Abstract
Patients meeting criteria for the risk syndrome for psychosis have treatment needs including positive and negative symptoms and cognitive impairment. These features could potentially respond to NMDA glycine-site agonists. The present objective was to determine which symptoms or domains of cognition promise to show the greatest response to glycine in risk syndrome patients. We conducted two short-term pilot studies of glycine used without adjunctive antipsychotic medication. In the first trial, 10 risk syndrome subjects received open-label glycine at doses titrated to 0.8 g/kg/d for 8 weeks, followed by discontinuation and 16 weeks of evaluation for durability of effects. In the second, 8 subjects were randomized to double-blind glycine vs. placebo for 12 weeks, followed by open-label glycine for another 12 weeks. Patients were evaluated every 1–2 weeks with the Scale Of Psychosis-risk Symptoms (SOPS) and before and after treatment with a neurocognitive battery. Within-group and between-group effect sizes were calculated. Effect sizes were large for positive (open-label within-group 1.10, double-blind between-group –1.11) and total (–1.39 and –1.15) symptoms and medium-to-large (–0.74 and –0.79) for negative symptoms. Medium or large effect sizes were also observed for several neurocognitive measures in the open-label study, although data were sparse. No safety concerns were identified. We conclude that glycine was associated with reduced symptoms with promising effect sizes in two pilot studies and a possibility of improvement in cognitive function. Further studies of agents that facilitate NMDA receptor function in risk syndrome patients are supported by these preliminary findings.
Keywords: Glycine, NMDA receptor, Risk syndrome, Prodrome, Psychosis, Schizophrenia
1. Introduction
The N-methyl-d-aspartate receptor (NMDAR) hypoactivity model is a leading hypothesis about the neurobiology of schizophrenia (Javitt and Zukin, 1991; Kantrowitz and Javitt, 2010; Kim et al., 1980; Krystal et al., 2002; Olney et al., 1999). This hypothesis is based in part on exacerbation of positive and negative symptoms and cognitive impairment in schizophrenia patients by NMDAR antagonists such as ketamine and the production of similar effects in healthy humans. Evidence suggests NMDAR hypoactivity may connect to other prominent models of psychosis (Feinberg, 1982; Howes and Kapur, 2009; McGlashan and Hoffman, 2000) by contributing to the development of dopamine hyperactivity in striatum (Carlsson et al., 1999; Laruelle et al., 2003) and cortical synaptic plasticity deficits (Collingridge and Singer, 1990; Newcomer and Krystal, 2001; Olney et al., 1999; Shi et al., 1999).
Over the past 15 years, researchers have attempted to identify patients in the prodromal phase of psychotic disorders prospectively, based primarily on subsyndromal psychotic-like or “attenuated” positive symptoms (Miller et al., 2002; Yung et al., 1996). Since the term “prodrome” traditionally carries a retrospective connotation, the alternative terms “risk syndrome for psychosis” (Woods et al., 2009), “at-risk mental state,” “ultra high risk,” “clinical high risk,” and most recently “attenuated psychosis syndrome” or “APS” (Carpenter and van Os, 2011) have been proposed. A recent meta-analysis of 27 studies suggested that the average rate of transition to full psychosis among such patients is 22% by one year and 36% by three years (Fusar-Poli et al., 2012). Structural thinning of cerebral cortex (Pantelis et al., 2003) and increased striatal uptake of dopamine precursor (Howes et al., 2011), neurobiological findings typical of established schizophrenia, have been reported at baseline in risk syndrome patients who later progress to psychosis, findings which increase in magnitude after progression to psychosis has occurred.
In addition to carrying substantial risk for transition to frank psychosis, risk syndrome patients meet general mental health standards for current illness (Ruhrmann et al., 2010) in that at presentation they display distressing current symptoms and functional and cognitive impairment (Woods et al., 2001, 2010). Intervention studies have begun to address these patients’ prevention needs (Amminger et al., 2010; McGlashan et al., 2006; McGorry et al., 2002; Morrison et al., 2004; Yung et al., 2011), and some have started to investigate current clinical state as a treatment target (Amminger et al., 2010; McGorry et al., 2002; Ruhrmann et al., 2007; Woods et al., 2003, 2007; Yung et al., 2011). Medication treatment studies have primarily focused on use of antipsychotics, but there is a compelling need for investigation of other treatments with fewer adverse effects such as the current effort and the recent omega-3 fatty acid study (Amminger et al., 2010).
Glycine is an amino acid neurotransmitter in brain that acts at the glycine/D-serine modulatory site on the NMDAR as a full coagonist with glutamate (Javitt, 2006). Based on the hypothesis that the risk syndrome may reflect an NMDAR hypofunction state, we tested the therapeutic effects of glycine in risk syndrome patients in two small, short-term pilot studies initiated in preparation for future more definitive trials.
2. Experimental procedures
The first pilot study assessed whether the size of any beneficial effect of glycine in this population promises to be clinically meaningful (Kraemer et al., 2006) and what might best be identified as the principal therapeutic target in future studies. An open-label design was employed. Since within-active-drug effect sizes in psychosis can be lower with placebo-controlled designs than when only active medication is employed (Woods et al., 2005), we also conducted a second small placebo-controlled study with similar aims. Glycine was used in both studies without adjunctive antipsychotic medication.
2.1. Subjects
Potential subjects or their families or providers were informed about the symptoms of the risk syndrome for psychosis through a variety of ongoing community education efforts and were invited to call our research clinic if concerned. Adult subjects gave written informed consent, and minors gave written informed assent with consent from a parent or guardian. Subjects were included in either study if they were treatment-seeking outpatients 14 to 35 years old who met diagnostic criteria for a possible risk syndrome (see below). Subjects were excluded for any of the following reasons: (1) past or current DSM IV criteria for any lifetime psychotic disorder, (2) judged clinically to suffer from a psychiatric disorder (e.g., mania, depression, ADHD) which could account for the inclusion symptoms, (3) presented with inclusion symptoms occurring primarily as sequelae to drug or alcohol use, (4) alcohol or drug abuse or dependence in the past three months, (5) use of antipsychotic medication in the previous three months, (6) change in dosage of any antidepressant, anxiolytic, psychostimulant, or mood stabilizer medication within eight weeks.
The Criteria of Psychosis-risk Syndromes (COPS) diagnostic criteria were used to identify subjects as eligible (McGlashan et al., 2010). The COPS criteria are based primarily on subthreshold levels of positive symptoms and operationally define three risk syndromes originally articulated by the Melbourne group (Yung et al., 1996): Attenuated Positive Symptom Syndrome, Brief Intermittent Psychotic Syndrome, and Genetic Risk and Recent Functional Decline Syndrome. The rationale for these syndromes, their definitions, and evidence for their reliability and validity have been published previously in detail (Addington et al., 2007; Miller et al., 2002, 2003; Woods et al., 2009). The Attenuated Positive Symptom Syndrome usually accounts for the preponderance of the cases. In general, positive symptoms are considered subsyndromal or “attenuated” when they remain relatively unformed, relatively infrequent, and are identified by the subject as possibly a trick of their imagination (McGlashan et al., 2010). Both studies required a minimum total score of 20 on the Scale of Psychosis-risk Symptoms (SOPS, see Assessments) for inclusion.
2.2. Study design
Subjects were enrolled in the first study between February 2003 and May 2004 and in the second study between March 2006 and May 2008. All subjects were enrolled in the PRIME (Prevention through Risk Identification Management and Education) Research Clinic at Yale University. The majority of subjects in the first pilot were seen in Hartford via collaboration with the Olin Center at the Institute of Living. All subjects in the second pilot were seen in New Haven. The Yale Human Investigation Committee institutional review board approved both protocols.
The first study used an open-label design over 8 weeks. Glycine was obtained from Ajinomoto USA Inc. as bulk powder. Glycine powder was dispensed under IND 41,851 (JHK) to subjects in prescription vials with instructions to dissolve the full contents of one vial in 8 ounces of fluid and take by mouth twice daily with meals. Glycine was discontinued after 8 weeks. Subjects were followed off study medication with biweekly visits for 16 more weeks.
The second study used a double-blind placebo-controlled design over 12 weeks. Since glycine is naturally sweet, sucrose (table sugar) was used as the placebo. Glycine and placebo were dispensed under IND 33,515 (DCJ) as one of two proprietary formulations developed by Glytech, Inc. Dosing was initiated with small microencapsulated beads or “sprinkles” (80% glycine by weight), with placebo consisting of microencapsulated sucrose. Recommended administration of the sprinkles was to spoon them onto pudding or applesauce and swallow them with minimal chewing. Since earlier product testing by Glytech revealed that a few individuals did not like the somewhat granular texture of the sprinkles, subjects could switch to a second Glytech formulation, consisting of proprietary pre-flavored glycine or sugar powders to be dissolved in 8 ounces of water. After 12 weeks all subjects had the option to receive open-label active glycine for a further 12 weeks.
In both studies glycine dosing was fixed at an initial dose of 0.2 g/kg q.h.s for 3 days, then 0.2 g/kg b.i.d. for 4 days, then 0.2 g/kg in the a.m. and 0.4 g/kg in the p.m. for 4 days, and finally 0.4 g/kg b.i.d. Subjects weighing > 100 kg were limited to a total daily dose of 80 g daily.
2.3. Assessments
All assessments were administered weekly for the first 6 weeks and then every 2 weeks thereafter. The principal outcome measure was the Scale Of Psychosis-risk Symptoms (SOPS) (McGlashan et al., 2010), a 19-item severity scale with five positive symptom items, six negative symptom items, four disorganization items, and four general symptom items designed to capture severity of subsyndromal symptoms. Each item is scaled 0–6, with 0–2 being the normal range, 3–5 being the risk syndrome range, and 6 being severe and psychotic for the positive symptoms and very severe for the other symptoms. Excellent reliability has been demonstrated across the range of symptoms (Lencz et al., 2003; Miller et al., 2003). Factor analysis supports the validity of the SOPS subscales (Hawkins et al., 2004). Both the SOPS total score and the SOPS positive symptom subscale subtotal have previously been shown to be sensitive to short-term treatment effects in the risk syndrome population (McGlashan et al., 2006; Woods et al., 2003). Treatment response was defined as all five SOPS positive symptom items being rated below the risk syndrome range (i.e. ≤2). The Montgomery–Asberg Depression Rating Scale (MADRS, see Supplementary file for citation) was used to rate depressive symptom severity at baseline and monthly. Global assessment of functioning (GAF, Supplementary file) was also acquired at baseline.
Neuropsychological assessments included tests of processing speed: Trails A and Stroop Color Word Test; verbal memory: Auditory Verbal Learning Task (AVLT), using alternate forms; executive functioning: Wisconsin Card Sort Test (WCS), semantic (category) fluency, and Controlled Oral Word Association (FAS) Test of phonemic fluency; and attention and working memory: The Continuous Performance Task (CPT) identical pairs version, letter–number sequencing, N-back, and Trails B. Citations for these tests are included in the Supplementary file. Follow-up neuropsychological assessments were conducted at 8 weeks in the open-label pilot and at 12 weeks in the double-blind pilot.
Safety was assessed by analyzing treatment-emergent adverse events (Systematic Assessment For Treatment Emergent Events, SAFTEE, specific inquiry method, see Supplementary file), vital signs, weight, and laboratory evaluations. Treatment-emergent adverse events were defined as those occurring at the moderate level or higher at any time point and representing an increase over baseline.
2.4. Data analysis
A descriptive approach has been recommended for statistical analysis of pilot studies rather than a hypothesis-testing approach (Lancaster et al., 2004), and a descriptive analysis of efficacy focusing on effect size provides a useful metric for comparison to previous treatment studies with other medications in risk syndrome patients and to glycine treatment studies of frank schizophrenia. Treatment effects were first evaluated by calculating last-observation-carried forward (LOCF) change scores, at the same 8-wk time point in both studies to facilitate comparison. Cohen's d effect sizes (Cohen, 1988) were then calculated, for within-group change from baseline, and in the case of double-blind glycine vs. double-blind placebo, between groups. Following Cohen, effect sizes were classified as “small” (0.2), “medium” (0.5), or “large” (0.8). Because some readers may be more familiar with hypothesis testing, we also indicate when the p-value for the paired or Student's t-test or Fisher's exact test was <0.05. Tests of differences at baseline compared open-label vs. double-blind glycine and double-blind glycine vs. placebo. Proportions endorsing treatment emergent increases in SAFTEE items were compared by visual inspection.
3. Results
3.1. Subject characteristics
Ten subjects enrolled in the first study, and eight in the second study. Demographic data are shown in Table 1. The double-blind glycine group did not differ significantly from the open-label glycine group or the double-blind placebo group on any measure. Subjects were generally in their midto-late teens and roughly three-quarters were male and roughly two-thirds Caucasian. GAF scores were in the midto-low 40s (severe impairment). All subjects met criteria for the SIPS-defined Attenuated Positive Symptom risk syndrome subtype. The subjects were all studied before the recently-proposed DSM-5 criteria (Carpenter and van Os, 2011) were drafted; thus it cannot be stated with certainty whether subjects would have met the DSM-5 criteria.
Table 1.
Demographic and treatment data at baseline.
| Study Group | Open-label Glycine (n=10) | Placebo-controlled |
|
|---|---|---|---|
| Glycine (n=4) | Placebo (n=4) | ||
| Characteristic | Mean(SD) | Mean(SD) | Mean(SD) |
| Age (yr) | 17.3 (3.3) | 15.3 (0.5) | 16.5 (2.4) |
| Current GAF | 44.3 (4.7) | 40.5 (4.0) | 39.0 (9.0) |
| No. (%) | No. (%) | No. (%) | |
| Male | 7 (70) | 3 (75) | 3 (75) |
| White nonHispanic | 6 (60) | 3 (75) | 2 (50) |
| Single | 10 (100) | 4 (100) | 4 (100) |
| Prodromal syndrome | |||
| APS | 10 (100) | 4 (100) | 4 (100) |
| BIPS | 1 (10) | 0 (0) | 0 (0) |
| GRD | 0 (0) | 0 (0) | 0 (0) |
| First degree family history | |||
| Psychosis | 0 (0) | 0 (0) | 0 (0) |
| Nonpsychotic major depression | 1 (10) | 1 (25) | 2 (50) |
| Nonpsychotic bipolar disorder | 2 (20) | 0 (0) | 0 (0) |
| Medication use at baseline* | |||
| Antidepressants | 3 (30) | 1 (25) | 1 (25) |
| Antipsychotics | 0 (0) | 0 (0) | 0 (0) |
| Benzodiazepines/hypnotics | 0 (0) | 0 (0) | 0 (0) |
| Mood stabilizers | 0 (0) | 0 (0) | 0 (0) |
| Stimulants | 0 (0) | 0 (0) | 0 (0) |
| Lifetime substance abuse/dependence | |||
| Marijuana | 1 (10) | 0 (0) | 0 (0) |
| Other (except nicotine)** | 1 (10) | 0 (0) | 0 (0) |
All comparisons n.s. GAF, Global Assessment of Functioning.
Patients taking antidepressants, anxiolytics, mood stabilizers, or stimulants were permitted to continue these medications but not to change dose. Doses were required to be stable ≥8 weeks at baseline.
Other in substance abuse/dependence includes: alcohol, sedatives, opioid, cocaine, hallucinogen and other.
Baseline psychotropic medication is also summarized in Table 1. Antidepressant medication had been prescribed to 25–30%. Although the entry criteria would have permitted patients to continue other psychotropic medications at baseline if doses were stable at least 8 weeks, none of the recruited subjects were prescribed other psychotropic medication at baseline. Entry criteria also would have permitted subjects to have received substantial prior anti-psychotic treatment so long as it was not in the past 3 months; however, lifetime antipsychotic histories in the recruited subjects were minimal. Subject 630 took one lifetime dose of antipsychotic 6 months prior to entry, and subject 718 took one lifetime day of antipsychotic a year prior to entry.
Table 2 shows baseline scores on clinical efficacy measures and Supplemental Table 1 those on cognitive measures. SOPS total scores were in the high 30s, similar to those from previous treatment studies from our group (Woods et al., 2003, 2007; Woods and McGlashan, 2010). Neuropsychological functioning scores at baseline also were similar to those from a previous treatment study (Woods et al., 2007), with the possible exceptions of lesser baseline impairment on the N-back tests in the open-label glycine group and on Trails B in both glycine groups. Baseline values for the double-blind glycine group differed significantly from the open-label glycine group or the double-blind placebo group only for WCS perseverative errors (more impaired than either comparison group) and for WCS categories completed (more impaired than for double-blind placebo).
Table 2.
Baseline scores on SOPS and MADRS measures.
| Domain | Study Treatment Measure | Open-label Glycine (n = 10) Mean (SD) | Double-blind |
|
|---|---|---|---|---|
| Glycine (n=4) Mean (SD) | Placebo (n=4) Mean (SD) | |||
| Prodromal symptoms | SOPS total | 39.7 (11.8) | 37.8 (15.3) | 37.5 (10.0) |
| SOPS positive | 11.3 (3.3) | 14.8 (2.2) | 13.0 (2.8) | |
| SOPS negative | 12.4 (5.5) | 11.0 (8.0) | 11.0 (7.2) | |
| SOPS disorganization | 6.5 (2.5) | 6.3 (3.1) | 4.0 (1.2) | |
| SOPS general | 9.5 (4.1) | 5.8 (5.7) | 9.5 (3.4) | |
| Depression | MADRS total | 13.8 (6.7)a | 13.0 (14.7) | 17.3 (7.0) |
n=9.
3.2. Subject disposition
Seven of 10 subjects (70%) completed 8 weeks in the open-label glycine study (Figure 1). The remaining subjects dropped at 2 weeks (lack of efficacy), at 3 weeks (also lack of efficacy), and at 5 weeks (lost to follow-up). There were no changes in concomitant medication. Three of four subjects (75%) completed 8 weeks of double-blind glycine; the fourth subject was withdrawn at 5 weeks due to nonadherence. Three of four subjects (75%) also completed 8 weeks of double-blind placebo; the one noncompleter was withdrawn at 3 weeks due to conversion to frank psychosis and institution of rescue antipsychotic medication. Two placebo subjects missed one or more rating visits (637 and 671). There were no changes in concomitant medication over the first 12 weeks.
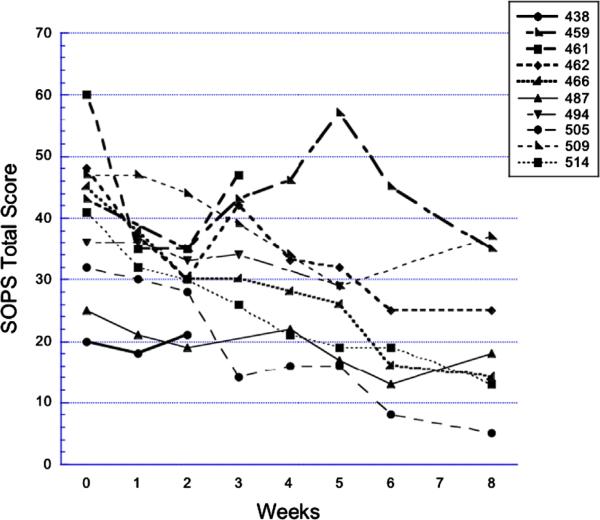
Figure 1.
Open-label glycine: SOPS total scores over time. Subject numbers indicate consecutive patients evaluated for risk syndrome research diagnoses who met criteria, consented, and began treatment with open-label glycine for 8 weeks. SOPS—Scale Of Psychosis-risk Symptoms.
3.3. Continuous efficacy outcomes at 8 weeks
Table 3 shows LOCF effect sizes for SOPS and MADRS measures at 8 weeks. In the open-label study the within-group effect size for the SOPS total score was large at –1.39. Effect sizes for most SOPS subscales were also large, with the lowest being medium-to-large for negative symptoms at –0.74. For the active glycine arm in the double-blind study, SOPS within-group effect sizes were generally medium or medium-to-large, with the positive symptom effect size being large and disorganization symptoms showing little change. For the placebo arm in the double-blind study, SOPS within-group effect sizes were generally in the medium range, in the direction of worsening. Glycine vs. placebo between-group effect sizes were large for the SOPS total score (–1.15) and large or medium-to-large for all subscales, with negative and disorganization symptoms being least strongly affected. Depressive symptoms on the MADRS showed only a medium effect size in the open-label study but larger effect sizes in the double-blind pilot.
Table 3.
Effect sizes and mean (and SD) endpoint change in SOPS and MADRS severity scores (LOCF).
| SOPS total | SOPS positive | SOPS negative | SOPS disorganized | SOPS general | MADRS total | |
|---|---|---|---|---|---|---|
| Open-label glycine (n= 10) | ||||||
| 8w Change | –15.3 (11.0)* | –5.7 (5.2)* | –3.0 (4.1)* | –3.1 (3.0)* | –3.5 (3.1)* | –2.1 (4.8)a* |
| Within group effect size | –1.39 | –1.10 | –0.74 | –1.05 | –1.12 | –0.44 |
| Double-blind active glycine (n=4) | ||||||
| 8w Change | –5.8 (8.1) | –2.3 (2.8) | –1.5 (2.5) | –0.3 (1.7) | –1.8 (2.4) | –5.8 (4.0) |
| Within group effect size | –0.71 | –0.82 | –0.60 | –0.15 | –0.74 | −1.43 |
| Double-blind placebo (n=4) | ||||||
| 8w Change | 4.5 (9.7) | 2.0 (4.7) | 0.3 (1.9) | 1.0 (2.0) | 1.3 (1.3) | 3.3 (4.9)b |
| Within group effect size | 0.46 | 0.43 | 0.13 | 0.50 | 0.99 | 0.68 |
| Double-blind glycine vs. placebo | ||||||
| Between groups effect size | –1.15 | –1.11 | –0.79 | –0.67 | –1.58 | –2.06* |
n=8.
n=3.
p<0.05 by paired or Student's t-test as appropriate.
Supplemental Table 2 shows endpoint change scores and effect sizes for neuropsychological functioning measures. Findings must be interpreted with caution due to small sample sizes and missing data. In the open-label pilot, three of four tests of processing speed showed medium or large within-group effect sizes in the direction of improvement, as did both tests of verbal memory, and three of four tests of executive functioning. Findings on attention and working memory showed less consistent effects, with a medium or large within-group effect size in the direction of improvement on four of 10 tests (2- and 3-digit CPT performance, LNS, and Trails B) but in the direction of worsening on two others (4-digit CPT performance and reaction time). Data are even sparser in the double-blind study, with only one subject on some measures due to missing data. In the active glycine arm, however, all four tests of processing speed showed medium or large within-group effect sizes in the direction of improvement, as did both tests of verbal memory. Two of four tests of executive functioning showed similar effects, but one (WCS perseverative errors) showed an opposite effect. As in the open-label study, double-blind active glycine findings showed a medium or large within-group effect size in the direction of improvement on 4/10 tests of attention and working memory. Between-group effect sizes in the large range favored glycine for two of four processing speed measures and for two of four executive functioning measures; in each case, however, one of the four measures showed a between-group effect size in the large range favoring placebo.
3.4. Categorical efficacy outcomes at 8 weeks
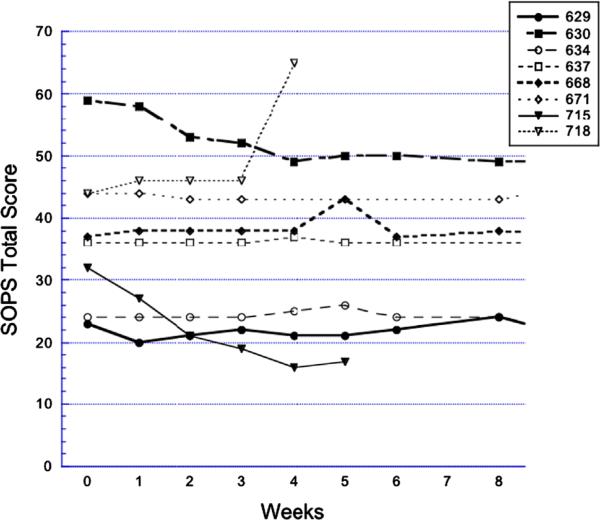
Of the seven completers in the open-label study, four met response criteria during the 8 weeks on glycine (cases 462, 466, 505, and 514; individual SOPS total scores are shown in Figure 1). Two of these had received antidepressant medication at baseline. In case 466 the antidepressant dose had been unchanged for 2 years; in case 514 the antidepressant dose was increased 8 weeks prior to baseline. In the double-blind pilot, no subjects met response criteria by 8 weeks. One glycine subject converted to psychosis at week 4 (Figure 2, case 718). None of the glycine-treated subjects converted to psychosis during the study.
Figure 2.
Double-blind glycine vs. placebo: SOPS total scores over time. Filled circles: glycine; open circles: placebo. Subject numbers indicate consecutive patients evaluated for risk syndrome research diagnoses who met criteria, consented, and began treatment with double-blind glycine vs. placebo for 12 weeks. The first 8 weeks of treatment are shown. SOPS—Scale Of Psychosis-risk Symptoms.
3.5. Efficacy outcomes after 8 weeks
After 8-wk completion and glycine discontinuation in the open-label pilot, seven subjects participated in off-drug follow-up, of whom six completed the full 16 additional weeks. Among the four subjects completing open-label glycine as responders, all maintained response throughout follow-up at all or nearly all visits. Two of the three on-drug nonresponders achieved response intermittently during off-drug follow-up. The third subject (459) underwent several antidepressant adjustments after 8 weeks; otherwise, there were no concomitant medication changes.
The three double-blind active glycine subjects and the two double-blind placebo subjects who continued on assignment from weeks 8 to 12 showed little additional change, so that the 12 wk LOCF between-group effect size for the SOPS total score was similar at –1.23. After 12 weeks, the three double-blind active glycine subjects continued on open-label glycine to 16, 20, and 24 weeks without meeting response criteria. One former placebo subject (634) began and completed 12 weeks of open-label glycine and also did not respond. Subject 630 underwent antidepressant dose changes during glycine extension; otherwise there were no concomitant medication changes.
Patients in the open-label pilot all contributed to the North American Prodromal Longitudinal Study phase one federated database (Woods et al. 2009), a project that provided for follow-up evaluations up to 24 months after the end of glycine study participation. Five of the 10 subjects converted to psychosis during this period; the remaining five had not converted at the time of last observation. Although patients in the double-blind pilot did not undergo structured follow-up after the study, one patient was known to have converted to psychosis after glycine study participation (630).
3.6. Adverse events (AEs)
Several AEs were endorsed as emerging during treatment in the open-label pilot (Table 4); however, none of these effects were endorsed with double-blind glycine and some were endorsed with placebo.
Table 4.
Treatment emergent adverse events in prodromal patients treated with glycinea.
| Study Treatment SAFTEE items | Open-label Glycine (n = 10) No. (%) | Double-blind |
|
|---|---|---|---|
| Glycine (n=4) No. (%) | Placebo (n=4) No. (%) | ||
| Irritability | 5 (50) | 1 (25) | |
| Insomnia | 4 (40) | ||
| Dry mouth | 3 (30) | ||
| Sedation | 2 (20) | 2 (50) | |
| Fatigue/weakness | 2 (20) | ||
| Memory impaired | 2 (20) | ||
| Sensory perception impaired | 2 (20) | ||
| Headache | 2 (20) | ||
| Orgasm dysfunction | 1 (25) | ||
| Disturbed sleep | 2 (50) | ||
| Malaise | 1 (25) | ||
| Mentation impaired | 1 (25) | ||
| Hallucinations | 1 (25) | ||
| Stomach discomfort | 1 (25) | ||
Proportion of patients endorsing adverse events as determined by the Schedule of Assessment For Treatment Emergent Effects (SAFTEE), using the specific inquiry method, at the medium level or higher at any time point in the first 8 weeks and representing an increase over baseline. Adverse events endorsed by only one patient for open-label glycine are not shown.
3.7. Vital signs, weight, clinical laboratory values
Table 5 shows effects on vital signs and weight (for baseline values see Supplemental Table 3). There was a medium-sized adverse effect of glycine vs. placebo on systolic blood pressure, however, the highest endpoint value in a double-blind glycine subject was 131 mmHg, and little effect was observed with open-label glycine. Supplemental Table 4 shows baseline values for clinical laboratory examinations, and Supplemental Table 5 shows endpoint change values. Laboratory examination data in the placebo-controlled pilot again was sparse, with only two subjects having complete data in the active arm and one in the placebo arm. Leukocytes fell in the two double-blind glycine subjects; these subjects however, entered with relatively high leukocyte counts (Supplemental Table 4) which then fell to the middle of the normal range during glycine treatment. Serum potassium showed adverse effect sizes in the medium range in the open-label pilot and in the large range in double-blind study; however, the lowest endpoint value in either study was 3.9 meq/L (normal range 3.5–5.0). Large adverse effect sizes for sodium and chloride in the double-blind study were similarly not associated with out-of-range individual values.
Table 5.
Effect sizes and mean (and SD) endpoint change in vital signs and weight.
| Systolic BP | Diastolic BP | Pulse | Weight (kg) | |
|---|---|---|---|---|
| Open-label glycine (n=10) | ||||
| 8w Change | –1 (23) | –3 (10) | 2 (8) | –1.0 (2.0) |
| Within group effect size | –0.04 | –0.33 | 0.27 | –0.52 |
| Double-blind active glycine (n=4) | ||||
| 8w Change | 2 (12) | 6 (6) | –3 (3) | –0.7 (2.0) |
| Within group effect size | 0.19 | 1.04 | –1.24 | –0.33 |
| Double-blind placebo (n=4) | ||||
| 8w Change | –4 (9) | 4 (13) | 17 (22) | 0.6 (1.3) |
| Within group effect size | –0.41 | 0.30 | 0.80 | 0.50 |
| Double-blind glycine vs. placebo Between groups effect size | 0.57 | 0.19 | –1.33 | –0.77 |
4. Discussion
The principal findings of these two small pilot studies are that short-term treatment of risk syndrome patients with glycine used as stand-alone medication led to beneficial symptom outcomes that were not seen in a small placebo sample. These beneficial outcomes generally were associated with medium or large effect sizes. The open-label study raises the possibility of cognitive benefits as well. These findings pave the way for pivotal studies to test efficacy and suggest that such studies could be powered for medium effect sizes with samples in the range of 60–70 per group and that such studies could anticipate definitive results.
Perhaps more so than for many pilot studies, the main limitation for interpreting the present findings is the small samples. This limitation is highlighted by consideration in Table 3 of the worsening in the double-blind placebo group, which contributes to the medium and large glycine vs. placebo between-group effect sizes. As Figure 2 shows, this group worsening is nearly all attributable to the one placebo-treated patient who converted to psychosis. Another limitation is that 25–30% of subjects were received antidepressants at baseline, although doses were required to be stable for 8 weeks or longer at enrollment. A strength of these pilots is that the subjects appear to be similar to those who volunteered for previous risk syndrome intervention studies. In particular, the average age of 15–17, the current GAF score in the 40's or high 30's, the male preponderance, and the SOPS total scores in the high 30's (Tables 1 and 2) are all consistent with previous studies (Woods et al., 2003, 2007), as are the MADRS scores in the teens (Woods et al., 2003).
The symptom effect sizes shown in Table 3 in these risk syndrome patients appear generally larger than those observed with glycine for established schizophrenia. A recent meta-analysis (Tsai and Lin, 2010) reported pooled glycine vs. placebo between-group effect sizes of –0.71 for total psychopathology (vs. –1.15 in Table 3), –0.42 for positive symptoms (vs. –1.11), –0.52 for negative symptoms (vs. –0.79), and –0.59 for depressive symptoms (vs. –2.06). Again, the small sample must be borne in mind. If the true effect sizes with glycine in risk syndrome patients turn out to be larger than those in schizophrenia patients, however, the difference might be due to a number of factors. First, the large effects we observed may have been related to our use of glycine as monotherapy. All previous glycine studies for established schizophrenia cited in the meta-analysis appear to be add-on studies, and addon treatment might be affected by floor effects such that smaller effect sizes are observed when patients are already partially treated with antipsychotic. Secondly, however, it is possible that glycine is simply more effective when it is given earlier in the course of illness, a possibility that has also been considered for omega-3 fatty acids (Amminger et al., 2010). Some authors have speculated that sustained NMDAR hypofunction could lead to structural pathology (Olney et al., 1999), which might then be more difficult to reverse. Thus, it is possible that adaptations to NMDAR hypofunction over periods of chronic illness could become entrenched and persist even after NMDAR hypofunction is addressed. In one study the cognitive impairments and altered basal and evoked dopamine release caused by subchronic administration of an NMDAR antagonist persisted after drug discontinuation (Jentsch et al., 1997). It is also interesting to note that the effect of glycine in our young risk syndrome patients appeared to extend across the symptom spectrum, with positive symptoms being more prominently reduced than negative symptoms, the reverse of the pattern suggested by the schizophrenia meta-analysis.
It must be noted, however, that effect sizes for risk symptoms on the SOPS at 8 weeks with open-label glycine (Table 3), while mostly large, were approximately half those seen in our previous open-label study with the antipsychotic medication aripiprazole (Woods et al., 2007). Further studies are needed before the benefits of these two classes of medication can be compared with confidence.
On the other hand, neuropsychological effects of glycine in the open-label study (Supplemental Table 2) despite the sparse data compared favorably with those from a previous open-label pilot of aripiprazole in the same population with the same battery (Woods et al., 2007), where only 3/20 measures showed medium or large effect sizes in the direction of improvement, compared to 11/20 for open-label glycine.
The beneficial effects of short-term glycine administration in chronic schizophrenic subjects have been shown to persist after discontinuation for at least 8 weeks in two studies (Heresco-Levy et al., 1996, 1999). Some evidence suggests the effect was no longer seen several months after glycine discontinuation (Heresco-Levy et al., 1999). We also noted a persistent beneficial effect after discontinuation, in that subjects who responded to 8 weeks of open-label glycine maintained response for 16 weeks after discontinuation. Conversely, subjects in the smaller double-blind trial who continued glycine beyond 8 weeks generally experienced little additional improvement. Future studies should take these findings, along with the small sample sizes, into account when designing the duration of active glycine dosing.
Safety findings (Tables 4 and 5 and Supplemental Table 5) do not indicate a specific concern in this population, although again the sample sizes were small. Generally glycine is considered to have a favorable adverse event profile, although nausea was reported more frequently with glycine than with placebo in the CONSIST study (Buchanan et al., 2007) and has also been noted occasionally in previous studies (Costa et al., 1990; Heresco-Levy et al., 1999). Nausea was not noted in the present pilots. In the double-blind trial, including the open-label extension, five subjects took glycine for at least 12 weeks, of whom three continued as long as 24 weeks with no ill effects.
Overall, the results of these two pilot studies suggest that future adequately-powered studies would likely be able to establish a beneficial effect of glycine used alone in risk syndrome patients and that the effect could be expected across the spectrum of psychotic-like symptoms and might possibly extend to neurocognitive impairment as well. The results also suggest that studies of other glycine-site agonists such as D-serine (Kantrowitz et al., 2010) and of glycine transporter inhibitors (D'souza et al., 2012; Umbricht et al., 2011) would appear to be similarly promising in this population.
Supplementary Material
Acknowledgments
Elizabeth M. Tully, M.D, assisted in managing some of the subjects, and Philip Markovich assisted with data preparation.
Role of funding source
Funding was provided by Stanley Foundation 02T-175 (open-label pilot), a NARSAD Distinguished Investigator Award (double-blind pilot), a research grant from Glytech Inc. (double-blind pilot), the Donaghue Foundation Early Schizophrenia Initiative (both pilots), and National Institutes of Health Grant U01 MH74356 (both pilots). None of these entities had any further role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the report for publication.
Footnotes
Trial Registration: These studies have the ClinicalTrials.gov identifiers: NCT00268749 and NCT00291226.
Contributors
Author Woods designed the studies, wrote the protocols, analyzed the data, and wrote the first draft. All authors contributed to and have approved the final manuscript.
Conflict of interest
Author Woods has applied for a patent “Method of treating prodromal schizophrenia by administering NMDA glycine-site agonists” and has received research support from Glytech Inc. and royalties from Oxford University Press. Author Javitt holds intellectual property rights for use of glycine, D-serine and glycine transport inhibitors in treatment of schizophrenia and related disorders and is CEO of Glytech Inc. Author McGlashan has received royalties from Oxford University Press. Author Krystal reports serving as a consultant at a rate each less than $10,000 per year for: Aisling Capital, LLC, Astellas Pharma Global Development, Inc., AstraZeneca Pharmaceuticals, Biocortech, Brintnall & Nicolini, Inc., Easton Associates, Gilead Sciences, Inc., GlaxoSmithKline, Janssen Pharmaceuticals, Lundbeck Research USA, Medivation, Inc., Merz Pharmaceuticals, MK Medical Communications, F. Hoffmann-La Roche Ltd., Sage Therapeutics, Inc., SK Holdings Co., Ltd., Sunovion Pharmaceuticals, Inc., Takeda Industries, and Teva Pharmaceutical Industries, Ltd.; on the scientific advisory board for: Abbott Laboratories, Bristol-Myers Squibb, CHDI Foundation, Inc., Eisai, Inc., Eli Lilly and Co., Forest Laboratories, Inc., Lohocla Research Corporation, Mnemosyne Pharmaceuticals, Inc., Naurex, Inc., Pfizer Pharmaceuticals, and Shire Pharmaceuticals; on the Board of Directors for: Coalition for Translational Research in Alcohol and Substance Use Disorders; as President of: American College of Neuropsychopharmacology; receiving research support from: Department of Veterans Affairs, Janssen Research Foundation; as editor of: Biol Psychiatry; and the following patents: (1) Seibyl J.P., Krystal J.H., Charney D.S. Dopamine and noradrenergic reuptake inhibitors in treatment of schizophrenia, Patent #:5,447,948, September 5, 1995, (2) co-inventor with Dr. Gerard Sanacora on a filed patent application by Yale University related to targeting the glutamatergic system for the treatment of neuropsychiatric disorders (PCTWO06108055A1), (3) Intranasal Administration of Keta-mine to Treat Depression (pending). Authors Walsh, Hawkins, Saksa, D'souza, and Pearlson report no financial or other relationship relevant to the subject of this article.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.euroneuro.2012.09.008.
References
- Addington J, Cadenhead KS, Cannon TD, Cornblatt B, McGlashan TH, Perkins DO, Seidman LJ, Tsuang M, Walker EF, Woods SW, Heinssen R. North American Prodrome Longitudinal Study: a collaborative multisite approach to prodromal schizophrenia research. Schizophr. Bull. 2007;33:665–672. doi: 10.1093/schbul/sbl075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amminger GP, Schafer MR, Papageorgiou K, Klier CM, Cotton SM, Harrigan SM, Mackinnon A, McGorry PD, Berger GE. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch. Gen. Psychiatry. 2010;67:146–154. doi: 10.1001/archgenpsychiatry.2009.192. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Javitt DC, Marder SR, Schooler NR, Gold JM, McMahon RP, Heresco-Levy U, Carpenter WT. The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): the efficacy of glutamatergic agents for negative symptoms and cognitive impairments. Am. J. Psychiatry. 2007;164:1593–1602. doi: 10.1176/appi.ajp.2007.06081358. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Waters N, Carlsson ML. Neurotransmitter interactions in schizophrenia—therarpeutic implications. Biol. Psychiatry. 1999;46:1388–1395. doi: 10.1016/s0006-3223(99)00117-1. [DOI] [PubMed] [Google Scholar]
- Carpenter WT, van Os J. Should attenuated psychosis syndrome be a DSM-5 diagnosis? Am. J. Psychiatry. 2011;168:460–463. doi: 10.1176/appi.ajp.2011.10121816. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Collingridge GL, Singer W. Excitatory amino-acid receptors and synaptic plasticity. Trends Pharmacol. Sci. 1990;11:290–296. doi: 10.1016/0165-6147(90)90011-v. [DOI] [PubMed] [Google Scholar]
- Costa J, Khaled E, Sramek J, Bunney W, Potkin SG. An open trial of glycine as an adjunct to neuroleptics in chronic treatment-refractory schizophrenics. J. Clin. Psychopharmacol. 1990;10:71–72. doi: 10.1097/00004714-199002000-00027. [DOI] [PubMed] [Google Scholar]
- D'Souza DC, Singh N, Elander J, Carbuto M, Pittman B, de Haes JU, Sjogren M, Peeters P, Ranganathan M, Schipper J. Glycine transporter inhibitor attenuates the psychotomimetic effects of ketamine in healthy males: preliminary evidence. Neuropsychopharmacology. 2012;37:1036–1046. doi: 10.1038/npp.2011.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J. Psychiatr. Res. 1982;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L, Barale F, Caverzasi E, McGuire P. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch. Gen. Psychiatry. 2012;69:220–229. doi: 10.1001/archgenpsychiatry.2011.1472. [DOI] [PubMed] [Google Scholar]
- Hawkins KA, Quinlan D, Miller TJ, McGlashan TH, Zipursky RB, Perkins DO, Addington J, Woods SW. Factorial structure of the scale of prodromal symptoms. Schizophr. Res. 2004;68:339–347. doi: 10.1016/S0920-9964(03)00053-7. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Javitt DC, Ermilov M, Mordel C, Horowitz A, Kelly D. Double-blind, placebo-controlled, crossover trial of glycine adjuvant therapy for treatment-resistant schizophrenia. Br. J. Psychiatry. 1996;169:610–617. doi: 10.1192/bjp.169.5.610. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Javitt DC, Ermilov M, Mordel C, Silipo G, Lichtenstein M. Efficacy of high-dose glycine in the treatment of enduring negative symptoms of schizophrenia. Arch. Gen. Psychiatry. 1999;56:29–36. doi: 10.1001/archpsyc.56.1.29. [DOI] [PubMed] [Google Scholar]
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr. Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Bose SK, Turkheimer F, Valli I, Egerton A, Valmaggia LR, Murray RM, McGuire P. Dopamine synthesis capacity before onset of psychosis: a prospective (18)F—DOPA PET imaging study. Am. J. Psychiatry. 2011;168:1311–1317. doi: 10.1176/appi.ajp.2011.11010160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencycli-dine model of schizophrenia [see comment]. Am. J. Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Is the glycine site half saturated or half unsaturated? Effects of glutamatergic drugs in schizophrenia patients. Curr. Opin. Psychiatry. 2006;19:151–157. doi: 10.1097/01.yco.0000214340.14131.bd. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Redmond DE, Jr., Elsworth JD, Taylor JR, Youngren KD, Roth RH. Enduring cognitive deficits and cortical dopamine dysfunction in monkeys after long-term administration of phencyclidine. Science. 1997;277:953–955. doi: 10.1126/science.277.5328.953. [DOI] [PubMed] [Google Scholar]
- Kantrowitz JT, Javitt DC. N-methyl-D-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain Res. Bull. 2010;83:108–121. doi: 10.1016/j.brainresbull.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantrowitz JT, Malhotra AK, Cornblatt B, Silipo G, Balla A, Suckow RF, D'Souza C, Saksa J, Woods SW, Javitt DC. High dose D-serine in the treatment of schizophrenia. Schizophr. Res. 2010;121:125–130. doi: 10.1016/j.schres.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Kornhuber HH, Schmidburgk W, Holzmuller B. Low cerbro-spinal glutamate in schizophrenic-patients and a new hypothesis on schizophrenia. Neurosci. Lett. 1980;20:379–382. doi: 10.1016/0304-3940(80)90178-0. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Mintz J, Noda A, Tinklenberg J, Yesavage JA. Caution regarding the use of pilot studies to guide power calculations for study proposals. Arch. Gen. Psychiatry. 2006;63:484–489. doi: 10.1001/archpsyc.63.5.484. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Anand A, Moghaddam B. Effects of NMDA receptor antagonists: implications for the pathophysiology of schizophrenia. Arch. Gen. Psychiatry. 2002;59:663–664. doi: 10.1001/archpsyc.59.7.663. [DOI] [PubMed] [Google Scholar]
- Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J. Eval. Clin. Pract. 2004;10:307–312. doi: 10.1111/j..2002.384.doc.x. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Kegeles LS, Abi-Dargham A. Glutamate, dopamine, and schizophrenia: from pathophysiology to treatment. Ann. N. Y. Acad. Sci. 2003;01:138–158. doi: 10.1196/annals.1300.063. [DOI] [PubMed] [Google Scholar]
- Lencz T, Smith CW, Auther AM, Correll CU, Cornblatt BA. The assessment of “prodromal schizophrenia”: unresolved issues and future directions. Schizophr. Bull. 2003;29:717–728. doi: 10.1093/oxfordjournals.schbul.a007041. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch. Gen. Psychiatry. 2000;57:637–648. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Zipursky RB, Perkins D, Addington J, Miller T, Woods SW, Hawkins KA, Hoffman RE, Preda A, Epstein I, Addington D, Lindborg S, Trzaskoma Q, Tohen M, Breier A. Randomized, double-blind trial of olanzapine versus placebo in patients prodromally symptomatic for psychosis. Am. J. Psychiatry. 2006;163:790–799. doi: 10.1176/ajp.2006.163.5.790. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Walsh BC, Woods SW. The Psychosis-Risk Syndrome: Handbook for Diagnosis and Follow-Up. Oxford University Press; New York: 2010. [Google Scholar]
- McGorry PD, Yung AF, Phillips LJ, Yuen HP, Francey S, Cosgrave EM, Germano D, Bravin J, Adlard S, McDonald T, Blair A, Adlard S, Jackson H. Randomized controlled trial of interventions designed to reduce the risk of progression to first-episode psychosis in a clinical sample with subthreshold symptoms. Arch. Gen. Psychiatry. 2002;59:921–928. doi: 10.1001/archpsyc.59.10.921. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Somjee L, Markovich PJ, Stein K, Woods SW. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am. J. Psychiatry. 2002;159:863–865. doi: 10.1176/appi.ajp.159.5.863. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, McFarlane W, Perkins DO, Pearlson GD, Woods SW. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr. Bull. 2003;29:703–715. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- Morrison AP, French P, Walford L, Lewis SW, Kilcommons A, Green J, Parker S, Bentall RP. Cognitive therapy for the prevention of psychosis in people at ultra-high risk. Randomized controlled trial. Br. J. Psychiatry. 2004;185:291–297. doi: 10.1192/bjp.185.4.291. [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Krystal JH. NMDA receptor regulation of memory and behavior in humans. Hippocampus. 2001;11:529–542. doi: 10.1002/hipo.1069. [DOI] [PubMed] [Google Scholar]
- Olney JW, Newcomer JW, Farber NB. NMDA receptor hypo-function model of schizophrenia. J. Psychiatr. Res. 1999;33:523–533. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, Yung AR, Bullmore ET, Brewer W, Soulsby B, Desmond PK, McGuire PK. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- Ruhrmann S, Bechdolf A, Kuhn KU, Wagner M, Schultze-Lutter F, Janssen B, Maurer K, Hafner H, Gaebel W, Moller HJ, Maier W, Klosterkotter J. Acute effects of treatment for prodromal symptoms for people putatively in a late initial prodromal state of psychosis. Br. J. Psychiatry Suppl. 2007;51:s88–s95. doi: 10.1192/bjp.191.51.s88. [DOI] [PubMed] [Google Scholar]
- Ruhrmann S, Schultze-Lutter F, Klosterkotter J. Probably at-risk, but certainly ill—advocating the introduction of a psychosis spectrum disorder in DSM-V. Schizophr. Res. 2010;120:23–37. doi: 10.1016/j.schres.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- Tsai GE, Lin P-Y. Strategies to enhance N-methyl-D-aspartate receptor-mediated neurotransmission in schizophrenia, a critical review and meta-analysis. Curr. Pharm. Des. 2010;16:522–537. doi: 10.2174/138161210790361452. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Martin-Facklam M, Pizzagalli F, Youssef E, Yoo K, Dorflinger E, Bausch A, Arrowsmith R, Alberati D, Santarelli L. Glycine transporter type 1 (Glyt1) inhibitor Rg1678: results of the proof-of-concept study for the treatment of negative symptoms in schizophrenia. Schizophr. Bull. 2011;37:324. [Google Scholar]
- Woods SW, Miller TJ, McGlashan TH. The “prodromal” patient: both symptomatic and at-risk. CNS Spectr. 2001;6:223–232. doi: 10.1017/s1092852900008609. [DOI] [PubMed] [Google Scholar]
- Woods SW, Breier A, Zipursky RB, Perkins DO, Addington J, Miller TJ, Hawkins KA, Marquez E, Lindborg SR, Tohen M, McGlashan TH. Randomized trial of olanzapine versus placebo in the symptomatic acute treatment of the schizophrenic prodrome. Biol. Psychiatry. 2003;54:453–464. doi: 10.1016/s0006-3223(03)00321-4. [DOI] [PubMed] [Google Scholar]
- Woods SW, Gueorguieva RV, Baker CB, Makuch RW. Control group bias in randomized atypical antipsychotic medication trials for schizophrenia. Arch. Gen. Psychiatry. 2005;62:961–970. doi: 10.1001/archpsyc.62.9.961. [DOI] [PubMed] [Google Scholar]
- Woods SW, Tully EM, Walsh BC, Hawkins KA, Callahan JL, Cohen SJ, Mathalon DH, Miller TJ, McGlashan TH. Aripiprazole in the treatment of the psychosis prodrome: an open label pilot study. Br. J. Psychiatry. 2007;191(51):s96–s101. doi: 10.1192/bjp.191.51.s96. [DOI] [PubMed] [Google Scholar]
- Woods SW, Addington J, Cadenhead KS, Cannon TD, Cornblatt BA, Heinssen R, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, McGlashan TH. Validity of the prodromal risk syndrome for psychosis: findings from North American Prodrome Longitudinal Study. Schizophr. Bull. 2009;35:894–908. doi: 10.1093/schbul/sbp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SW, McGlashan TH. Fish Oil to Prevent Psychosis (Electronic Letter). Arch Gen Psychiatry 23 February 2010. 2010 [Google Scholar]
- Woods SW, Walsh BC, Saksa JR, McGlashan TH. The case for including Attenuated Psychotic Symptoms Syndrome in DSM-5 as a psychosis risk syndrome. Schizophr. Res. 2010;123:199–207. doi: 10.1016/j.schres.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung AR, McGorry PD, McFarlane CA, Jackson HJ, Patton GC, Rakkar A. Monitoring and care of young people at incipient risk of psychosis. Schizophr. Bull. 1996;22:283–303. doi: 10.1093/schbul/22.2.283. [DOI] [PubMed] [Google Scholar]
- Yung AR, Phillips LJ, Nelson B, Francey SM, PanYuen H, Simmons MB, Ross ML, Kelly D, Baker K, Amminger GP, Berger G, Thompson AD, Thampi A, McGorry PD. Randomized controlled trial of interventions for young people at ultra high risk for psychosis: 6-month analysis. J. Clin. Psychiatry. 2011;72:430–440. doi: 10.4088/JCP.08m04979ora. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.