Abstract
Introduction
Only about one third of patients at high risk for psychosis based on current clinical criteria convert to a psychotic disorder within a 2.5-year follow-up period. Targeting clinical high-risk (CHR) individuals for preventive interventions could expose many to unnecessary treatments, underscoring the need to enhance predictive accuracy with non-clinical measures. Candidate measures include event-related potential (ERP) components with established sensitivity to schizophrenia. Here we examined the mismatch negativity (MMN) component of the ERP elicited automatically by auditory deviance in CHR and early illness schizophrenia (ESZ) patients. We also examined whether MMN predicted subsequent conversion to psychosis in CHR patients.
Method
MMN to auditory deviants (duration, frequency, and duration+frequency “double deviant”) were assessed in 44 healthy controls (HC), 19 ESZ, and 38 CHR patients. Within CHR patients, 15 converters to psychosis were compared to 16 non-converters with at least 12 months of clinical follow-up. Hierarchical Cox regression examined the ability of MMN to predict time to psychosis onset in CHR patients.
Results
Irrespective of deviant type, MMN was significantly reduced in ESZ and CHR patients relative to HC, and in CHR converters relative to non-converters. MMN did not significantly differentiate ESZ and CHR patients. The duration+frequency double deviant MMN, but not the single deviant MMNs, significantly predicted the time to psychosis onset in CHR patients.
Conclusions
Neurophysiological mechanisms underlying automatic processing of auditory deviance, as reflected by the duration+frequency double deviant MMN, are compromised prior to psychosis onset, and can enhance the prediction of psychosis risk among CHR patients.
Keywords: event-related potential, schizophrenia, mismatch negativity, clinical high risk for psychosis, psychosis, auditory cortex, longitudinal
Among individuals at clinical high-risk (CHR) for psychosis, 29–36% will convert to a psychotic disorder within a 2–3 year follow-up period (1, 2). Targeting CHR patients for early intervention based solely on clinical criteria could expose many patients to unnecessary treatments, underscoring the need to enhance predictive accuracy with non-clinical measures. Candidate measures include electroencephalography (EEG)-based event-related potential (ERP) components with established sensitivity to schizophrenia. Among such ERP components, the mismatch negativity (MMN) shows promise as a biomarker of psychosis risk (3–7).
Auditory MMN, a neurophysiological measure of stimulus feature analysis (8), is a negative ERP component elicited automatically by any discriminable deviant sound occurring during a series of repeated “standard” sounds (9). MMN is thought to reflect “echoic” memory because the detection of deviance depends on a short-term representation of the preceding sequence of standard sounds (9, 10). Larger MMN amplitude is associated with greater feature deviance and lower deviance probability (9). Importantly, MMN is pre-attentive; its elicitation does not require attention to the auditory stream or to the deviant stimulus (9, 11, 12). As such, it allows study of auditory pathophysiology in schizophrenia while minimizing cognitive, attentional, and motivational confounds.
MMN amplitude is reduced in schizophrenia (13, 14), including chronic (4, 15–30), recent onset (4, 5, 18, 28, 31), and unmedicated (3, 15, 21, 32, 33, but see 34) patients. Studies of MMN in first episode schizophrenia are mixed, with some finding reduced frequency-deviant (22) or duration-deviant (3, 20, 35, 36) MMN, at least in a subgroup with no college education (18), but others finding normal duration- (26) or frequency-deviant (3, 26, 30, 34, 37) MMN amplitudes that subsequently decline over 2.5 months (34) to 1.5 years (38), in one case in association with left Heschel’s gyrus volume decline (38). Genetic studies are also mixed, with some showing MMN deficits in first-degree relatives of schizophrenia probands (39–41) but others showing no familial effects (26, 42–44) or weak genetic effects based on patient twin data (44, 45). In addition, reduced MMN has recently been observed in CHR patients (3–7), indicating that MMN is compromised prior to psychosis onset. Importantly, two of these CHR studies (3, 6) found greater MMN deficits in CHR patients who subsequently converted to psychosis.
Although MMN deficits in schizophrenia are not always observed (13), study results may depend on the type of MMN elicited. Indeed, MMN is not a unitary ERP component; rather, distinct neural generators contribute to MMN depending on which stimulus features are deviant (46–51). There is some evidence that duration-deviant MMN is more sensitive to schizophrenia than frequency-deviant MMN (13, 19, 31). All previous CHR studies (3–7) examined duration-deviant MMN, but only one examined frequency-deviant MMN (3), finding it to be normal in CHR patients. Nonetheless, frequency-deviant MMN deficits have been observed in schizophrenia (13, 18, 38), suggesting that pathophysiological heterogeneity across patient samples may also contribute to inconsistent MMN findings. If some schizophrenia or CHR patients are more deficient in duration-deviant MMN, while others are more deficient in frequency-deviant MMN, single-deviant MMN paradigms may yield weak and inconsistent group effects. Multi-deviant paradigms, in which two or more deviant types are presented along with standards within a single sequence, have been used to overcome this limitation (31, 52). Another approach, adopted in the current study, is to combine deviance features such as duration and frequency within a single stimulus, potentially facilitating detection of MMN deficits regardless of which MMN type is more deficient in a given patient. Prior studies (53–57) have shown that when two features of a stimulus are deviant, the deviant features are processed in parallel, with MMN showing additive (53, 55) or at least enhanced (55–57) amplitude relative to the amplitudes of corresponding single-deviant MMNs.
The current study examined MMN in CHR patients, in early illness schizophrenia patients (ESZ), and in healthy controls. Both duration- and frequency-deviant MMNs were assessed in two single-deviant paradigms. Based on prior studies (3, 13, 18, 21, 30, 38), we hypothesized that duration-deviant MMN would be more deficient than frequency-deviant MMN in both CHR and ESZ patients. In addition, we implemented a third paradigm that combined duration and frequency deviance within a single stimulus. We hypothesized that this “double-deviant” MMN, relative to the single-deviant MMNs, would show enhanced sensitivity to ESZ and CHR patients, and further, would be the best predictor of subsequent psychosis among CHR patients.
Method
Participants
Participants included 19 early illness schizophrenia patients (ESZ; within 5 years of initial hospitalization or initiation of antipsychotic medication) based on the Structured Clinical Interview for DSM-IV (SCID) (58), 38 clinical high risk (CHR) patients meeting the Criteria of Prodromal States (COPS) (59) based on the Structured Interview for Prodromal Syndromes (59, 60), and 44 healthy controls (HC). CHR patients who converted to a psychotic disorder within 24-months of study entry (converters n=15) were compared to CHR non-converters (n=16) who’d been followed clinically for at least 12 months. CHR patients (n=7) who dropped out of the study before the 12-month follow-up were excluded from converter vs. non-converter sub-group comparisons but were included in survival analyses predicting time to psychosis onset. Mean time from ERP assessment to psychosis onset in CHR converters was 10.4 months (sd=9.0). In non-converters, the mean clinical follow-up interval was 28.6 months (sd=8.8). Interviews were conducted by a trained research assistant, psychiatrist, or clinical psychologist. HC with a past or current DSM-IV Axis I disorder (based on a SCID) or a first-degree relative with a psychotic disorder were excluded. Exclusion criteria for all groups included substance dependence or abuse within the past year, a history of significant medical or neurological illness or head injury resulting in loss of consciousness, and abnormal audiometric testing. CHR patients were recruited from the Yale Psychosis Prodrome Research Clinic. ESZ patients were referred by community clinicians. HC were recruited by advertisements and word-of-mouth. The study was approved by the institutional review board of Yale University. Adult participants and parents of minors provided written informed consent, and minors provided written assent.
Symptom Ratings
ESZ symptoms were rated using the Positive and Negative Syndrome Scale (PANSS) (61) within 1 month (mean ± sd = 15.7 ± 10.4 days) of ERP assessment; CHR symptoms were rated using the Scale of Prodromal Symptoms (SOPS) (59, 60) within 2 weeks (mean ± sd = 11.4 ± 3.1 days) of ERP assessment. Ratings were based on consensus of a trained research assistant and a psychiatrist or clinical psychologist.
MMN Paradigm
Auditory stimuli were presented to participants at 78 dB SPL (sound pressure level) via Etymotic ER3-A insert earphones. Each MMN paradigm consisted of two runs, with each run comprising a fixed pseudorandom sequence of 875 tones, of which 90% were standards (50ms, 633 Hz) and 10% were deviants: For duration (DUR) MMN, deviants were 100ms, 633 Hz; for frequency (FREQ) MMN, deviants were 50ms, 1000 Hz; for double-deviant (DBL) MMN, deviants were 100ms, 1000 Hz. All tones had 5ms rise/fall times and were presented with a 510 ms stimulus onset asynchrony. The order of MMN paradigms (DUR, FREQ, DBL) was fixed. Participants were instructed to ignore auditory stimuli while they silently read a book.
Data Acquisition and Pre-processing
EEG was recorded from a 20-channel (standard 10–20 scalp locations) electrode cap (Physiometrix, Inc.) and additional mastoid and nose electrodes, using a linked-ears reference and an FPz ground. EEG was digitized at 1000 Hz and bandpass filtered between 0.01 and 100 Hz during acquisition using a Neuroscan Synamps amplifier (Neuroscan, Herndon, VA). Subsequently, continuous EEG data were high-pass filtered at 1 Hz and parsed into 600 ms epochs (−100 to 500 ms) for each stimulus type. Vertical and horizontal electro-oculograms, recorded from electrodes above and below the left eye and at the outer canthi of both eyes, respectively, were used to correct EEG for eye movement and blink artifacts (62). After baseline-correction (−50 to 0 ms pre-stimulus baseline), EEG epochs containing amplitudes exceeding +/−100 µV in any fronto-central electrode (F3, Fz, F4, C3, Cz, C4) were rejected. Next, ERP averages for standards and deviants were generated using a sorted averaging technique previously shown to reduce noise in the MMN waveform by averaging over the subset of trials that optimizes the estimated signal to noise ratio (eSNR) (63). Briefly, single-epoch root mean squared (RMS) amplitude values for each trial are calculated and sorted in ascending order for each stimulus type. The subset of sorted trials selected for ERP averaging are associated with the largest eSNR, which is the ratio of the number of trials to the variance of the amplitude values across trials. Details of this method are presented in Supplemental Materials. The number of trials contributing to ERP averages did not differ between groups (all ps >.59). ERPs for standards and deviants were then low-pass filtered at 30 Hz and subtracted to derive a deviant-standard difference wave. MMN amplitude was defined as the most negative peak between 90 and 290 ms in each participant’s difference wave.
Statistical Correction for Normal Aging Effects
In order to compare ESZ patients with the younger CHR patients on MMN, we needed to control for normal brain development and aging effects (27). Accordingly, normal aging effects on MMN were modeled in the HC (age range 12 – 38 years) by regressing MMN amplitudes on age separately for each deviant type and electrode. Resulting regression equations were used to derive predicted “normal” MMN amplitudes for each participant (patients and HC) based on his/her specific age. Differences between observed and age-specific predicted MMN amplitudes were then divided by the standard error of regression (from the HC regression model), yielding age-corrected MMN z-scores for all groups. This method, which has been used in previous brain imaging and ERP patient studies to control for normal aging effects (64–66), is preferable to ANCOVA models because it preserves pathological aging effects (e.g., abnormal brain maturation trajectories) while only removing normal aging effects. The resulting age-corrected MMN z-score expresses, in standard units, the degree to which a participant’s MMN amplitude deviates from the normal value expected for his/her age.
Statistical Analysis
Group differences in MMN z-scores were assessed using a 4-way repeated measures analysis of variance (ANOVA) with Group (ESZ, CHR, HC) or Conversion Group (Converter, Non-Converter) as the between-subjects factor and Deviant Type (DUR, FREQ, DBL), Fronto-Central Lead (Frontal, Central), and Lateral Lead (Left, Midline, Right) as within-subjects factors. Significant effects were parsed using post-hoc Tukey-Kramer tests. Greenhouse-Geisser non-sphericity correction was applied to within-subjects effects with more than two levels. In addition, four Bonferroni-corrected (alpha = .05/4 = .0125) planned contrasts compared converter and non-converter CHR sub-groups to HC and ESZ groups on MMN z-scores averaged across the deviant types and six fronto-central leads.
Hierarchical Cox regression was performed to model the relationship between MMN amplitude and the time to psychosis onset (i.e., “survival time”) among CHR patients. To test whether the DBL MMN has greater predictive power than the DUR and FREQ MMNs, raw MMN amplitudes (in microvolts) averaged over the six fronto-central leads were entered hierarchically in two separate regression models. In one model, DUR and FREQ MMNs were entered as a block in the first step, then DBL MMN was entered second. In the other model, DBL MMN was entered first followed by entry of DUR and FREQ MMN as a block in the second step. All CHR patients were included, with censoring of those who did not convert to psychosis. Alpha was set to p=.05.
To assess correlations with symptom severity, raw MMN amplitudes for each deviant type (average of the six fronto-central leads) were correlated with positive and negative symptom subscales from the PANSS in the ESZ group and from the SOPS in the CHR group.
Because only a minority of CHR patients was taking antipsychotic medication, all analyses were repeated with 27 antipsychotic-free CHR patients.
RESULTS
Demographic Differences Between Groups
Demographic data are shown in Table 1. Gender and handedness (67) did not differ between groups. Age significantly differed between groups. Post-hoc tests showed ESZ to be significantly older than both CHR and HC groups, whereas the CHR vs. HC age difference was not significant. All group comparisons of MMN used age-corrected z-scores. Parental socioeconomic status (PSES) (68) significantly differed between groups, with post-hoc tests showing lower PSES in the two patient groups relative to the HC group. Accordingly, all ANOVAs were repeated using PSES as a covariate. CHR converters did not differ from non-converters in gender, handedness, age, or PSES. For baseline SOPS ratings, converters had marginally more severe positive symptoms and significantly more severe negative symptoms than non-converters.
Table 1.
Group Demographic Data
| Demographic/Clinical Measure | Early
Illness Schizophrenia Patients n = 19 |
Clinical High-Risk Patients n= 38 |
Healthy Controls n = 44 |
Clinical High-Risk Patients | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Converters n= 15 |
Non-Converters n = 16 |
||||||||||||||
| N | % | N | % | N | % | χ2 | p-value |
Post-hoc contrasts |
N | % | N | % | χ2 | p-value | |
| Gender | 2.2 | 0.34 | 0.05 | 0.83 | |||||||||||
| female | 4 | 21.1 | 15 | 39.5 | 17 | 38.6 | 6 | 40.0 | 7 | 43.8 | |||||
| male | 15 | 78.9 | 23 | 60.5 | 27 | 61.4 | 9 | 60.0 | 9 | 56.3 | |||||
| Handednessa | 1.8 | 0.78 | 1.01 | 0.60 | |||||||||||
| right | 16 | 84.2 | 31 | 81.6 | 37 | 84.1 | 13 | 86.7 | 12 | 75.0 | |||||
| left | 1 | 5.3 | 3 | 7.9 | 5 | 11.4 | 1 | 6.7 | 1 | 6.3 | |||||
| ambidextrous | 2 | 10.5 | 4 | 10.5 | 2 | 4.5 | 1 | 6.7 | 3 | 18.8 | |||||
| Diagnostic Subtype | |||||||||||||||
| paranoid | 11 | 57.9 | |||||||||||||
| disorganized | 1 | 5.3 | |||||||||||||
| undifferentiated | 2 | 10.5 | |||||||||||||
| catatonic | 1 | 5.3 | |||||||||||||
| residual | 1 | 5.3 | |||||||||||||
| schizoaffective | 3 | 15.7 | |||||||||||||
| Clinical High-Risk Syndrome (COPS)b | |||||||||||||||
| APS | 38 | 100.0 | 15 | 100.0 | 16 | 100.0 | |||||||||
| BIPS | 1 | 2.6 | 1 | 6.7 | 0 | 0.0 | |||||||||
| GRD | 1 | 2.6 | 1 | 6.7 | 0 | 0.0 | |||||||||
| Antipsychotic type | |||||||||||||||
| atypical alone | 13 | 68.4 | 10 | 26.3 | 5 | 33.3 | 3 | 18.8 | |||||||
| typical alone | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| atypical and typical | 3 | 15.8 | 1 | 2.6 | 0 | 0 | 0 | 0 | |||||||
| none | 2 | 10.5 | 27 | 71.1 | 10 | 66.7 | 13 | 81.3 | |||||||
| unknown | 1 | 5.3 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| M | SD | M | SD | M | SD | F | p-value |
Post-hoc contrasts |
M | SD | M | SD | F | p-value | |
| Age, yearsc | 23.91 | 6.17 | 17.4 | 3.5 | 19.99 | 5.5 | 10.83 | <0.001 | ESZ>HC† ESZ>CHR† CHR=HC | 17.47 | 2.2 | 15.88 | 3.3 | 2.48 | 0.13 |
| Parental Socioeconomic Statusd | 40.44 | 9.06 | 36.6 | 15.22 | 28.02 | 13.17 | 6.96 | 0.002 | HC<ESZ† HC<CHR† CHR=ESZ | 36.70 | 13.4 | 35.34 | 18.0 | 0.06 | 0.82 |
| PANSS Positive Symptom Totale | 18.71 | 5.78 | |||||||||||||
| PANSS Negative Symptom Totale | 17.14 | 6.11 | |||||||||||||
| SOPS Positive Symptom Total | 11.03 | 4.96 | 12.45 | 5.07 | 9.0 | 4.91 | 3.74 | 0.06 | |||||||
| SOPS Negative Symptom Total | 10.74 | 6.35 | 14.40 | 5.05 | 6.69 | 5.71 | 15.77 | <0.001 | |||||||
Note. Values are given as number and percentage of subjects for gender, handedness, diagnostic subtype, prodromal criteria, and antipsychotic type. Group means with the standard deviation for age, parental socioeconomic status, PANSS, and SOPS are reported. Gender and handedness were analyzed with Pearson chi-square tests. Age and parental socioeconomic status were analyzed with one-way ANOVA and Tukey-Kramer post hoc tests.
Abbreviations: HC, healthy controls; PD, prodromal patients; ESZ, early illness schizophrenia patients; PANSS, Positive and Negative Syndrome Scale; SOPS, Scale of Prodromal Symptoms; APS, Attenuated Positive Symptoms; BIPS, Brief Intermittent Psychotic Symptoms; GRD, Genetic Risk and Deterioration.
The Crovitz-Zener (1962) questionnaire was used to measure handedness.
Prodromal criteria APS, BIPS, and GRD are not mutually exclusive.
Age range (years): ESZ = 13.8–37.5 years; CHR = 12.4–26.6 years; HC = 12.4–37.9 years; Converters = 14.8–21.4 years; Non-Converters = 12.4–24.2 years.
The Hollingshead (1975) four-factor index of parental socioeconomic status (SES) is based on a composite of maternal education, paternal education, maternal occupational status, and paternal occupational status. Lower values signify higher socioeconomic status. SES data could not be retrieved from two early illness patients.
PANSS symptom ratings were collected from 14 of the 19 early illness schizophrenia patients.
Significant at p<0.05, two-tailed.
Correlations Among MMN Deviant Types
DUR and FREQ MMN z-scores were moderately correlated in each group (HC r=.39, p<.01; ESZ r=.38,p=.11; CHR r=.41, p=.01). DBL MMN correlated more highly with FREQ MMN (HC r=.75, p<.001; ESZ r=.82, p<.001; CHR r=.79, p<.001) than with DUR MMN (HC r=.41, p<.01; ESZ r=.39, p=.10; CHR r=.51, p=.001), suggesting a greater contribution of frequency deviance than duration deviance to the double-deviant MMN.
Group Differences in MMN
Nose-referenced ERP difference waves showing the expected MMN polarity reversal at mastoid leads are presented in Supplementary Materials. Grand average ERP difference waves, scalp topography maps, and mean amplitude values (raw voltage and z-score) for DUR, FREQ, and DBL MMN are presented in Figure 1. These figures show MMN amplitude across deviant types to be reduced in both ESZ and CHR groups relative to the HC group. In the ANOVA of MMN z-scores, a significant Group effect emerged, with post-hoc tests showing significantly smaller MMN amplitudes in the ESZ and CHR groups compared to the HC group, but no difference between the ESZ and CHR groups (see Table 2). There were also marginally significant Group×Lateral Lead and Group × Fronto-Central Lead × Deviant Type effects. These interactions mainly involved variation in the strength of Group effects and or Deviant Type effects across scalp electrode sites. In no case did they reveal a noteworthy absence of a Group effect or a significant dependence of the Group effect on MMN Deviant type. Accordingly, we do not present a full description of these interaction effects here. Instead, a full parsing of these interaction effects is presented in the Supplemental Materials and in Table 2.
Figure 1. Mismatch Negativity (MMN) for each Group and Deviant Type.
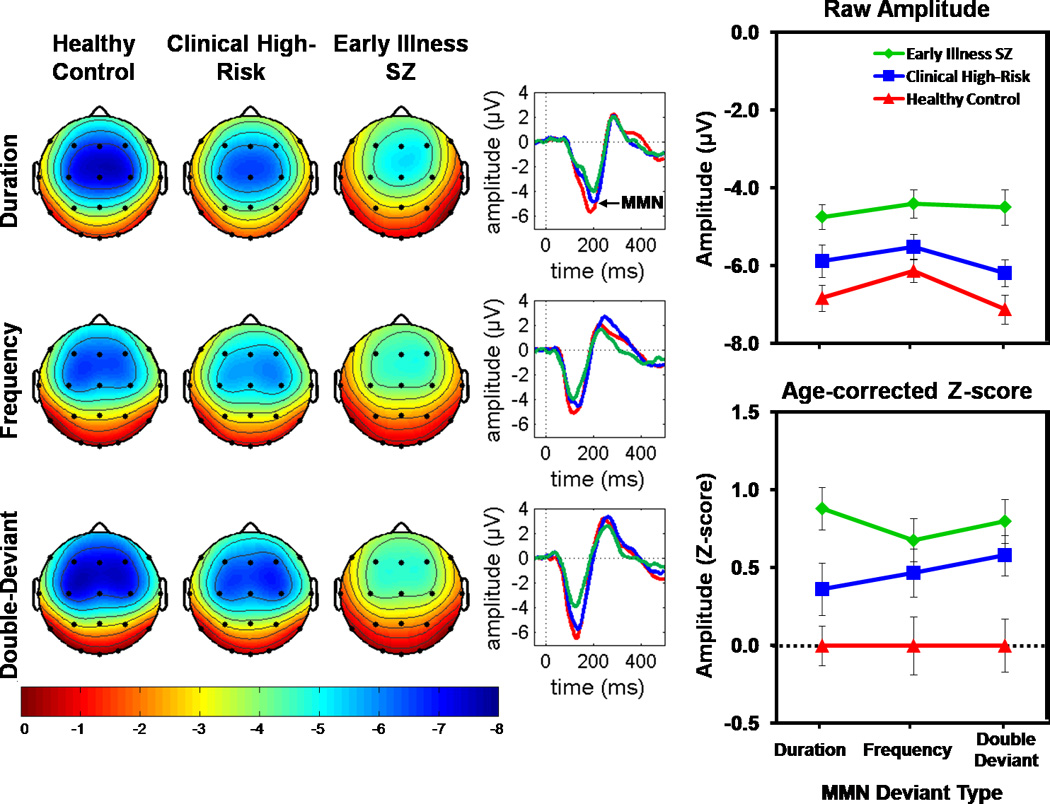
On the left, scalp voltage topography maps of MMN amplitudes are shown for each group and deviant type. MMN topography maps show the group means of MMN amplitudes associated with subject-specific median peak latency across the six fronto-central leads (F3, Fz, F4, C3, Cz, C4). All maps are plotted on the same voltage scale (µV) as indicated in the legend. In the center, ear-referenced ERP difference waveforms averaged across the six fronto-central leads for Duration, Frequency, and Double-Deviant MMN are shown for each group. On the right, line graphs show group means and standard errors for raw MMN amplitude in microvolts (top) and MMN age-corrected z-scores (bottom). Healthy Controls are shown in red, Clinical High Risk patients in blue, and Early Illness Schizophrenia (SZ) patients in green. MMN is reduced in Early Illness SZ and Clinical High Risk patients relative to Healthy Controls across deviant type.
Table 2.
Analyses of Diagnostic Group and Psychosis Conversion Effects on Mismatch Negativity.
| Group Effects (HC, CHR, ESZ)a | Conversion Group Effects (Converter, Non-converter)b |
|||||||
|---|---|---|---|---|---|---|---|---|
| Effect | df | F | p-value | Follow-Up Testsc | df | F | p-value | Follow-Up Testsc |
| Group | 2, 98 | 8.63 | <.001 | CHR>HC*, ESZ>HC***, CHR=ESZ | 1,29 | 4.07 | 0.053 | Converter>Non-converter* |
| Deviant Type (DUR, FREQ, DBL) | 2,196 | 0.37 | 0.639 | 2, 58 | 0.74 | 0.441 | ||
| Fronto-Central Lead (Frontal, Central) | 1,98 | 0.02 | 0.889 | 1,29 | 1.66 | 0.208 | ||
| Lateral Lead (Left, Midline, Right) | 2,196 | 1.61 | 0.205 | 2,58 | 6.45 | 0.009 | Left>Midline*, Midline>Right* | |
| Group x Deviant Type | 4,196 | 0.67 | 0.579 | 2, 58 | 0.57 | 0.512 | ||
| Group x Fronto-Central Lead | 2,98 | 2.38 | 0.098 | 1,29 | 0.10 | 0.756 | ||
| Group x Lateral Lead | 4,196 | 2.52 | 0.053 | 2, 58 | 1.26 | 0.284 | ||
| Lateral Lead effect in HC | 2, 86 | 0.00 | 1.000 | |||||
| Lateral Lead effect in CHR | 2,74 | 7.73 | 0.004 | Left>Midline*, Midline>Right* | ||||
| Lateral Lead effect in ESZ | 2,36 | 0.21 | 0.747 | |||||
| Group effect at Left (F3, C3) leads | 2,98 | 10.23 | <.001 | CHR>HC***, ESZ>HC***, CHR=ESZ | ||||
| Group effect at Midline (Fz, Cz) leads | 2, 98 | 8.25 | <.001 | CHR>HC*, ESZ>HC***, CHR=ESZ | ||||
| Group effect at Right (F4, C4) leads | 2, 98 | 6.62 | 0.002 | CHR=HC, ESZ>HC**, CHR=ESZ | ||||
| Group x Lateral Lead x Deviant Type | 8,392 | 1.15 | 0.330 | 4,116 | 0.52 | 0.681 | ||
| Group x Fronto-Central Lead x Deviant Type | 4,196 | 2.50 | 0.054 | 2,58 | 1.54 | 0.226 | ||
| Group x Fronto-Central Lead for DUR | 1,98 | 1.27 | 0.285 | |||||
| Group x Fronto-Central Lead for DBL | 1,98 | 1.75 | 0.180 | |||||
| Group x Fronto-Central Lead for FREQ | 1,98 | 4.39 | 0.015 | |||||
| Fronto-Central Lead effect in HC | 1,43 | 0.00 | 0.999 | |||||
| Fronto-Central Lead effect in ESZ | 1,18 | 0.24 | 0.627 | |||||
| Fronto-Central Lead effect in CHR | 1,37 | 9.67 | 0.004 | Frontal>Central** | ||||
| Group effect at Frontal Leads | 2,98 | 5.59 | 0.005 | CHR>HC*, ESZ>HC*, CHR=ESZ | ||||
| Group effect at Central Leads | 2,98 | 3.64 | 0.030 | CHR=HC, ESZ>HC*, CHR=ESZ | ||||
| Fronto-Central Lead x Deviant Type in HC | 2,86 | 0.00 | 1.000 | |||||
| Fronto-Central Lead x Deviant Type in ESZ | 2,36 | 1.67 | 0.204 | |||||
| Fronto-Central Lead x Deviant Type in CHR | 2,74 | 7.31 | 0.003 | |||||
| Deviant Type effect at Frontal Leads | 2,74 | 2.51 | 0.108 | |||||
| Deviant Type effect at Central Leads | 2,74 | 0.78 | 0.461 | |||||
| Fronto-Central Lead effect for DUR | 1,37 | 0.34 | 0.566 | |||||
| Fronto-Central Lead effect for FREQ | 1,37 | 9.67 | 0.004 | Frontal>Central** | ||||
| Fronto-Central Lead effect for DBL | 1,37 | 2.63 | 0.113 | |||||
| Group x Deviant Type at Frontal Leads | 2,196 | 0.99 | 0.401 | |||||
| Group x Deviant Type at Central Leads | 2,196 | 0.61 | 0.621 | |||||
| Group x Fronto-Central Lead x Lateral Lead | 4,196 | 1.75 | 0.148 | 2, 58 | 3.87 | 0.035 | ||
| Group x Fronto-Central Lead effect on Left | 1,29 | 0.24 | 0.629 | |||||
| Group x Fronto-Central Lead effect on Midline | 1,29 | 1.31 | 0.262 | |||||
| Group x Fronto-Central Lead effect on Right | 1,29 | 0.40 | 0.535 | |||||
| Group x Lateral Lead effect at Frontal leads | 1,29 | 0.19 | 0.764 | |||||
| Group x Lateral Lead effect at Central leads | 1,29 | 2.37 | 0.122 | |||||
| Fronto-Central Lead x Lateral Lead in Nonconverters | 1,15 | 2.25 | 0.132 | |||||
| Fronto-Central Lead x Lateral Lead in Converters | 1,14 | 5.55 | 0.016 | |||||
| Lateral Lead effect at Frontal leads | 2,28 | 3.59 | 0.051 | Left=Midline, Midline>Right* | ||||
| Lateral Lead effect at Central leads | 2,28 | 3.58 | 0.049 | Left>Midline*, Midline=Right | ||||
| Fronto-central Lead effect on Left | 1,14 | 0.44 | 0.518 | |||||
| Fronto-central Lead effect on Midline | 1,14 | 4.95 | 0.043 | Frontal>Central* | ||||
| Fronto-central Lead effect on Right | 1,14 | 0.19 | 0.670 | |||||
| Group x Deviant Type x Fronto-Central x Lateral Lead | 8,392 | 0.94 | 0.476 | 4,116 | 0.73 | 0.537 | ||
Repeated measures ANOVA comparing Healthy Control (HC), Clinical High Risk (CHR), and Early Illness Schizophrenia (ESZ) groups across the three deviant types (DUR, FREQ, DBL) using age-corrected mismatch negativity (MMN) z-scores. DUR=Duration deviant type; FREQ=Frequency deviant type; DBL=Double-deviant type.
Repeated measures ANOVA comparing converters and non-converters to psychosis across DUR, FREQ, and DBL deviant types using age-corrected MMN z-scores. More positive MMN z-scores indicate greater MMN deficits relative to HC.
Between group comparisons are based on post-hoc Tukey tests. Between lead comparisons are based on Helmert contrasts.
p ≤ 0.05,
p ≤ 0.01,
p ≤ 0.001
ANOVAs were repeated in the subset of antipsychotic-free CHR patients (n=27) and in the full sample using PSES as a covariate. Both re-analyses yielded results similar to those presented above.
Conversion Effects in MMN
Grand average ERP difference waves, scalp topography maps, and mean amplitude values (raw voltage and z-score) for DUR, FREQ, and DBL MMN show CHR converters to have smaller (i.e., less negative) MMN amplitudes than non-converters (Figure 2). Results of the 4-way (Converter Group × Deviant Type×Fronto-Central Lead × Lateral Lead) ANOVA of MMN z-scores, are presented in Table 2. A marginally significant Conversion Group effect indicated that converters had greater MMN deficits than non-converters. While the Conversion Group effect did not interact with Deviant Type, it significantly interacted with the Fronto-Central Lead × Lateral Lead effect. Since the basic finding of a Conversion Group effect that did not significantly depend on MMN Deviant Type was not altered by the interaction effects involving scalp topography factors, these interaction effects are not presented here. Instead, a full parsing of these interaction effects is presented in the Supplemental Materials and in Table 2.
Figure 2. Mismatch Negativity (MMN) for each Clinical High-Risk Conversion Group and Deviant Type.
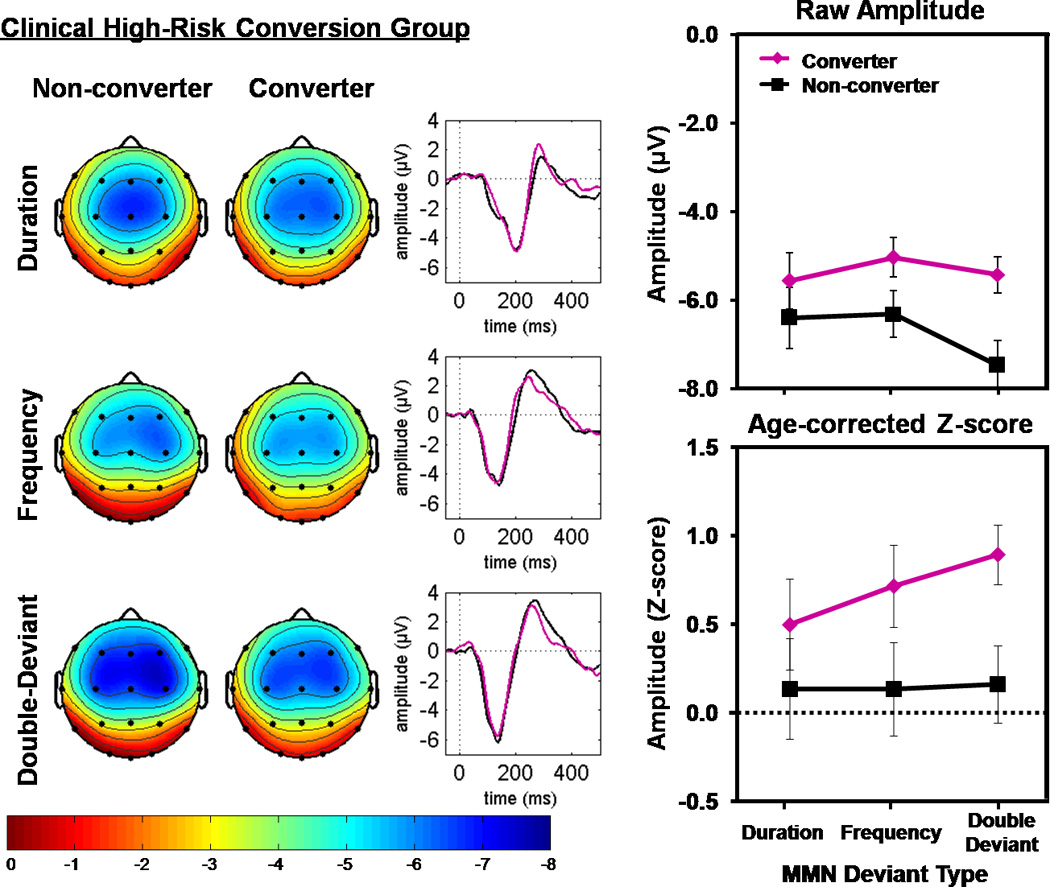
On the left, scalp voltage topography maps of MMN amplitudes are shown for each group and deviant type. MMN topography maps show the group means of MMN amplitudes associated with subject-specific median peak latency across the six fronto-central leads (F3, Fz, F4, C3, Cz, C4). In the center, ear-referenced ERP difference waveforms averaged across the six fronto-central leads for Duration, Frequency, and Double-Deviant MMN are shown for both groups. On the right, line graphs show (top) group means and standard errors for raw MMN amplitude in microvolts and (bottom) MMN age-corrected z-scores. Converters to psychosis are shown in magenta and non-converters are shown in black. MMN is reduced in converters relative to non-converters across deviant type. Individual subject butterfly plots are shown in Figure S3 of the Supplementary Materials.
MMN z-scores averaged over leads and deviant types were subjected to a one-way ANOVA with planned comparisons of the CHR conversion sub-groups with the ESZ and HC groups. Converters (p=.002), but not non-converters (p=.506), showed a significant MMN deficit relative to HC. In addition, ESZ patients showed significant MMN deficits relative to non-converters (p=.011) but not converters (p=.740).
Converters were also compared to the smaller subgroup (n=11) of non-converters who were followed for at least 24 months. For MMN z-scores (averaged over six fronto-central leads), the Conversion Group effect only showed a trend (F(1,24)=3.58, p=.070). However, for MMN raw amplitudes, the Conversion Group effect reached significance (F(1,29)=4.78, p=0.037), with no significant effects for Deviant Type (F(2,28)=1.89, p=0.18) or Conversion Group × Deviant Type (F(2,28)=1.16, p=0.31).
Survival Function for Conversion to Psychosis
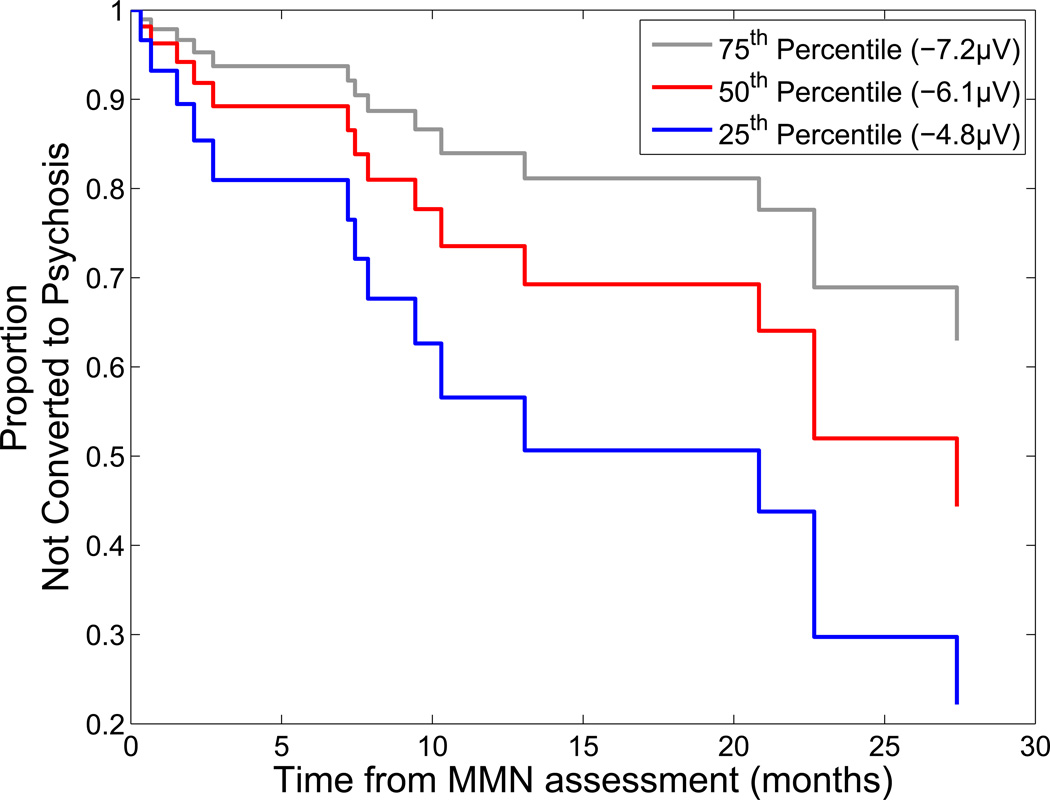
In the first of two hierarchical Cox regression models predicting the time from ERP assessment to psychosis conversion in CHR patients, DUR and FREQ MMN raw amplitudes entered as a block in Step 1 did not produce a significant overall increment in prediction (χ2=2.63, p=0.27), and neither MMN produced a significant predictive increment over and above the other (DUR: Wald(1)=0.17, p=.68, Exp(B)=1.06, FREQ: Wald(1)=1.59, p=.21, Exp(B)=1.24). However, at Step 2, entry of DBL MMN significantly improved prediction of time to psychosis onset ( χ2=8.41, p=0.004, DUR: Wald(1)=0.07, p=0.79, Exp(B)=0.97, FREQ: Wald(1)=1.86, p=0.17, Exp(B)=0.7,DBL: Wald(1)=7.3, p=0.007, Exp(B)=2.23). In the second model, DBL MMN entered at Step 1 significantly predicted time to psychosis onset (χ2=9.06, p=0.003; Exp(B)=1.63, Wald(1)=7.53, p=.006), and DUR and FREQ MMNs failed to significantly improve this prediction when entered as a block in Step 2 (χ2=1.99, p=0.37). In the final model, with all three MMN deviant types entered as predictors, a significant hazard ratio was produced by the DBL MMN (Exp(B)=2.23, p=.007) but not the DUR (Exp(B)=0.97, p=.79) or FREQ (Exp(B)=0.70, p=.17) MMNs. When DBL MMN was the sole predictor, the significant hazard ratio indicated that a unit decrease in MMN (i.e., 1 microvolt less negative MMN amplitude) is associated with a 1.63-fold increase in the risk for conversion to psychosis. As suggested by Hosmer and Lemeshow (69), we illustrate this effect by showing the estimated cumulative survival functions, indicating the probability of not converting to psychosis, for the three MMN values corresponding to the quartiles of the DBL MMN in the CHR sample (see Figure 3).
Figure 3. Estimated Survival Functions for Quartiles of the Double-Deviant Mismatch Negativity (MMN) in Clinical High-Risk Patients Showing Psychosis Conversion Risk.
A Cox regression analysis shows that a greater Double-Deviant MMN deficit in CHR patients is associated with an earlier transition to psychosis. To illustrate this finding, estimated cumulative survival functions are plotted for the amplitude values corresponding to the quartiles of the double-deviant MMN in the CHR group: Lower quartile (25th percentile) of MMN = −4.8 µV (blue line); Middle quartile (50th percentile) of MMN = −6.1 µV (red line); Upper quartile (75th percentile) of MMN = −7.2 µV (gray line).
Correlations with Clinical Symptoms
MMN amplitudes did not significantly correlate with PANSS or SOPS positive and negative symptom scores in ESZ or CHR groups, respectively.
Discussion
This study directly compared the sensitivity of MMN elicited by three deviant types to schizophrenia and to a putatively prodromal clinical syndrome associated with high risk for psychosis. ESZ and CHR patients showed reduced MMN, irrespective of deviant type, relative to healthy controls, but did not differ from each other. CHR patients who converted to psychosis within 24 months of their ERP assessment showed a significant MMN deficit, again irrespective of deviant type, relative to CHR non-converters. Whereas the MMN deficit in CHR converters was comparable to the ESZ MMN deficit, MMN in the non-converters was essentially normal. In addition, while group differences in MMN did not depend on deviant type, the double deviant MMN, but not the single deviant MMNs, significantly predicted the time from ERP assessment to psychosis onset in CHR patients.
Our results corroborate five previous studies (3–7) reporting MMN deficits in CHR patients, providing strong evidence that deficient automatic processing of auditory deviance, possibly reflecting compromised sensory echoic memory (9, 10), predictive coding (70), synaptic plasticity (71), and/or glutamatergic NMDA receptor function (72–76), predates psychosis onset. Moreover, our observation of MMN deficits in the subset of antipsychotic-free CHR patients who converted to psychosis is consistent with prior studies showing that MMN deficits are not related to anti-psychotic use (3, 15, 21, 32, 33, but see 34) or dose (77).
Among individuals meeting CHR criteria, only 35% are expected to convert to psychosis within a 2–3 year follow-up period (1, 2). Of the remaining 65%, most will never develop a psychotic disorder. This relatively low conversion rate tempers enthusiasm for early interventions in CHR patients, particularly with drugs that have significant side effects such as antipsychotics. Preventive interventions with CHR patients would not only expose many patients to unnecessary treatments, they also potentially expose patients to societal stigma (78), making the clinical utility of the CHR syndrome a matter of ongoing debate (79–82). If consideration of biomarkers can improve the predictive validity of the CHR syndrome, the risk-benefit ratio may be tipped in favor of early intervention with the subset of CHR patients at greatest risk for psychosis. Consistent with two prior reports (3, 6), our study indicates that reduced MMN in CHR patients is associated with increased risk for imminent conversion to psychosis. Challenges remain in translating these findings into a clinically useful prognostic test, but their replicability across three different CHR samples suggests that the time is ripe for future studies to address these challenges.
From the standpoint of distinguishing CHR vs. HC and converter vs. non-converter groups, the three types of MMN assessed (duration, frequency, duration+frequency double deviant) produced comparable group effects. These results did not support the hypothesis, based on prior schizophrenia studies (3, 13, 18, 21, 30, 38), that duration-deviant MMN is more sensitive than frequency-deviant MMN to early schizophrenia and its putative prodrome. Moreover, our finding of reduced frequency-deviant MMN in CHR patients is inconsistent with findings of normal frequency-deviant MMN in first episode schizophrenia (3, 26, 30, 34, 37). It is also inconsistent with one report showing normal frequency-deviant MMN in CHR patients (3), although the other CHR MMN studies only examined duration-deviant MMN (4–7). MMN paradigm differences and patient heterogeneity within and between studies may have contributed to these inconsistencies. However, relatively few first-episode and CHR studies to date have directly compared frequency- and duration-deviant MMN, and most have relied on relatively small samples. Accordingly, more research is needed before definitive conclusions can be drawn about the status of frequency- and duration-deviant MMN in CHR and first-episode patients.
The double-deviant MMN did not yield significantly larger group effects than the single-deviant MMNs, inconsistent with our hypothesis. However, when the time from ERP assessment to psychosis conversion among CHR patients was considered, only the double-deviant MMN significantly predicted psychosis onset after controlling for the correlations among the three MMN deviant types. This finding, which replicates and extends findings from Bodatsch and colleagues (3), suggests that the neuroanatomically distinct MMN generators associated with processing different dimensions of auditory deviance (54, 57, 83) may be heterogeneously compromised across schizophrenia (84) and CHR patients, with no single-deviant MMN being optimally sensitive to disease in all patients (52). While the double-deviant MMN was more strongly related to the frequency MMN than the duration MMN, its ability to predict psychosis onset over and above the single deviant MMNs suggests that it may be particularly useful for purposes of clinical prediction in CHR patients. Moreover, the double-deviant MMN appears to assess neurophysiological processes associated with multi-feature auditory deviance detection that are not assessed by separate assessment of MMN to each deviance feature, at least from the standpoint of processes related to psychosis risk.
There were no associations between MMN and symptoms in either patient group, consistent with much of the prior literature (13, 14) but inconsistent with several studies showing MMN to correlate with negative symptoms (15, 28, 85) or hallucinations (85–88). Variability in symptom correlations across studies may arise for many reasons, including failure to distinguish contributions of trait severity and clinical state fluctuations to symptom ratings, as well as attenuation of relationships associated with studying medicated patients (89).
In conclusion, this study demonstrates MMN reduction in and CHR patients. Moreover, among CHR patients, the greater the MMN reduction, the more imminent the risk of psychosis. MMN is a promising biomarker of psychosis risk that may improve the accuracy of psychosis prediction when combined with clinical risk criteria. Future studies should extend the follow-up period in order to track within-patient clinical and neurophysiological illness progression from the prodrome through the early stages of schizophrenia.
Supplementary Material
Acknowledgements
This study was supported by grants from the National Institute of Health (R01 MH076989, U01 MH082022, K02 MH067967, and T32 MH089920) and from the Brain and Behavior Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures. Drs. Perez, Woods, Ford, McGlashan, Srihari, and Mr. Roach report no biomedical financial interests or poten tial conflicts of interest. Dr. Mathalon is a consultant for Bristol-Myers Squibb and Amgen.
References
- 1.Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fusar-Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69:220–229. doi: 10.1001/archgenpsychiatry.2011.1472. [DOI] [PubMed] [Google Scholar]
- 3.Bodatsch M, Ruhrmann S, Wagner M, Muller R, Schultze-Lutter F, Frommann I, et al. Prediction of psychosis by mismatch negativity. Biological psychiatry. 2011;69:959–966. doi: 10.1016/j.biopsych.2010.09.057. [DOI] [PubMed] [Google Scholar]
- 4.Jahshan C, Cadenhead KS, Rissling AJ, Kirihara K, Braff DL, Light GA. Automatic sensory information processing abnormalities across the illness course of schizophrenia. Psychol Med. 2011:1–13. doi: 10.1017/S0033291711001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atkinson RJ, Michie PT, Schall U. Duration Mismatch Negativity and P3a in First-Episode Psychosis and Individuals at Ultra-High Risk of Psychosis. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 6.Shaikh M, Valmaggia L, Broome MR, Dutt A, Lappin J, Day F, et al. Reduced mismatch negativity predates the onset of psychosis. Schizophrenia research. 2012;134:42–48. doi: 10.1016/j.schres.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 7.Shin KS, Kim JS, Kang DH, Koh Y, Choi JS, O'Donnell BF, et al. Pre-attentive auditory processing in ultra-high-risk for schizophrenia with magnetoencephalography. Biological psychiatry. 2009;65:1071–1078. doi: 10.1016/j.biopsych.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 8.Naatanen R, Gaillard AW, Mantysalo S. Early selective-attention effect on evoked potential reinterpreted. Acta Psychol (Amst) 1978;42:313–329. doi: 10.1016/0001-6918(78)90006-9. [DOI] [PubMed] [Google Scholar]
- 9.Naatanen R, Teder W, Alho K, Lavikainen J. Auditory attention and selective input modulation: a topographical ERP study. Neuroreport. 1992;3:493–496. doi: 10.1097/00001756-199206000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Naatanen R, Jacobsen T, Winkler I. Memory-based or afferent processes in mismatch negativity (MMN): a review of the evidence. Psychophysiology. 2005;42:25–32. doi: 10.1111/j.1469-8986.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- 11.Fischer C, Morlet D, Bouchet P, Luaute J, Jourdan C, Salord F. Mismatch negativity and late auditory evoked potentials in comatose patients. Clin Neurophysiol. 1999;110:1601–1610. doi: 10.1016/s1388-2457(99)00131-5. [DOI] [PubMed] [Google Scholar]
- 12.Naatanen R, Alho K. Generators of electrical and magnetic mismatch responses in humans. Brain Topogr. 1995;7:315–320. doi: 10.1007/BF01195257. [DOI] [PubMed] [Google Scholar]
- 13.Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophrenia research. 2005;76:1–23. doi: 10.1016/j.schres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Naatanen R, Kahkonen S. Central auditory dysfunction in schizophrenia as revealed by the mismatch negativity (MMN) and its magnetic equivalent MMNm: a review. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2009;12:125–135. doi: 10.1017/S1461145708009322. [DOI] [PubMed] [Google Scholar]
- 15.Catts SV, Shelley AM, Ward PB, Liebert B, McConaghy N, Andrews S, et al. Brain potential evidence for an auditory sensory memory deficit in schizophrenia. Am J Psychiatry. 1995;152:213–219. doi: 10.1176/ajp.152.2.213. [DOI] [PubMed] [Google Scholar]
- 16.Shelley AM, Ward PB, Catts SV, Michie PT, Andrews S, McConaghy N. Mismatch negativity: an index of a preattentive processing deficit in schizophrenia. Biological psychiatry. 1991;30:1059–1062. doi: 10.1016/0006-3223(91)90126-7. [DOI] [PubMed] [Google Scholar]
- 17.Javitt DC, Doneshka P, Zylberman I, Ritter W, Vaughan HG., Jr Impairment of early cortical processing in schizophrenia: an event-related potential confirmation study. Biological psychiatry. 1993;33:513–519. doi: 10.1016/0006-3223(93)90005-x. [DOI] [PubMed] [Google Scholar]
- 18.Umbricht DS, Bates JA, Lieberman JA, Kane JM, Javitt DC. Electrophysiological indices of automatic and controlled auditory information processing in first-episode, recent-onset and chronic schizophrenia. Biological psychiatry. 2006;59:762–772. doi: 10.1016/j.biopsych.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 19.Michie PT, Budd TW, Todd J, Rock D, Wichmann H, Box J, et al. Duration and frequency mismatch negativity in schizophrenia. Clin Neurophysiol. 2000;111:1054–1065. doi: 10.1016/s1388-2457(00)00275-3. [DOI] [PubMed] [Google Scholar]
- 20.Oades RD, Wild-Wall N, Juran SA, Sachsse J, Oknina LB, Ropcke B. Auditory change detection in schizophrenia: sources of activity, related neuropsychological function and symptoms in patients with a first episode in adolescence, and patients 14 years after an adolescent illness-onset. BMC Psychiatry. 2006;6:7. doi: 10.1186/1471-244X-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brockhaus-Dumke A, Tendolkar I, Pukrop R, Schultze-Lutter F, Klosterkotter J, Ruhrmann S. Impaired mismatch negativity generation in prodromal subjects and patients with schizophrenia. Schizophr Res. 2005;73:297–310. doi: 10.1016/j.schres.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 22.Oknina LB, Wild-Wall N, Oades RD, Juran SA, Ropcke B, Pfueller U, et al. Frontal and temporal sources of mismatch negativity in healthy controls, patients at onset of schizophrenia in adolescence and others at 15 years after onset. Schizophrenia research. 2005;76:25–41. doi: 10.1016/j.schres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Rasser PE, Schall U, Todd J, Michie PT, Ward PB, Johnston P, et al. Gray matter deficits, mismatch negativity, and outcomes in schizophrenia. Schizophrenia bulletin. 2011;37:131–140. doi: 10.1093/schbul/sbp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Light GA, Braff DL. Stability of mismatch negativity deficits and their relationship to functional impairments in chronic schizophrenia. The American journal of psychiatry. 2005;162:1741–1743. doi: 10.1176/appi.ajp.162.9.1741. [DOI] [PubMed] [Google Scholar]
- 25.Light GA, Braff DL. Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Archives of general psychiatry. 2005;62:127–136. doi: 10.1001/archpsyc.62.2.127. [DOI] [PubMed] [Google Scholar]
- 26.Magno E, Yeap S, Thakore JH, Garavan H, De Sanctis P, Foxe JJ. Are auditory-evoked frequency and duration mismatch negativity deficits endophenotypic for schizophrenia? High-density electrical mapping in clinically unaffected first-degree relatives and first-episode and chronic schizophrenia. Biological psychiatry. 2008;64:385–391. doi: 10.1016/j.biopsych.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiang M, Braff DL, Sprock J, Light GA. The relationship between preattentive sensory processing deficits and age in schizophrenia patients. Clin Neurophysiol. 2009;120:1949–1957. doi: 10.1016/j.clinph.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Javitt DC, Shelley AM, Silipo G, Lieberman JA. Deficits in auditory and visual context-dependent processing in schizophrenia: defining the pattern. Arch Gen Psychiatry. 2000;57:1131–1137. doi: 10.1001/archpsyc.57.12.1131. [DOI] [PubMed] [Google Scholar]
- 29.Javitt DC, Grochowski S, Shelley AM, Ritter W. Impaired mismatch negativity (MMN) generation in schizophrenia as a function of stimulus deviance, probability, and interstimulus/interdeviant interval. Electroencephalography and clinical neurophysiology. 1998;108:143–153. doi: 10.1016/s0168-5597(97)00073-7. [DOI] [PubMed] [Google Scholar]
- 30.Salisbury DF, Shenton ME, Griggs CB, Bonner-Jackson A, McCarley RW. Mismatch negativity in chronic schizophrenia and first-episode schizophrenia. Arch Gen Psychiatry. 2002;59:686–694. doi: 10.1001/archpsyc.59.8.686. [DOI] [PubMed] [Google Scholar]
- 31.Todd J, Michie PT, Schall U, Karayanidis F, Yabe H, Naatanen R. Deviant matters: duration, frequency, and intensity deviants reveal different patterns of mismatch negativity reduction in early and late schizophrenia. Biological psychiatry. 2008;63:58–64. doi: 10.1016/j.biopsych.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 32.Kirino E, Inoue R. The relationship of mismatch negativity to quantitative EEG and morphological findings in schizophrenia. J Psychiatr Res. 1999;33:445–456. doi: 10.1016/s0022-3956(99)00012-6. [DOI] [PubMed] [Google Scholar]
- 33.Korostenskaja M, Dapsys K, Siurkute A, Maciulis V, Ruksenas O, Kahkonen S. Effects of olanzapine on auditory P300 and mismatch negativity (MMN) in schizophrenia spectrum disorders. Progress in neuro-psychopharmacology & biological psychiatry. 2005;29:543–548. doi: 10.1016/j.pnpbp.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 34.Devrim-Ucok M, Keskin-Ergen HY, Ucok A. Mismatch negativity at acute and post-acute phases of first-episode schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2008;258:179–185. doi: 10.1007/s00406-007-0772-9. [DOI] [PubMed] [Google Scholar]
- 35.Hermens DF, Ward PB, Hodge MA, Kaur M, Naismith SL, Hickie IB. Impaired MMN/P3a complex in first-episode psychosis: cognitive and psychosocial associations. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:822–829. doi: 10.1016/j.pnpbp.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 36.Kaur M, Battisti RA, Ward PB, Ahmed A, Hickie IB, Hermens DF. MMN/P3a deficits in first episode psychosis: comparing schizophrenia-spectrum and affective-spectrum subgroups. Schizophrenia research. 2011;130:203–209. doi: 10.1016/j.schres.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 37.Valkonen-Korhonen M, Purhonen M, Tarkka IM, Sipila P, Partanen J, Karhu J, et al. Altered auditory processing in acutely psychotic never-medicated first-episode patients. Brain Res Cogn Brain Res. 2003;17:747–758. doi: 10.1016/s0926-6410(03)00199-x. [DOI] [PubMed] [Google Scholar]
- 38.Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64:521–529. doi: 10.1001/archpsyc.64.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jessen F, Fries T, Kucharski C, Nishimura T, Hoenig K, Maier W, et al. Amplitude reduction of the mismatch negativity in first-degree relatives of patients with schizophrenia. Neurosci Lett. 2001;309:185–188. doi: 10.1016/s0304-3940(01)02072-9. [DOI] [PubMed] [Google Scholar]
- 40.Michie PT, Innes-Brown H, Todd J, Jablensky AV. Duration mismatch negativity in biological relatives of patients with schizophrenia spectrum disorders. Biol Psychiatry. 2002;52:749–758. doi: 10.1016/s0006-3223(02)01379-3. [DOI] [PubMed] [Google Scholar]
- 41.Sevik AE, Anil Yagcioglu AE, Yagcioglu S, Karahan S, Gurses N, Yildiz M. Neuropsychological performance and auditory event related potentials in schizophrenia patients and their siblings: a family study. Schizophr Res. 2011;130:195–202. doi: 10.1016/j.schres.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 42.Schreiber H, Stolz-Born G, Kornhuber HH, Born J. Event-related potential correlates of impaired selective attention in children at high risk for schizophrenia. Biological psychiatry. 1992;32:634–651. doi: 10.1016/0006-3223(92)90294-a. [DOI] [PubMed] [Google Scholar]
- 43.Bramon E, Croft RJ, McDonald C, Virdi GK, Gruzelier JG, Baldeweg T, et al. Mismatch negativity in schizophrenia: a family study. Schizophrenia research. 2004;67:1–10. doi: 10.1016/s0920-9964(03)00132-4. [DOI] [PubMed] [Google Scholar]
- 44.Ahveninen J, Jaaskelainen IP, Osipova D, Huttunen MO, Ilmoniemi RJ, Kaprio J, et al. Inherited auditory-cortical dysfunction in twin pairs discordant for schizophrenia. Biological psychiatry. 2006;60:612–620. doi: 10.1016/j.biopsych.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 45.Hall MH, Rijsdijk F, Picchioni M, Schulze K, Ettinger U, Toulopoulou T, et al. Substantial shared genetic influences on schizophrenia and event-related potentials. The American journal of psychiatry. 2007;164:804–812. doi: 10.1176/ajp.2007.164.5.804. [DOI] [PubMed] [Google Scholar]
- 46.Paavilainen P, Alho K, Reinikainen K, Sams M, Naatanen R. Right hemisphere dominance of different mismatch negativities. Electroencephalogr Clin Neurophysiol. 1991;78:466–479. doi: 10.1016/0013-4694(91)90064-b. [DOI] [PubMed] [Google Scholar]
- 47.Alho K. Cerebral generators of mismatch negativity (MMN) and its magnetic counterpart (MMNm) elicited by sound changes. Ear Hear. 1995;16:38–51. doi: 10.1097/00003446-199502000-00004. [DOI] [PubMed] [Google Scholar]
- 48.Giard MH, Perrin F, Pernier J, Bouchet P. Brain generators implicated in the processing of auditory stimulus deviance: a topographic event-related potential study. Psychophysiology. 1990;27:627–640. doi: 10.1111/j.1469-8986.1990.tb03184.x. [DOI] [PubMed] [Google Scholar]
- 49.Deouell LY, Bentin S, Giard MH. Mismatch negativity in dichotic listening: evidence for interhemispheric differences and multiple generators. Psychophysiology. 1998;35:355–365. [PubMed] [Google Scholar]
- 50.Csepe V. On the origin and development of the mismatch negativity. Ear Hear. 1995;16:91–104. doi: 10.1097/00003446-199502000-00007. [DOI] [PubMed] [Google Scholar]
- 51.Molholm S, Martinez A, Ritter W, Javitt DC, Foxe JJ. The neural circuitry of pre-attentive auditory change-detection: an fMRI study of pitch and duration mismatch negativity generators. Cereb Cortex. 2005;15:545–551. doi: 10.1093/cercor/bhh155. [DOI] [PubMed] [Google Scholar]
- 52.Naatanen R, Pakarinen S, Rinne T, Takegata R. The mismatch negativity (MMN): towards the optimal paradigm. Clin Neurophysiol. 2004;115:140–144. doi: 10.1016/j.clinph.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 53.Takegata R, Paavilainen P, Naatanen R, Winkler I. Independent processing of changes in auditory single features and feature conjunctions in humans as indexed by the mismatch negativity. Neurosci Lett. 1999;266:109–112. doi: 10.1016/s0304-3940(99)00267-0. [DOI] [PubMed] [Google Scholar]
- 54.Levanen S, Hari R, McEvoy L, Sams M. Responses of the human auditory cortex to changes in one versus two stimulus features. Exp Brain Res. 1993;97:177–183. doi: 10.1007/BF00228828. [DOI] [PubMed] [Google Scholar]
- 55.Paavilainen P, Valppu S, Naatanen R. The additivity of the auditory feature analysis in the human brain as indexed by the mismatch negativity 1+1 approximately 2 but 1+1+1<3. Neurosci Lett. 2001;301:179–182. doi: 10.1016/s0304-3940(01)01635-4. [DOI] [PubMed] [Google Scholar]
- 56.Schroger E. Processing of auditory deviants with changes in one versus two stimulus dimensions. Psychophysiology. 1995;32:55–65. doi: 10.1111/j.1469-8986.1995.tb03406.x. [DOI] [PubMed] [Google Scholar]
- 57.Wolff C, Schroger E. Human pre-attentive auditory change-detection with single, double, and triple deviations as revealed by mismatch negativity additivity. Neurosci Lett. 2001;311:37–40. doi: 10.1016/s0304-3940(01)02135-8. [DOI] [PubMed] [Google Scholar]
- 58.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID), Research Version, Patient Edition with Psychotic Screen. New York: Biometrics Research, New York State Psychiatric Institute; 1997. [Google Scholar]
- 59.Miller TJ, McGlashan TH, Rosen JL, Somjee L, Markovich PJ, Stein K, et al. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry. 2002;159:863–865. doi: 10.1176/appi.ajp.159.5.863. [DOI] [PubMed] [Google Scholar]
- 60.Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29:703–715. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- 61.Kay S, Opler L. The positive-negative dimension in schizophrenia: its validity and significance. Psychiatr Dev. 1987;5:79–103. [PubMed] [Google Scholar]
- 62.Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- 63.Rahne T, von Specht H, Muhler R. Sorted averaging--application to auditory event-related responses. J Neurosci Methods. 2008;172:74–78. doi: 10.1016/j.jneumeth.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 64.Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, et al. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcohol Clin Exp Res. 1992;16:1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- 65.Perez VB, Ford JM, Roach BJ, Woods SW, McGlashan TH, Srihari VH, et al. Error monitoring dysfunction across the illness course of schizophrenia. J Abnorm Psychol. 2011 doi: 10.1037/a0025487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perez VB, Ford JM, Roach BJ, Loewy RL, Stuart BK, Vinogradov S, et al. Auditory Cortex Responsiveness During Talking and Listening: Early Illness Schizophrenia and Patients at Clinical High-Risk for Psychosis. Schizophr Bull. 2011 doi: 10.1093/schbul/sbr124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crovitz HF, Zener K. A group-test for assessing hand- and eye-dominance. Am J Psychol. 1962;75:271–276. [PubMed] [Google Scholar]
- 68.Hollingshead AA. Unpublished manuscript. New Haven, CT: Yale University; 1975. Four-factor index of social status. [Google Scholar]
- 69.Hosmer DW, Lemeshow S. Applied survival analysis: Regression modeling of time to event data. New York: John Wiley & Sons, Inc.; 1999. [Google Scholar]
- 70.Garrido MI, Kilner JM, Stephan KE, Friston KJ. The mismatch negativity: a review of underlying mechanisms. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2009;120:453–463. doi: 10.1016/j.clinph.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stephan KE, Baldeweg T, Friston KJ. Synaptic plasticity and dysconnection in schizophrenia. Biological psychiatry. 2006;59:929–939. doi: 10.1016/j.biopsych.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 72.Javitt DC, Steinschneider M, Schroeder CE, Arezzo JC. Role of cortical N-methyl-D-aspartate receptors in auditory sensory memory and mismatch negativity generation: implications for schizophrenia. Proc Natl Acad Sci U S A. 1996;93:11962–11967. doi: 10.1073/pnas.93.21.11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Umbricht D, Vollenweider FX, Schmid L, Grubel C, Skrabo A, Huber T, et al. Effects of the 5-HT2A agonist psilocybin on mismatch negativity generation and AX-continuous performance task: implications for the neuropharmacology of cognitive deficits in schizophrenia. Neuropsychopharmacology. 2003;28:170–181. doi: 10.1038/sj.npp.1300005. [DOI] [PubMed] [Google Scholar]
- 74.Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69–108. doi: 10.1016/S0074-7742(06)78003-5. [DOI] [PubMed] [Google Scholar]
- 75.Carlson GC, Talbot K, Halene TB, Gandal MJ, Kazi HA, Schlosser L, et al. From the Cover: Dysbindin-1 mutant mice implicate reduced fast-phasic inhibition as a final common disease mechanism in schizophrenia. Proc Natl Acad Sci U S A. 2011;108:E962–E970. doi: 10.1073/pnas.1109625108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26:365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Javitt DC, Doneshka P, Grochowski S, Ritter W. Impaired mismatch negativity generation reflects widespread dysfunction of working memory in schizophrenia. Arch Gen Psychiatry. 1995;52:550–558. doi: 10.1001/archpsyc.1995.03950190032005. [DOI] [PubMed] [Google Scholar]
- 78.Yang LH, Wonpat-Borja AJ, Opler MG, Corcoran CM. Potential stigma associated with inclusion of the psychosis risk syndrome in the DSM-V: an empirical question. Schizophrenia research. 120:42–48. doi: 10.1016/j.schres.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Woods SW, Walsh BC, Saksa JR, McGlashan TH. The case for including Attenuated Psychotic Symptoms Syndrome in DSM-5 as a psychosis risk syndrome. Schizophrenia research. 123:199–207. doi: 10.1016/j.schres.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Corcoran CM, First MB, Cornblatt B. The psychosis risk syndrome and its proposed inclusion in the DSM-V: a risk-benefit analysis. Schizophrenia research. 120:16–22. doi: 10.1016/j.schres.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fleischhacker WW, DeLisi LE. Should a 'psychosis risk syndrome' be a separate diagnosis in DSM-5? Curr Opin Psychiatry. 25:327–328. doi: 10.1097/YCO.0b013e3283535921. [DOI] [PubMed] [Google Scholar]
- 82.Nelson B, Yung AR. Should a risk syndrome for first episode psychosis be included in the DSM-5? Curr Opin Psychiatry. 24:128–133. doi: 10.1097/YCO.0b013e32834190cd. [DOI] [PubMed] [Google Scholar]
- 83.Ritter W, Gomes H, Cowan N, Sussman E, Vaughan HG., Jr Reactivation of a dormant representation of an auditory stimulus feature. J Cogn Neurosci. 1998;10:605–614. doi: 10.1162/089892998563004. [DOI] [PubMed] [Google Scholar]
- 84.Winterer G, Coppola R, Goldberg TE, Egan MF, Jones DW, Sanchez CE, et al. Prefrontal broadband noise, working memory, and genetic risk for schizophrenia. Am J Psychiatry. 2004;161:490–500. doi: 10.1176/appi.ajp.161.3.490. [DOI] [PubMed] [Google Scholar]
- 85.Hirayasu Y, Potts GF, O'Donnell BF, Kwon JS, Arakaki H, Akdag SJ, et al. Auditory mismatch negativity in schizophrenia: topographic evaluation with a high-density recording montage. Am J Psychiatry. 1998;155:1281–1284. doi: 10.1176/ajp.155.9.1281. [DOI] [PubMed] [Google Scholar]
- 86.Schall U, Catts SV, Karayanidis F, Ward PB. Auditory event-related potential indices of fronto-temporal information processing in schizophrenia syndromes: valid outcome prediction of clozapine therapy in a three-year follow-up. Int J Neuropsychopharmacol. 1999;2:83–93. doi: 10.1017/S1461145799001418. [DOI] [PubMed] [Google Scholar]
- 87.Youn T, Park HJ, Kim JJ, Kim MS, Kwon JS. Altered hemispheric asymmetry and positive symptoms in schizophrenia: equivalent current dipole of auditory mismatch negativity. Schizophrenia research. 2003;59:253–260. doi: 10.1016/s0920-9964(02)00154-8. [DOI] [PubMed] [Google Scholar]
- 88.Fisher DJ, Labelle A, Knott VJ. The right profile: mismatch negativity in schizophrenia with and without auditory hallucinations as measured by a multi-feature paradigm. Clin Neurophysiol. 2008;119:909–921. doi: 10.1016/j.clinph.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 89.Mathalon DH, Ford JM. Neurobiology of schizophrenia: search for the elusive correlation with symptoms. Front Hum Neurosci. 2012;6:136. doi: 10.3389/fnhum.2012.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.