Abstract
Background
Persistent fibroblast activation initiated by transforming growth factor β (TGF-β) is a fundamental event in the pathogenesis of systemic sclerosis (SSc), and its pharmacological inhibition represents a potential therapeutic strategy. The nuclear receptor peroxisome proliferator-activated receptor-gamma (PPAR-γ) exerts potent fibrotic activities. The synthetic triterpenoid oleanane 2-cyano-3,12-dioxoolean-1,9-dien-28-oic (CDDO) is a PPAR-γ agonist with potential effects on TGF-β signaling and dermal fibrosis.
Objective
To examine the modulation of fibrogenesis by CDDO in explanted fibroblasts, skin organ cultures and murine models of scleroderma.
Material and methods
The effects of CDDO on experimental fibrosis induced by bleomycin injection or by overexpression of type I constitutively active TGF-β receptor was evaluated. Modulation of fibrotic gene expression was examined in human skin organ cultures. To delineate the mechanisms underlying the anti-fibrotic effects of CDDO, explanted skin fibroblasts cultured in 2-dimensional monolayers or in 3-dimensional full-thickness human skin equivelants were studied.
Results
CDDO significantly ameliorated dermal fibrosis in two complementary mouse models of scleroderma, as well as in human skin organ cultures and in 3-dimensional human skin equivalents. In 2-dimensional monolayer cultures, CDDO abrogated fibrogenic responses in explanted normal human skin fibroblasts. These CDDO effects occurred via disruption of Smad-dependent transcription and were associated with inhibition of Akt activation. In scleroderma fibroblasts, CDDO attenuated collagen synthesis. Remarkably, the anti-fibrotic effects of CDDO were independent of PPAR-γ.
Conclusion
The PPAR-γ agonist triterpenoid CDDO attenuates fibrogenesis by antagonistically targeting canonical TGF-β/Smad and Akt signaling in a PPAR-γ-independent manner. These findings identify this synthetic triterpenoid as a potential new therapy for the control of fibrosis.
Keywords: CDDO, triterpenoid, fibrosis, PPAR-γ, TGF-β, fibroblast, murine scleroderma
Introduction
Systemic sclerosis (SSc) or scleroderma is characterized by the characteristic triad infalmmation, obliterative microvasculopathy and fibrosis1. Fibrosis, the distinguishing pathological hallmark of late-stage SSc, is caused by overproduction of collagen and other extracellular matrix (ECM) components by activated fibroblasts and α-smooth muscle actin (α-SMA)-positive myofibroblasts2. Fibroblast activation and myofibroblast transformation result from a complex series of events initiated and orchestrated by the multifunctional cytokine transforming growth factor-β (TGF-β)3. Microarray-based expression profiling of SSc skin biopsies has identified a distinct subset with a “TGF-β-activated gene signature” that is characterized by extensive skin involvement4, 5. However, the clinical benefits of targeting TGF-β for SSc therapy remain to be demonstrated
Multiple physiologic mechanisms exist to regulate fibroblast activation in order to prevent excessive tissue remodeling and fibrosis. Recent studies have identified the nuclear receptor peroxisome proliferator-activated receptor-γ (PPAR-γ) as an important endogenous anti-fibrotic defense mechanism6. Widely expressed in mammalian tissues, PPAR-γ regulates the expression of genes involved in lipid uptake and synthesis, lipolysis, glucose metabolism, cell differentiation, survival and proliferation, as well as immune and inflammatory responses7, 8. Recent evidences suggest an important novel role for PPAR-γ in the negative regulation of connective tissue biosynthesis during both physiologic and pathological matrix remodeling9. We have shown that natural and synthetic PPAR-γ ligands effectively blocked fibrotic responses elicited by TGF-β in explanted fibroblasts, and attenuated bleomycin-induced skin fibrosis in vivo10, 11. Subsequent studies in a variety of cell types and model systems have confirmed and expanded upon these findings12–14. Moreover, treatment of mice with synthetic PPAR-γ ligands was shown to attenuate experimentally-induced hepatic15, cardiac16 and kidney17 fibrosis. At the same time, genetic deletion of PPAR-γ in fibroblasts was associated with exaggerated fibrosis in mice injected with bleomycin18. Importantly, lesional tissue expression of PPAR-γ is markedly diminished in various rodent models of fibrosis and scleroderma11, 19, 20, as well as in fibrotic skin from patients with cicatrical (scarring) alopecia21 and SSc22. These observations suggest that restoring normal PPAR-γ activity or function in these fibrotic conditions might represent a viable approach to preventing or attenuating persistent fibroblast activation.
Traditional PPAR-γ agonists such as thiazolidinediones have been widely used in type 2 diabetes because of their potent glucose-lowering activity. Recent concerns regarding the safety of this class of drugs have prompted interest in the development of selective PPAR-γ modulators23, 24. The synthetic pentacycline oleanane triterpenoid 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid (CDDO) was originally developed to inhibit nitric oxide synthase25. Subsequent studies showed that CDDO binds to and stimulates the activity of PPAR-γ26. Further investigation of CDDO has uncovered a broad range of biological activities, including anti-angiogenic, anti-oxidant, anti-inflammatory and anti-proliferative effects that make this compound highly attractive as a potential therapeutic agent for a multiplicity of chronic diseases27–29. Moreover, recent studies have shown that CDDO inhibited the activation and differentiation of lung and corneal fibroblasts, although the underlying mechanisms remain unknown30, 31. In light of the myriad salutary biological properties associated with CDDO, the present studies sought to explore its effects in experimental models of scleroderma in vivo and in fibroblasts in vitro.
Materials and Methods
Cell cultures
Primary cultures of human fibroblasts were established by explantation from neonatal foreskin or from skin biopsies from the dorsal forearm of five healthy adults and five patients with diffuse cutaneous SSc32. Skin biopsies were performed upon informed consent and in compliance with Northwestern University Institutional Review Board for Human Studies. The clinical characteristics of the patients are available upon request. Cells were maintained Dulbecco’s Modified Eagle’s Medium (DMEM) (BioWhittaker, Walkersville, MD), and studied between passages 4–833. Mouse 3T3-L1 preadipocytes (ATCC) were maintained in maintenance medium (PM-1, Zen-Bio, Research Triangle Park, NC). Adipogenic differentiation of these cells was evaluated by perilipin-1 staining (see Supplementary text). When cells reached early confluence, fresh media containing indicated concentrations of CDDO (RAID, NCI, Rockville, MD) or human recombinant TGF-β2 (Peprotech, Rocky Hill, NJ) were added to the cultures and incubation continued for up 48 h. In selected experiments, cultures were pretreated with rosiglitazone or GW9662 (both from Cayman Chemical, Ann Arbor, MI). At the end of the incubation periods, cultures were harvested and modulation of fibrotic responses was examined by real-time qPCR, Western analysis and luciferase assays. Cytotoxicity was evaluated using LDH cytotoxicity assay kits (Biovision, Mountain View, CA) and by Trypan blue dye exclusion.
Human skin organ cultures and fibroblasts in 3-dimensional organotypic raft skin cultures
Foreskin biopsies were cut into 0.5 cm × 0.5 cm sections and placed in six-well plates. Explants were maintained in an air-medium interface with the epidermal layer exposed to air, and culture media were replaced every other day. The cultures were incubated in media with 10 ng/ml TGF-β2 for six days, and CDDO (5 μM) was added 15 min before or 48 h following the addition of TGF-β. In organotypic raft skin cultures, normal human dermal fibroblasts (3×105 cells) were resuspended in 1.5 ml reconstitution buffer with rat tail Type I collagen (4 mg/ml, BD Biosciences, San Jose, CA), seeded in a 12-well plates and incubated at 37°C for 48 h to solidify the collagen plug34. Keratinocytes isolated from foreskin (6×105 cells) were suspended in E medium supplemented with 5 ng/ml epidermal growth factor and seeded on the collagen plug35. Following 48 h incubation, the 3-dimensional organotypic cultures were placed on a metal grid (McMaster-Carr, Atlanta, GA) and maintained at an air-medium interface with fresh E medium every other day for 5 days. Organotypic cultures were then incubated with CDDO (5 μM) for 24 h, followed by TGF-β (5 ng/ml) for a further six days. Cultures were then harvested for RNA analysis, or fixed in formalin and processed for histology. Paraffin-embedded sections (4 μm) were examined by Sirius Red staining or Masson’s trichrome staining.
Mouse models of scleroderma
Animal protocols were institutionally approved by the Animal Care and Use Committee of Northwestern University or the University of Erlangen-Nuremberg. Skin fibrosis was induced by two distinct approaches: daily subcutaneous injections of bleomycin, or intracutaneous injections of an adenovirus expressing a constitutively-active mutant of the Type I TGF-β receptor (TβRIca).
In the first protocol, six- to eight-week-old female C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) were given daily subcutaneous injections of bleomycin (10 mg/kg) for 14 consecutive days and sacrificed on day 28. In the second protocol, replication-deficient type 5 adenovirus encoding TβRIca or LacZ (6.7 × 107 pfu/mouse) was injected intracutaneously on two occasions separated by four weeks, and mice were sacrificed four weeks following the second injection36. Lesional skin was harvested and analyzed as previously described 11(see Supplementary text). In both protocols, CDDO (5 mg/kg, dissolved in 80% PBS, 10% DMSO, and 10% Cremophor-EL, Sigma, St. Louis, MO) was injected intraperitoneally (i.p.) daily. In other experiments, the effects of CDDO on modulating established fibrosis were investigated. For this purpose, scleroderma was induced by bleomycin injections as above, and CDDO (5 mg/kg/day) was injected i.p. daily starting at day 15.
Statistical analysis
Data are presented as means ± SD. The significance of differences between experimental and control groups was determined by Student’s t-test. In the animal studies, differences between the groups were evaluated using non-parametric Mann–Whitney U test. p<0.05 was considered statistically significant.
Results
CDDO induces PPAR-γ activity and stimulates adipogenesis
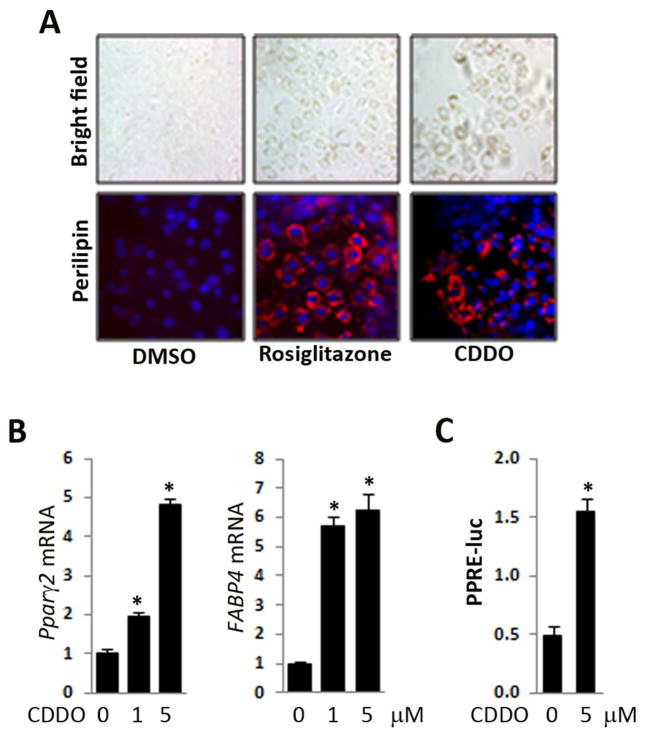
In initial studies, we sought to examine the effect of CDDO on PPAR-γ signaling in mesenchymal cells. For this purpose, multipotent mouse 3T3-L1 preadipocytes were incubated with CDDO, or the synthetic PPAR-γ ligand rosiglitazone in parallel, for up to seven days. Phase contrast microscopy showed a substantial time-dependent accumulation of cytosolic oil droplets induced by both rosiglitazone and CDDO in these cells (Fig. 1A). Both ligands enhanced the expression of perilipin, a lipid droplet-associated adipocyte marker that is a target of PPAR-γ37. Moreover, CDDO stimulated the expression of adipogenic markers PPAR-γ2 and FABP4 mRNA in a dose-dependent manner, with a maximal 5–6 fold increase at day 7 (Fig. 1B). To directly evaluate the effect of CDDO on PPAR-γ activity, confluent human skin fibroblasts transiently transfected with PPRE-luc were incubated with CDDO. Following a 24 h incubation, whole cell lysates were assayed for their luciferase activities. The results showed that CDDO caused a >3-fold increase in PPRE promoter activity in transfected fibroblasts (Fig. 1C). The concentration of CDDO used in these experiments was not toxic to 3T3 L1 cells or fibroblasts (data not shown). Taken together, these results demonstrate the CDDO can function as a potent PPAR-γ agonist that induces PPAR-γ-dependent adipogenic responses in both preadipocytes and skin fibroblasts.
Figure 1. CDDO induces PPAR-γ activation and promotes adipogenesis.
A, B. Confluent 3T3L1 preadipocytes were incubated with CDDO (5 μM or indicated concentration) or rosiglitazone (10 μM) for 7 days before harvesting. A. Cultures were immunostained with antibody to perilipin-1. Upper panels, phase contrast microscopy; lower panels, immunofluorescence. Representative images. Original magnification x 200. B. RNA was examined by real-time qPCR. The results, normalized with 36B4, represent the means ±SD of triplicate determinations from a representative experiment. C. Human skin fibroblasts transiently transfected with PPRE-luc were incubated in media with or without CDDO for 24 h. Cell lysates were assayed for their luciferase activities. The results, normalized with renilla luciferase, represent the means ± SD of three transfection assays in triplicate. * p<0.05.
CDDO ameliorates dermal fibrosis in the mouse
In light of the anti-fibrotic activity ascribed to conventional PPAR-γ ligands10, 11, we evaluated the effects of CDDO on fibrosis using two complementary mouse models of scleroderma. In the first approach, skin fibrosis was induced in C57BL/6J mice by daily s.c. injections with bleomycin for 14 consecutive days. Mice were given concurrent CDDO (5 mg/kg) or vehicle by i.p. injections from day 1 through day 27. Mice were sacrificed after 28 days, and lesional skin was harvested and analyzed. Injection of CDDO was well tolerated, and no significant weight loss or other signs of toxicity were noted. After 28 days, the thickness of the dermis was markedly increased in mice injected with bleomycin compared to PBS (322±72 μm vs 136±34 μm, 5 mice/group) (Fig. 2A). Both excessive collagen deposition and dermal thickness were markedly ameliorated (by 53%, p=0.01) when CDDO was administered concomitantly with bleomycin. Moreover, the dramatic loss of the subcutaneous adipose layer accompanying dermal fibrosis in mice injected with bleomycin alone was substantially attenuated in mice treated with CDDO. Furthermore, up-regulated expression of multiple fibrotic marker genes in lesional skin was attenuated in CDDO-treated mice (Fig. 2B).
Figure 2. CDDO ameliorates dermal fibrosis in mouse models of scleroderma.
Mice were given s.c. injections of bleomycin daily for 14 days (A,B, F,G), or Ad-TβRIca or control adenovirus twice separated by two weeks (C–E) along with daily i.p. injections of CDDO from days 1–27 (A–E) or from days 15–27 (F,G). At the end of the experiments, lesional skin was harvested for analysis. A, F. Left panels, Trichrome (original magnification 200 x). Arrows delineate the dermis. Right panel, quantitation of dermal thickness. The results, representing the means ± SD of 5 determinations/hpf from 5 mice/group, are shown as -fold change compared to control (PBS-treated) mice. B, G. RNA was isolated and examined by real-time qPCR. The results, normalized with 36b4 or β-actin, represent the means ± SD of triplicate determinations from 3–5 mice/group. * p<0.05. C. Left panels, hematoxylin and eosin (original magnification 200 x). Right panel, quantitation of dermal thickness. The results, representing the means ± SEM of 6–8 mice/group, are shown as fold change compared to control (Ad-lacZ). D. Hydroxyproline assays. The results, representing the means ± SEM of triplicate determinations from 6–8 mice/group, are shown as -fold change compared to control (Ad-lacZ). E. Immunohistochemistry. α-SMA positive cells were quantified as described under Materials and Methods. Results are the means ± SEM. * p<0.05.
In a complementary experimental approach, C57BL/6J mice were given two intradermal injections of Ad-TβRIca or Ad-LacZ separated by four weeks, along with daily i.p. injections of CDDO or vehicle as above. Mice were sacrificed four weeks following the second adenovirus injection, and lesional skin was harvested for analysis. Ectopic expression of TβRIca induced local TGF-β signaling that was associated with significant dermal thickening, increased collagen deposition, and myofibroblast accumulation (Figs. 2C–E, 6–8 mice/group). Concomitant treatment of the mice with CDDO almost completely abrogated these fibrotic responses, and attenuated the induction of TGF-β target genes (data not shown).
To evaluate the effects of CDDO on established fibrosis, treatment was started on day 15 of daily bleomycin injections. In this model of fibrosis regression, CDDO resulted in significant reduction of dermal thickness in lesional skin compared to mice injected with bleomycin plus vehicle (Fig. 2F, n=5). Moreover, CDDO markedly attenuated the increased levels of α-SMA expression (Fig. 2G). The results from these complementary mouse models of scleroderma indicate that CDDO antagonized fibrotic responses triggered by bleomycin, or by activation of TGF-β signaling in the skin.
CDDO inhibits fibrotic responses induced by TGF-β
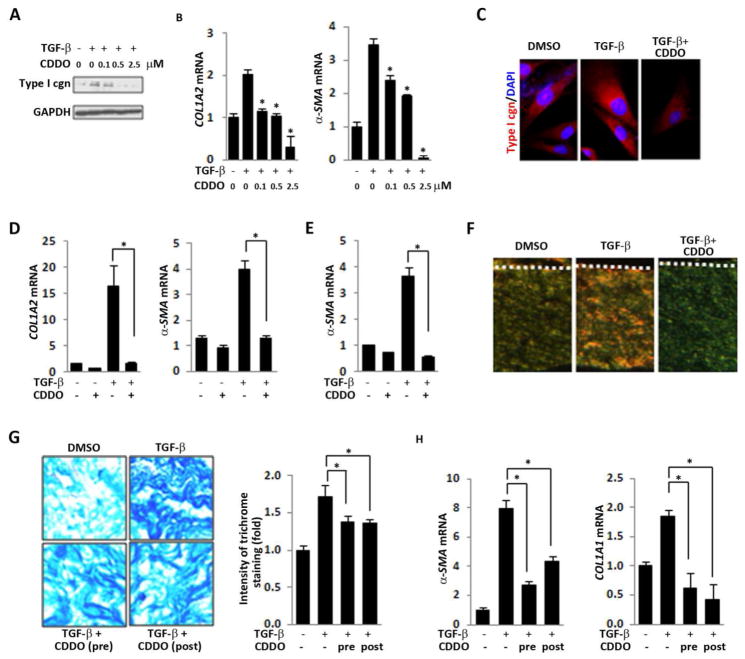
To explore the mechanism underlying the anti-fibrotic and anti-TGF-β effects of CDDO noted in two complementary mouse models of scleroderma, in vitro studies using 2-dimensional cultures of explanted normal human skin fibroblasts were undertaken. While TGF-β markedly enhanced Type I collagen and α-SMA accumulation and mRNA expression in these cells, pre-incubation with CDDO abrogated each of the TGF-β-induced fibrotic responses in a dose-dependent manner (Figs. 3A–C). Importantly, CDDO was capable of reversing the stimulation of Type I collagen and α-SMA even when it was added to the cultures up to 24 h following TGF-β (Fig. 3D). To further investigate the effects of CDDO on collagen gene regulation, fibroblasts transiently transfected with Col1A2-luc were pre-incubated with CDDO, followed by TGF-β for 24 h. Stimulation of Col1A2 promoter activity in TGF-β-stimulated fibroblasts was significantly attenuated by CDDO (data not shown).
Figure 3. CDDO abrogates stimulation of collagen synthesis and myofibroblast differentiation.
A–D. Confluent skin fibroblasts were incubated with CDDO (2.5 μM unless otherwise indicated) and TGF-β (10 ng/ml) following (A–C) or preceding (D) CDDO. A. Whole cell lysates were examined by Western analysis. Type I cgn, Type I collagen. Representative images. B, D. RNA was examined by real-time qPCR. The results, normalized with GAPDH, are the means ± SD of triplicate determinations from a representative experiment. * p<0.05. C. Immunofluorescence. Red, Type I collagen. Blue, DAPI. Representative images. Original magnification, 400 X. E, F. 3-dimensional human skin equivalents populated with skin fibroblasts were incubated with CDDO (5 μM) for 24 h, followed by TGF-β (5 ng/ml) for 5 days, and rafts were harvested. E. Total RNA was isolated and examined by real-time qPCR. The results, normalized with GAPDH, are the means ± SD of triplicate determinations from a representative experiment. * p<0.05. F. 4-μM thick paraffin-embedded sections were stained with Picrosirius Red. Mature collagen fibers in the dermal compartment appear red when visualized under polarized light, whereas less mature collagen fibers containing fewer cross-links appear yellow/green. Representative images (original magnification, x 40). Dashed lines, dermal-epidermal junction. G, H. Human skin organ cultures were incubated in media with TGF-β (10 ng/ml) for six days. CDDO (5 μM) was added to the cultures 15 min before or 48 h following addition of TGF-β. G. Four-μM thick paraffin-embedded sections were stained with Masson’s trichrome. Left panels, representative images (original magnification, x 400); right panel, relative -fold change in staining intensity. H. Skin tissues were harvested, and total RNA was isolated and examined by real-time qPCR. The results, normalized with GAPDH, are the means ± SD of triplicate determinations from a representative experiment. * p<0.05.
To evaluate the effects of CDDO on fibroblast activity and function in a context morphologically relevant for the skin, we utilized an organotypic human skin raft model comprising epidermal keratinocytes and dermal fibroblasts. In these skin rafts, fibroblasts embedded in a collagen-rich 3-dimensional matrix are, in comparison to 2-dimensional monolayer cultures grown on plastic dishes, subjected to substantially lower levels of mechanical stress approximately those of normal dermis (unpublished results). As the topography and biomechanical properties of the organotypic skin raft mimic those of human skin, the 3-dimensional skin equivalent serves as an attractive tractable model system to study tissue fibrosis38, 39. Incubation of the skin rafts in media with TGF-β for 5 days resulted in a marked increase in the expression of COL1A1, COL1A2 and α-SMA in fibroblasts within the dermal compartment (Fig. 3E and data not shown). Treatment of the rafts with CDDO significantly attenuated the upregulation of each of these genes. Picrosirius Red staining of four μm thick sections showed that TGF-β induced a notable increase red birefringence, indicating the accumulation of highly cross-linked collagen, in the dermal compartment (Fig. 3F). Pretreatment of the rafts with CDDO prevented collagen fiber maturation, with a predominance of green color collagen fibers representing attenuated cross-linking (Fig. 3F)40.
To further characterize the modulation of cutaneous fibrotic responses by CDDO, experiments using human skin organ cultures were performed. Incubation of the organ cultures with TGF-β resulted in increased collagen accumulation, and pre-incubation with CDDO markedly attenuated this response (Fig. 3G). Similar results were seen even when CDDO was added to the cultures 48 h following TGF-β. The stimulation of COL1A1 and α-SMA mRNA expression by TGF-β was also significantly suppressed by CDDO (Fig. 3H).
Epithelial-mesenchymal transition (EMT) has been considered to play an important role in fibrosis1. CDDO markedly attenuated TGF-β-induced EMT in human A540 epithelial cells (Fig. S1).
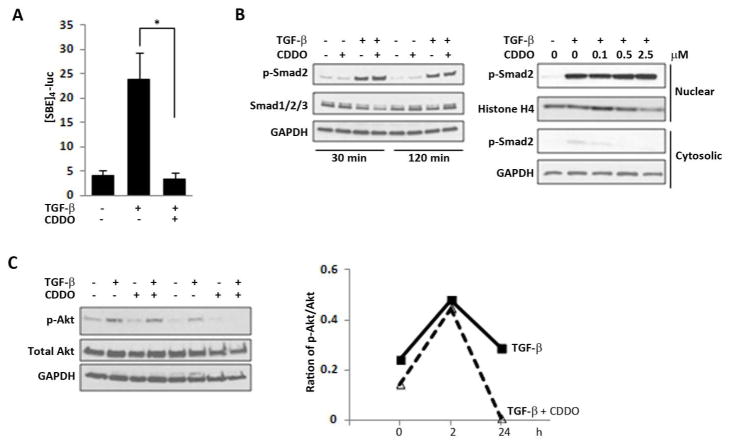
CDDO abrogates TGF-β/Smad and Akt signaling
To delineate the TGF-β signaling pathways that are targeted by CDDO, fibroblasts in 2-dimensional monolayer cultures were transiently transfected with the Smad-responsive [SBE]4-luc followed by TGF-β in the presence or absence of CDDO. The results of transient transfection assays showed that stimulation of [SBE]4-luc activity by TGF-β was completely abrogated in the presence of CDDO (Fig. 4A). Surprisingly however, there was no change in TGF-β-induced Smad2 phosphorylation or nuclear translocation in CDDO-treated fibroblasts (Fig. 4 B). These results indicate that CDDO blocked TGF-β signaling by disrupting Smad-dependent transcription but without preventing Smad2/3 activation.
Figure 4. CDDO blocks Smad-dependent transcription and Akt activation.
Confluent skin fibroblasts were transiently transfected with [SBE]4-luc (A), or left untransfected (B, C). Cultures were preincubated with CDDO (2.5 μM or indicated concentrations), followed by further incubation with TGF-β (10 ng/ml) for up to 24 h (A) or indicated time periods (B). A. Whole cell lysates were assayed for their luciferase activities. The results, normalized with renilla luciferase, represent the means ± SD of triplicate experiments. * p<0.05. B. Whole cell lysates (left panel) or cytosolic and nuclear fractions (right panel) were examined by Western analysis. Representative images. C. Whole cell lysates were examined by Western analysis. Bands were quantitated by densitometry. Relative phospho-Akt levels normalized with total Akt are shown below the images. Representative images.
In addition to canonical Smad signaling, TGF-β also induces Smad-independent cellular pathways that are implicated in fibrotic responses. To investigate the modulation of non-canonical TGF-β signaling by CDDO, we focused on the Akt pathway previously shown to be regulated by CDDO in lung fibroblasts41. Confluent dermal fibroblasts were incubated with TGF-β for up to 24 h in the presence or absence of CDDO, and whole cell lysates were examined. The results of Western analysis showed that while TGF-β induced a ~2-fold increase in phospho-Akt, perincubation of the cultures with CDDO had little effects on Akt activation at 120 min, but completely abrogated the response at 24 h (Fig. 4C). Together, these results indicated that CDDO was able to disrupt both canonical and non-canonical pathways mediating profibrotic TGF-β responses.
CDDO ameliorates collagen overproduction in explanted scleroderma fibroblasts
We previously demonstrated that explanted scleroderma fibroblasts maintained their activated phenotype ex vivo with constitutive TGF-β signaling even in the absence of exogenous ligand42. Evaluation of the anti-fibrotic effects of CDDO in fibroblasts explanted from five patients with diffuse scleroderma showed 50–60% reduction in levels of cellular and secreted Type I collagen (Fig. 5A), and >50% reduction COL1A1 and COL1A2 mRNA, in each SSc cell line (Fig. 5B).
Figure 5. CDDO normalizes collagen overproduction in scleroderma fibroblasts.
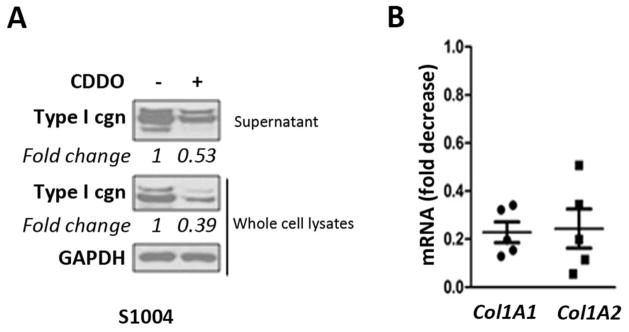
Skin fibroblasts explanted from SSc patients (n=5) were incubated with CDDO (5 μM) for 24 h. A. Whole cell lysates and culture supernatants were analyzed by Western analysis. Bands were quantitated by densitometry. Relative levels normalized with GAPDH are shown below the images. Representative images. B. RNA was examined by real-time qPCR. Results are expressed as dot plots of mRNA levels relative to untreated cultures.
Inhibition of profibrotic responses by CDDO is PPAR-γ-independent
Since CDDO has agonist activity on PPAR-g signaling, we sought to explore the potential role of PPAR-γ in mediating the inhibitory effects of CDDO on TGF-β-dependent fibrotic responses. To this end, GW9662, a PPAR-γ ligand that irreversibly blocks PPAR-γ signaling was used43. Pre-incubation of confluent normal fibroblasts with GW9662 failed to rescue TGF-β-induced stimulation of collagen synthesis or [SBE]4-luc activity in the presence of inhibitory CDDO, while effectively blocking the inhibitory effects of rosiglitazone (Fig. 6 and data not shown). We conclude on the basis of these experiments that in contrast to the traditional PPAR-γ ligand rosiglitazone, CDDO inhibition of TGF-β-induced fibrotic responses was largely PPAR-γ independent.
Figure 6. Inhibition of profibrotic responses is PPAR-γ independent.
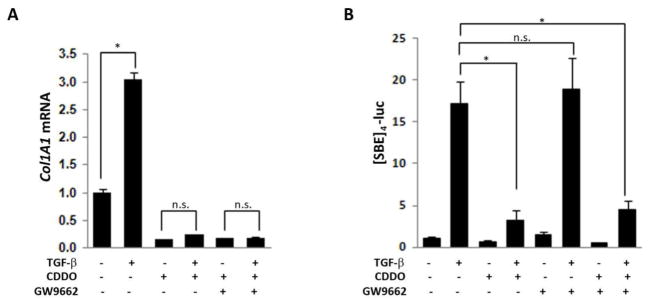
Confluent skin fibroblasts untransfected (A), or transiently transfected with [SBE]4-luc (B), were preincubated GW9662, followed by CDDO (2.5 μM) and TGF-β (10 ng/ml) for 24 h. A. RNA was examined by real-time qPCR. The results, normalized with GAPDH, represent the means ± SD of triplicate determinations from a representative experiment. * p<0.05. B. Luciferase activities, normalized with renilla luciferase in each sample, are means ± SD of triplicate experiments. * p<0.05.
Discussion
Since activation of fibroblasts and myofibroblasts by TGF-β underlies the initiation and progression of fibrosis in SSc, disrupting TGF-β activity represents an appealing approach to fibrosis therapy 44. We showed previously that synthetic or natural ligands of PPAR-γ blocked Smad-dependent TGF-β responses in mesenchymal cells10, 11, 45, suggesting that activation of PPAR-γ could represent a pharmacological strategy to modulate fibrosis. Although the thiazolidinedione class of drug are potent PPAR-γ ligands, their clinical use has been associated with diverse side effects, including cardiovascular complications 23, making the discovery of novel PPAR-γ ligands the focus of intensive research.
The synthetic oleanane triterpenoid CDDO was previously shown to enhance PPAR-γ activity in mouse progenitor cells and human lung fibroblasts26, 41. Here, we confirmed the ability of CDDO to enhance PPAR-γ signaling and to promote adipogenesis in mesenchymal cell types. We evaluated the effects of CDDO on fibrotic responses in vivo using two experimental approaches to model fibrosis. These studies showed that CDDO attenuated skin fibrosis in a bleomycin-induced model characterized by inflammatory cells infiltration in the dermis that mimics the inflammatory stage of SSc46. Since CDDO has anti-inflammatory effects, the attenuation of skin fibrosis observed in the present studies might reflect immunosuppression or inhibition of fibrotic TGF-β signaling, or both. Previous studies have shown that CDDO can either synergize with or antagonize TGF-β in a context-dependent manner47, 48. To evaluate the effects of CDDO on TGF-β-induced fibrotic responses directly, we used a complementary model of scleroderma induced by constitutive TGF-b signaling that is independent of inflammation. The results showed that CDDO abrogated dermal thickening and myofibroblast accumulation in this inflammation-independent scleroderma model36. Moreover, in monolayer cultures of explanted skin fibroblasts, CDDO dramatically attenuated the stimulation of fibrotic responses elicited by TGF-β, indicating that CDDO directly antagonizes TGF-β activity in fibroblasts. The anti-fibrotic properties of CDDO were further investigated using a 3-dimensional human skin equivalent culture system, where fibroblasts are embedded in a relatively soft and mechanically unstrained collagen matrix in topographic proximity to a stratified squamous epidermal layer. Incubation of this organotypic skin raft culture with TGF-β results in stimulation of resident fibroblast with activated Smad2/3 signaling, increased secretion of collagen, and transdifferentiation into α-SMA-positive myofibroblasts. Moreover, over the course of a 7-day incubation period, the dermal compartment develops sclerosis and evidence of collagen fiber maturation accompanied by increased stiffness. Thus, these activated human skin equivalents acquire the biochemical, biomechnical and morphologic features of scleroderma. In this ex vivo scleroderma model, CDDO attenuated the up-regulation of fibrotic gene expression and alleviate other hallmark features of cutaneous fibrosis. Moreover, CDDO abrogated fibrotic responses induced ex vivo by TGF-β in human skin organ cultures.
To explore the mechanisms underlying the antagonistic effects of CDDO on TGF-β responses, a series of experiments using explanted dermal fibroblasts were pursued. The results showed that CDDO abrogated Smad-dependent transcriptional activity without preventing Smads phosphorylation and nuclear translocation. In contrast, other studies using lung and corneal fibroblasts showed that CDDO had no significant effects on Smad signaling30. Since dermal fibroblasts explanted from scleroderma patients show enhanced Smad2/3 activation that might account for increased ECM production in vitro, it was not surprising that CDDO reduced collagen gene expression in scleroderma fibroblasts. In agreement with other studies41, we found that CDDO attenuated activation of Akt induced by TGF-β. Although CDDO served as a potent stimulus for PPAR-γ activation in fibroblasts, we showed using the irreversible PPAR-γ antagonist GW9662 that the antifibrotic effects of CDDO were PPAR-γ-independent. Synthetic olenane triterpenoids are known to be the most potent inducers of the transcription factor nuclear factor (erythroid-derived 2)-like 2 (Nrf2), which is the master regulator of anti-oxidative and cytoprotective responses in variety cell types27. Expression of Nrf2 is reduced in lung fibroblasts from patients with idiopathic pulmonary fibrosis49. Intriguingly, we found that Nrf2 was stabilized by CDDO treatment in dermal fibroblasts (data not shown). The involvement of the Nrf2 pathway in mediating the antifibrotic effects of CDDO is currently under investigation.
Triterpenoids are derived from isopentenyl pyrophosphate oligomers that comprise the largest group of natural plant products28. Synthetic oleanane triterpenoids such as CDDO have both cytoprotective and anti-cancer activities and have been shown to inhibit liver, lung and prostate cancer in preclinical models50–53. By targeting multiple signaling pathways in addition to Nrf2, PPAR-γ, and PI3K/Akt, including JAK/STAT, mTOR, and NF-κB, synthetic oleananes regulate cellular stress responses54. Several CDDO derivatives are currently in clinical trials. A methyl ester derivative of CDDO, bardoxolone methyl is currently in Phase I clinical trials for the treatment of leukemia, solid tumors, and other non-neoplastic diseases (www.clinicaltrials.gov). In a recent Phase II randomized, placebo-controlled trial, bardoxolone methyl was shown to increase glomerular filtration rate in diabetic patients with impaired renal function55, 56. Synthetic derivatives of CDDO might have utility in a variety of chronic inflammatory and neurodegenerative conditions as well.
In summary, we show that the synthetic pentacyclic triterpenoid CDDO, a PPAR-γ agonist and potent activator of the Nrf2 pathway, ameliorated experimental skin fibrosis in two complementary mouse models of scleroderma, and inhibited TGF-β-induced profibrotic responses by blocking Smad and Akt signaling in a PPAR-γ independent manner. While the precise mechanism of the CDDO-Smad antagonism remains to be elucidated, CDDO is known to directly interact with a large number of cellular targets, with context-dependent biological outcomes determined by the cell types and their differentiation states, as well as dose54. Ultimately, the clinical efficacy of CDDO may be determined by its ability to simultaneously modulate entire signaling networks rather than a single target. Together, these observations suggest that CDDO and its related olenane derivatives represent novel orally active agents with excellent safety profiles and plausible clinical potential in the therapy of complex chronic diseases such as SSc.
Supplementary Material
Acknowledgments
We thank members of the Scleroderma Research Group for helpful discussions and Spiro Getsios and Paul Hoover (Skin Disease Research Center at Northwestern University) for valuable technical help.
Funding Supported by grants to J.V. from the National Institutes of Health (AR-49025) and M. T. from CMH Research Project (00000023728).
Footnotes
Contributors Design of the study: JW, JHWD, JV. Acquisition of data: JW, HZ, KK, GL, MT, WW, SD, ZT, MH. Interpretation of data: JW, HZ, KK, MT, MH, JHWD, JV. Manuscript preparation: JW, JV. All authors approved the final version.
Competing interests None.
References
- 1.Bhattacharyya S, Wei J, Varga J. Understanding fibrosis in systemic sclerosis: shifting paradigms, emerging opportunities. Nat Rev Rheumatol. 2012;8(1):42–54. doi: 10.1038/nrrheum.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenbloom J, Castro SV, Jimenez SA. Narrative review: fibrotic diseases: cellular and molecular mechanisms and novel therapies. Ann Intern Med. 2010;152(3):159–66. doi: 10.7326/0003-4819-152-3-201002020-00007. [DOI] [PubMed] [Google Scholar]
- 3.Varga J, Pasche B. Transforming growth factor beta as a therapeutic target in systemic sclerosis. Nat Rev Rheumatol. 2009;5(4):200–6. doi: 10.1038/nrrheum.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sargent JL, Milano A, Bhattacharyya S, et al. A TGFbeta-responsive gene signature is associated with a subset of diffuse scleroderma with increased disease severity. J Invest Dermatol. 2010;130(3):694–705. doi: 10.1038/jid.2009.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milano A, Pendergrass SA, Sargent JL, et al. Molecular subsets in the gene expression signatures of scleroderma skin. PLoS One. 2008;3(7):e2696. doi: 10.1371/journal.pone.0002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei J, Bhattacharyya S, Varga J. Peroxisome proliferator-activated receptor gamma: innate protection from excessive fibrogenesis and potential therapeutic target in systemic sclerosis. Curr Opin Rheumatol. 2010;22(6):671–6. doi: 10.1097/BOR.0b013e32833de1a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005;123(6):993–9. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 8.Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 9.Wei J, Bhattacharyya S, Jain M, et al. Regulation of matrix remodeling by peroxisome proliferator-activated receptor-gamma: a novel link between metabolism and fibrogenesis. The Open Rheumatology Journal. 2012 doi: 10.2174/1874312901206010103. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh AK, Bhattacharyya S, Lakos G, et al. Disruption of transforming growth factor beta signaling and profibrotic responses in normal skin fibroblasts by peroxisome proliferator-activated receptor gamma. Arthritis Rheum. 2004;50(4):1305–18. doi: 10.1002/art.20104. [DOI] [PubMed] [Google Scholar]
- 11.Wu M, Melichian DS, Chang E, et al. Rosiglitazone abrogates bleomycin-induced scleroderma and blocks profibrotic responses through peroxisome proliferator-activated receptor-gamma. Am J Pathol. 2009;174(2):519–33. doi: 10.2353/ajpath.2009.080574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng F, Fornoni A, Elliot SJ, et al. Upregulation of type I collagen by TGF-beta in mesangial cells is blocked by PPARgamma activation. Am J Physiol Renal Physiol. 2002;282(4):F639–48. doi: 10.1152/ajprenal.00189.2001. [DOI] [PubMed] [Google Scholar]
- 13.Burgess HA, Daugherty LE, Thatcher TH, et al. PPARgamma agonists inhibit TGF-beta induced pulmonary myofibroblast differentiation and collagen production: implications for therapy of lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2005;288(6):L1146–53. doi: 10.1152/ajplung.00383.2004. [DOI] [PubMed] [Google Scholar]
- 14.Shi-wen X, Eastwood M, Stratton RJ, et al. Rosiglitazone alleviates the persistent fibrotic phenotype of lesional skin scleroderma fibroblasts. Rheumatology (Oxford) 49(2):259–63. doi: 10.1093/rheumatology/kep371. [DOI] [PubMed] [Google Scholar]
- 15.Galli A, Crabb DW, Ceni E, et al. Antidiabetic thiazolidinediones inhibit collagen synthesis and hepatic stellate cell activation in vivo and in vitro. Gastroenterology. 2002;122(7):1924–40. doi: 10.1053/gast.2002.33666. [DOI] [PubMed] [Google Scholar]
- 16.Shiomi T, Tsutsui H, Hayashidani S, et al. Pioglitazone, a peroxisome proliferator-activated receptor-gamma agonist, attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation. 2002;106(24):3126–32. doi: 10.1161/01.cir.0000039346.31538.2c. [DOI] [PubMed] [Google Scholar]
- 17.Kawai T, Masaki T, Doi S, et al. PPAR-gamma agonist attenuates renal interstitial fibrosis and inflammation through reduction of TGF-beta. Lab Invest. 2009;89(1):47–58. doi: 10.1038/labinvest.2008.104. [DOI] [PubMed] [Google Scholar]
- 18.Kapoor M, McCann M, Liu S, et al. Loss of peroxisome proliferator-activated receptor gamma in mouse fibroblasts results in increased susceptibility to bleomycin-induced skin fibrosis. Arthritis Rheum. 2009;60(9):2822–9. doi: 10.1002/art.24761. [DOI] [PubMed] [Google Scholar]
- 19.Miyahara T, Schrum L, Rippe R, et al. Peroxisome proliferator-activated receptors and hepatic stellate cell activation. J Biol Chem. 2000;275(46):35715–22. doi: 10.1074/jbc.M006577200. [DOI] [PubMed] [Google Scholar]
- 20.Yang L, Chan CC, Kwon OS, et al. Regulation of peroxisome proliferator-activated receptor-gamma in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2006;291(5):G902–11. doi: 10.1152/ajpgi.00124.2006. [DOI] [PubMed] [Google Scholar]
- 21.Karnik P, Tekeste Z, McCormick TS, et al. Hair follicle stem cell-specific PPARgamma deletion causes scarring alopecia. J Invest Dermatol. 2009;129(5):1243–57. doi: 10.1038/jid.2008.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei J, Ghosh AK, Sargent JL, et al. PPARgamma downregulation by TGFbeta in fibroblast and impaired expression and function in systemic sclerosis: a novel mechanism for progressive fibrogenesis. PLoS One. 2010;5(11):e13778. doi: 10.1371/journal.pone.0013778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356(24):2457–71. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 24.Higgins LS, Depaoli AM. Selective peroxisome proliferator-activated receptor gamma (PPARgamma) modulation as a strategy for safer therapeutic PPARgamma activation. Am J Clin Nutr. 2010;91(1):267S–72S. doi: 10.3945/ajcn.2009.28449E. [DOI] [PubMed] [Google Scholar]
- 25.Honda T, Rounds BV, Gribble GW, et al. Design and synthesis of 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, a novel and highly active inhibitor of nitric oxide production in mouse macrophages. Bioorg Med Chem Lett. 1998;8(19):2711–4. doi: 10.1016/s0960-894x(98)00479-x. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Porter WW, Suh N, et al. A synthetic triterpenoid, 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid (CDDO), is a ligand for the peroxisome proliferator-activated receptor gamma. Mol Endocrinol. 2000;14(10):1550–6. doi: 10.1210/mend.14.10.0545. [DOI] [PubMed] [Google Scholar]
- 27.Liby K, Hock T, Yore MM, et al. The synthetic triterpenoids, CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and Nrf2/ARE signaling. Cancer Res. 2005;65(11):4789–98. doi: 10.1158/0008-5472.CAN-04-4539. [DOI] [PubMed] [Google Scholar]
- 28.Petronelli A, Pannitteri G, Testa U. Triterpenoids as new promising anticancer drugs. Anticancer Drugs. 2009;20(10):880–92. doi: 10.1097/CAD.0b013e328330fd90. [DOI] [PubMed] [Google Scholar]
- 29.Sogno I, Vannini N, Lorusso G, et al. Anti-angiogenic activity of a novel class of chemopreventive compounds: oleanic acid terpenoids. Recent Results Cancer Res. 2009;181:209–12. doi: 10.1007/978-3-540-69297-3_19. [DOI] [PubMed] [Google Scholar]
- 30.Ferguson HE, Kulkarni A, Lehmann GM, et al. Electrophilic peroxisome proliferator-activated receptor-gamma ligands have potent antifibrotic effects in human lung fibroblasts. Am J Respir Cell Mol Biol. 2009;41(6):722–30. doi: 10.1165/rcmb.2009-0006OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuriyan AE, Lehmann GM, Kulkarni AA, et al. Electrophilic PPARgamma ligands inhibit corneal fibroblast to myofibroblast differentiation in vitro: A potentially novel therapy for corneal scarring. Exp Eye Res. 2012;94(1):136–45. doi: 10.1016/j.exer.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mori Y, Ishida W, Bhattacharyya S, et al. Selective inhibition of activin receptor-like kinase 5 signaling blocks profibrotic transforming growth factor beta responses in skin fibroblasts. Arthritis Rheum. 2004;50(12):4008–21. doi: 10.1002/art.20658. [DOI] [PubMed] [Google Scholar]
- 33.Wei J, Ghosh AK, Sargent JL, et al. PPARgamma downregulation by TGFss in fibroblast and impaired expression and function in systemic sclerosis: a novel mechanism for progressive fibrogenesis. PLoS One. 2010;5(11):e13778. doi: 10.1371/journal.pone.0013778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyers C, Laimins LA. In vitro systems for the study and propagation of human papillomaviruses. Curr Top Microbiol Immunol. 1994;186:199–215. doi: 10.1007/978-3-642-78487-3_11. [DOI] [PubMed] [Google Scholar]
- 35.Getsios S, Simpson CL, Kojima S, et al. Desmoglein 1-dependent suppression of EGFR signaling promotes epidermal differentiation and morphogenesis. J Cell Biol. 2009;185(7):1243–58. doi: 10.1083/jcb.200809044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akhmetshina A, Palumbo K, Dees C, et al. Activation of canonical Wnt signalling is required for TGF-beta-mediated fibrosis. Nat Commun. 2012;3:735. doi: 10.1038/ncomms1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arimura N, Horiba T, Imagawa M, et al. The peroxisome proliferator-activated receptor gamma regulates expression of the perilipin gene in adipocytes. J Biol Chem. 2004;279(11):10070–6. doi: 10.1074/jbc.M308522200. [DOI] [PubMed] [Google Scholar]
- 38.Zahouani H, Pailler-Mattei C, Sohm B, et al. Characterization of the mechanical properties of a dermal equivalent compared with human skin in vivo by indentation and static friction tests. Skin Res Technol. 2009;15(1):68–76. doi: 10.1111/j.1600-0846.2008.00329.x. [DOI] [PubMed] [Google Scholar]
- 39.Auxenfans C, Fradette J, Lequeux C, et al. Evolution of three dimensional skin equivalent models reconstructed in vitro by tissue engineering. Eur J Dermatol. 2009;19(2):107–13. doi: 10.1684/ejd.2008.0573. [DOI] [PubMed] [Google Scholar]
- 40.Bradshaw AD, Puolakkainen P, Dasgupta J, et al. SPARC-null mice display abnormalities in the dermis characterized by decreased collagen fibril diameter and reduced tensile strength. J Invest Dermatol. 2003;120(6):949–55. doi: 10.1046/j.1523-1747.2003.12241.x. [DOI] [PubMed] [Google Scholar]
- 41.Kulkarni AA, Thatcher TH, Olsen KC, et al. PPAR-gamma ligands repress TGFbeta-induced myofibroblast differentiation by targeting the PI3K/Akt pathway: implications for therapy of fibrosis. PLoS One. 2011;6(1):e15909. doi: 10.1371/journal.pone.0015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mori Y, Chen SJ, Varga J. Expression and regulation of intracellular SMAD signaling in scleroderma skin fibroblasts. Arthritis Rheum. 2003;48(7):1964–78. doi: 10.1002/art.11157. [DOI] [PubMed] [Google Scholar]
- 43.Huang JT, Welch JS, Ricote M, et al. Interleukin-4-dependent production of PPAR-gamma ligands in macrophages by 12/15-lipoxygenase. Nature. 1999;400(6742):378–82. doi: 10.1038/22572. [DOI] [PubMed] [Google Scholar]
- 44.Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest. 2007;117(3):557–67. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghosh AK, Bhattacharyya S, Wei J, et al. Peroxisome proliferator-activated receptor-{gamma} abrogates Smad-dependent collagen stimulation by targeting the p300 transcriptional coactivator. Faseb J. 2009 doi: 10.1096/fj.08-128736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Batteux F, Kavian N, Servettaz A. New insights on chemically induced animal models of systemic sclerosis. Curr Opin Rheumatol. 2011;23(6):511–8. doi: 10.1097/BOR.0b013e32834b1606. [DOI] [PubMed] [Google Scholar]
- 47.Suh N, Roberts AB, Birkey Reffey S, et al. Synthetic triterpenoids enhance transforming growth factor beta/Smad signaling. Cancer Res. 2003;63(6):1371–6. [PubMed] [Google Scholar]
- 48.Liao D, Liu Z, Wrasidlo WJ, et al. Targeted therapeutic remodeling of the tumor microenvironment improves an HER-2 DNA vaccine and prevents recurrence in a murine breast cancer model. Cancer Res. 2011;71(17):5688–96. doi: 10.1158/0008-5472.CAN-11-1264. [DOI] [PubMed] [Google Scholar]
- 49.Artaud-Macari E, Goven D, Brayer S, et al. Nrf2 nuclear translocation induces myofibroblastic dedifferentiation in idiopathic pulmonary fibrosis. Antioxid Redox Signal. 2012 doi: 10.1089/ars.2011.4240. [DOI] [PubMed] [Google Scholar]
- 50.Yates MS, Kwak MK, Egner PA, et al. Potent protection against aflatoxin-induced tumorigenesis through induction of Nrf2-regulated pathways by the triterpenoid 1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole. Cancer Res. 2006;66(4):2488–94. doi: 10.1158/0008-5472.CAN-05-3823. [DOI] [PubMed] [Google Scholar]
- 51.Ling X, Konopleva M, Zeng Z, et al. The novel triterpenoid C-28 methyl ester of 2-cyano-3, 12-dioxoolen-1, 9-dien-28-oic acid inhibits metastatic murine breast tumor growth through inactivation of STAT3 signaling. Cancer Res. 2007;67(9):4210–8. doi: 10.1158/0008-5472.CAN-06-3629. [DOI] [PubMed] [Google Scholar]
- 52.Vannini N, Lorusso G, Cammarota R, et al. The synthetic oleanane triterpenoid, CDDO-methyl ester, is a potent antiangiogenic agent. Mol Cancer Ther. 2007;6(12 Pt 1):3139–46. doi: 10.1158/1535-7163.MCT-07-0451. [DOI] [PubMed] [Google Scholar]
- 53.Deeb D, Gao X, Liu Y, et al. Synthetic triterpenoid CDDO prevents the progression and metastasis of prostate cancer in TRAMP mice by inhibiting survival signaling. Carcinogenesis. 2011;32(5):757–64. doi: 10.1093/carcin/bgr030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liby KT, Sporn MB. Synthetic oleanane triterpenoids: multifunctional drugs with a broad range of applications for prevention and treatment of chronic disease. Pharmacol Rev. 2012;64(4):972–1003. doi: 10.1124/pr.111.004846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomas MC, Cooper ME. Diabetes: bardoxolone improves kidney function in type 2 diabetes. Nat Rev Nephrol. 2011;7(10):552–3. doi: 10.1038/nrneph.2011.114. [DOI] [PubMed] [Google Scholar]
- 56.Pergola PE, Raskin P, Toto RD, et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011;365(4):327–36. doi: 10.1056/NEJMoa1105351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.