Dolan RN. Multiple levels of visual object constancy revealed by event-related fMRI of repetition priming. Nat Neurosci. 2002;5:491–499. doi: 10.1038/nn839. [DOI] [PubMed] [Google Scholar]
- Wig GS, Buckner RL, Schacter DL. Repetition priming influences distinct brain systems: Evidence from task-evoked and resting-state correlations. J Neurophysiol. 2009;101:2632–2648. doi: 10.1152/jn.91213.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wig GS, Grafton ST, Demos KE, Kelley WM. Reductions in neural activity underlie behavioral components of repetition priming. Nat Neurosci. 2005;9:1227–1233. doi: 10.1038/nn1515. [DOI] [PubMed] [Google Scholar]
- Wiggs C, Weisberg J, Martin A. Repetition priming across the adult lifespan – The long and short of it. Aging, Neurospsychol Cogn. 2006;13:308–325. doi: 10.1080/138255890968718. [DOI] [PubMed] [Google Scholar]
- Xue G, Dong Q, Chen C, Lu Z, Mumford JA, Poldrack RA. Greater neural pattern similarity across repetitions is associated with better memory. Science. 2010;330 (6000):97–101. doi: 10.1126/science.1193125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zago L, Freske MJ, Aminoff E, Bar M. The rise and fall of priming: How visual exposure shapes cortical representations of objects. Cereb Cortex. 2005;16:1655–1665. doi: 10.1093/cercor/bhi060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.