Summary
Using US SEER17 Registry data, age-specific melanoma incidence rates were calculated and comparisons were made between males and females. Relative Risk (RR) for males and females in each age group was computed and compared with that from Nordic Cancer Registry data set and to that for non-melanoma skin cancer (NMSC). For age groups 44 and younger, females showed higher incidence rates, with a peak difference at age 20–24 (RR = 2.01, 95% CI = 1.21–3.33). Males exhibited higher incidence rates after age 44. The same bimodal gender difference was confirmed by the Nordic Cancer Registry data set, but it was not observed for NMSC, which is known to be strongly associated with cumulative exposure to solar UV radiation. We conclude that exposure to solar ultraviolet (UV) radiation is the major causative factor for melanoma at older age (>44 yr), but that other factors may play a role in early onset melanomas, particularly in females.
Keywords: melanoma, gender, hormones, epidemiology, disparity
Introduction
Melanoma incidence rates ranked number 5 in both men and women in the United States during 2004–2008, and it is one of the few cancers that has consistently been increasing in recent decades (Howlader et al., 2010). Epidemiologic and molecular studies have demonstrated that solar ultraviolet (UV) radiation is an important environmental risk factor for melanoma (Fears and Tucker, 2005; Leiter and Garbe, 2008; Markovic et al., 2007). In addition, genetic factors, particularly those that determine skin type, hair color, number of nevi, and a family history of melanoma have been found to be associated with increased risk of melanoma (Caini et al., 2009). Other environmental factors may be implicated, but the evidence is less defined, which include exposure to heavy metals and insecticides (Dennis et al., 2010; Meyskens and Yang, 2011). Hormones are implicated in the risk and progression of several gender-specific malignancies such as breast cancer and prostate cancer (Ahmad and Kumar, 2011; Cordera and Jordan, 2006; Kassi and Moutsatsou, 2011; Perry et al., 2006). Considerable evidence has documented that the melanocytes are responsive to neuroendocrine signals and sex hormones (Slominski, 2009; Slominski and Wortsman, 2000; Slominski et al., 2004). Over 30 years ago, Lee and Storer reported that at younger ages, the incidence and mortality from melanoma was higher in British women than for British men, and that the differences diminished with age (Lee and Storer, 1980); however, no explanation for this finding was clearly demonstrated although a great deal of scientific inquiring into the issue of endocrine responsiveness of melanoma was initiated including our own work (Fuller and Meyskens, 1981; Lens and Bataille, 2008; Meyskens and Salmon, 1981).
Recent studies using the California Cancer Registry data revealed that higher socioeconomic status was associated with increased melanoma risk, especially in young women (Hausauer et al., 2011). The cause was attributed, at least in part, to UV exposure including both solar UV and artificial UV tanning bed use (Coelho and Hearing, 2009). The melanoma incidence rate in young women has been increasing over the past three decades (age-adjusted rate from 8.1 in 1975 to 17.4 in 2008, age 20–49, from SEER 9 data) (Bradford et al., 2010; Purdue et al., 2008); but the incidence rate for young men did not increase as much (age-adjusted from 8.3 in 1975 to 12.5 in 2008, age 20–49, SEER 9 data). However, for all ages combined, the age-adjusted melanoma incidence rate is higher in men than in women. The purpose of this study is to examine the difference in age-specific melanoma incidence rates between men and women and examine possible gender-specific risk factors in addition to UV radiation. Our study suggests that factors other than UV exposure may affect the onset of melanoma in young women.
Results
Bimodal gender difference in melanoma across age groups
Analysis of all skin melanoma data from SEER17 revealed that there was an age-related bimodal distribution pattern for both genders. The age and sex distributions of the SEER17 population are described in Figure 1A. The race ethnicity distribution of melanoma cases included 88.1% White, 5.8% Black, and 6.1% ‘Other’. Figure 1B listed the sex and age distribution from the Nordic population.
Figure 1.
The study populations. (A) The gender and age distribution of SEER17 population from 1973 to 2008 (in personyears). (B) The gender and age distribution of Nordic population (1980–2006, in personyears).
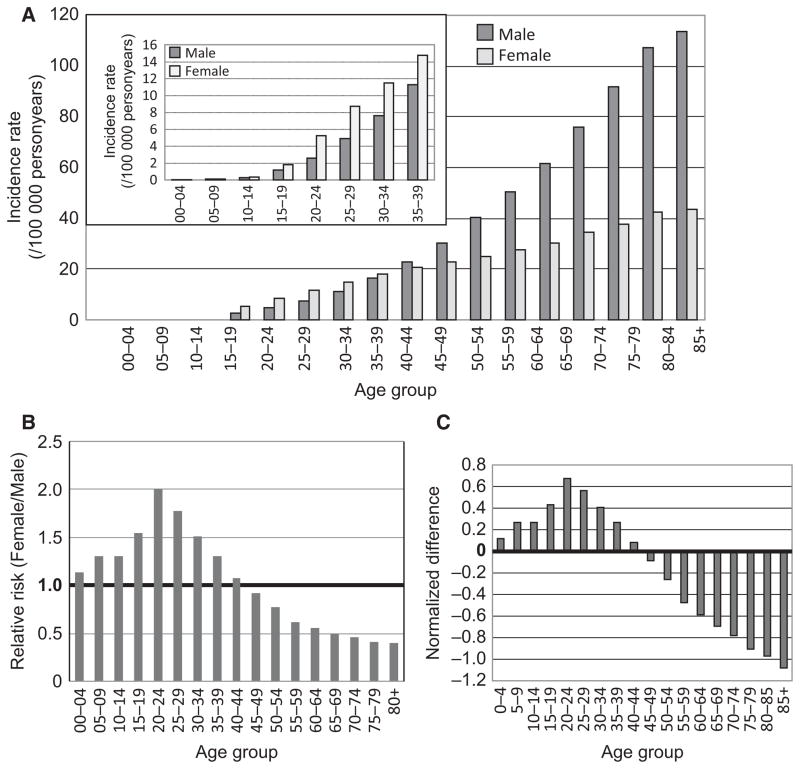
The female incidence rates are higher than that in males for all age groups from birth to age 44 (Table 1 and Figure 2A). Starting from age 10–14, the difference is significant (P < 0.05, Table 1). The age-specific incidence rates (IR) for females (IR = 5.23) in the 20–24 yr age group was double of that in males in the same age group (IR = 2.61) (Table 1). Figure 2A shows the graphic difference of the age-specific incidence rates between males and females. Figure 2B shows the relative risk (RR) for females as compared to males in each age group, graphed from Table 1. Females in the 20–24 age group showed twice the risk for developing melanoma compared with males in this age group.
Table 1.
SEER 17 melanoma case numbers, incidence rates, RRs and 95% CI of RRs, NDs and 95% CI of NDs
| Age group | Case number
|
Incidence rates
|
P-value | RR (F/M)
|
ND (F versus M)
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | M | F | RR | 95% CI | ND | 95% CI | ||||
| 00–04 | 39 | 42 | 0.07 | 0.08 | 0.592 | 1.13 | 0.66 | 1.92 | 0.119 | −16.25 | 16.51 |
| 05–09 | 56 | 70 | 0.11 | 0.14 | 0.133 | 1.31 | 0.77 | 2.2 | 0.268 | −7.09 | 7.57 |
| 10–14 | 147 | 184 | 0.27 | 0.36 | 0.014 | 1.31 | 0.78 | 2.19 | 0.27 | −3.53 | 4.10 |
| 15–19 | 623 | 918 | 1.16 | 1.8 | <0.001 | 1.55 | 0.93 | 2.57 | 0.432 | −0.75 | 1.62 |
| 20–24 | 1 414 | 2 706 | 2.61 | 5.23 | <0.0001 | 2.01 | 1.21 | 3.33 | 0.675 | 0.19 | 1.16 |
| 25–29 | 2 749 | 4 739 | 4.9 | 8.72 | <0.0001 | 1.78 | 1.07 | 2.96 | 0.564 | 0.14 | 0.98 |
| 30–34 | 4 257 | 6 307 | 7.61 | 11.51 | <0.0001 | 1.51 | 0.91 | 2.51 | 0.409 | −0.07 | 0.89 |
| 35–39 | 6 071 | 7 884 | 11.25 | 14.74 | <0.0001 | 1.31 | 0.79 | 2.18 | 0.269 | −0.35 | 0.89 |
| 40–44 | 8 391 | 9 160 | 16.48 | 17.84 | <0.0001 | 1.08 | 0.65 | 1.8 | 0.079 | −1.79 | 1.95 |
| 45–49 | 10 418 | 9 769 | 22.59 | 20.72 | <0.0001 | 0.92 | 0.55 | 1.52 | −0.087 | −1.69 | 1.51 |
| 50–54 | 12 080 | 9 648 | 30.07 | 23.05 | <0.0001 | 0.77 | 0.46 | 1.27 | −0.265 | −0.77 | 0.24 |
| 55–59 | 13 459 | 8 847 | 40.25 | 24.89 | <0.0001 | 0.62 | 0.37 | 1.03 | −0.475 | −0.76 | −0.19 |
| 60–64 | 13 488 | 8 158 | 50.53 | 27.78 | <0.0001 | 0.55 | 0.33 | 0.91 | −0.589 | −0.83 | −0.35 |
| 65–69 | 13 184 | 7 671 | 61.62 | 30.51 | <0.0001 | 0.50 | 0.3 | 0.82 | −0.694 | −0.91 | −0.48 |
| 70–74 | 12 938 | 7 527 | 75.82 | 34.58 | <0.0001 | 0.46 | 0.27 | 0.76 | −0.782 | −0.98 | −0.59 |
| 75–79 | 11 862 | 6 932 | 92.12 | 37.73 | <0.0001 | 0.41 | 0.25 | 0.68 | −0.904 | −1.09 | −0.72 |
| 80–84 | 8 762 | 5 774 | 107.35 | 42.61 | <0.0001 | 0.40 | 0.24 | 0.66 | −0.967 | −1.18 | −0.76 |
| 85+ | 6 295 | 5 466 | 113.39 | 43.42 | <0.0001 | 0.38 | 0.23 | 0.64 | −1.079 | −1.32 | −0.85 |
| All age | 126 233 | 101 802 | 18.15 | 14.25 | <0.0001 | 0.79 | 0.47 | 1.3 | −0.241 | −0.41 | −0.07 |
M, Male; F, Female.
Figure 2.
The gender difference of melanoma in different age groups. (A) age-specific incidence rates (per 100 000 personyears) for males and females from US SEER17 data set. The figure inside is on a different scale. (B) Age-specific relative risks for females as compared to males. (C) normalized difference (ND = (IRF−IRM)/IRT)) between male and female for each age group.
Melanoma incidence rate increases with age (Jemal et al., 2009), as also shown in our data (Figure 2A). We used a ‘normalized difference’ (ND) to describe the difference between male and female incidence rates. Specifically, ND was obtained by using the IR for females (IRF) minus IR for males (IRM) for each age group; this difference was then divided by the total incidence rate for both sexes (IRT) for that age group (ND = (IRF−IRM)/IRT). ND enables us to directly compare the IR difference between genders among different age groups (Figure 2C, Table 1). The ND increases with age up to 40–44 yr age group, with a peak ND at 20–24 yr; and then drops after age 45–49 (Figure 2C).
The bimodal gender difference persists in various diagnosis years, melanoma body sites, and histologic types
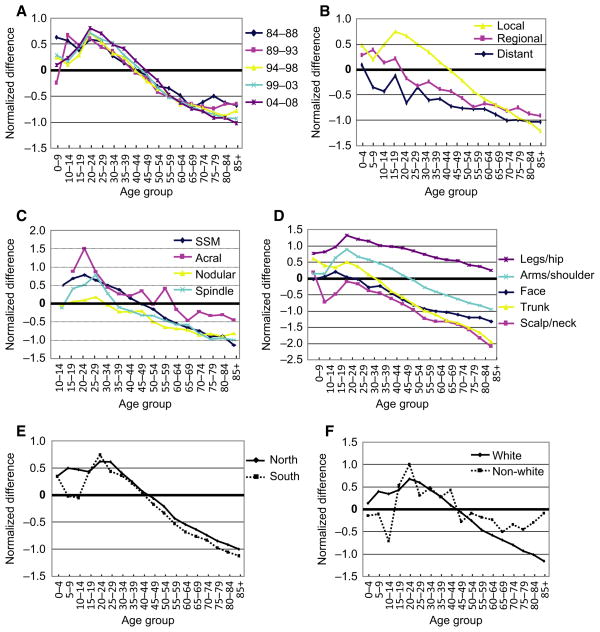
A bimodal gender difference in ND is observed in all years examined (1984–2008) (Figure 3A), which was also observed in localized melanomas, with the same peak difference in the 20–24 yr group (Figure 3B). No bimodal pattern was observed in cases with regional or distant disease (Figure 3B). In both regional and distant cases, the NDs exhibited a continuous decreasing trend for all ages (Figure 3B). In addition, as shown in Figure 3C, superficial spreading melanoma (SSP) (ICD-0-3 code 8743/3), acral melanoma (ICD-0-3 code 8744/3), and spindle cell melanoma (ICD-0-3 codes 8770, 8771, 8772) all exhibited bimodal patterns for ND along the age axis. In contrast, nodular melanoma did not show such a bimodal pattern.
Figure 3.
Detailed analysis of SEER17 data set for different years of diagnosis, histology subtypes, disease stages, body sites, geographic location, and ethnic groups. Normalized gender difference in incidence rates for (A), every 5-yr intervals from 1984 to 2008; (B) different disease stages; (C) different histology subtypes; (D) different body sites; (E) northern and southern areas; and (F) Caucasian and non-Caucasian groups.
Anatomical sites of melanoma are linked to various degrees of sun exposure (Curtin et al., 2005). As shown in Figure 3D, the incidence rates for hips and legs are higher in females than in males for all age groups. Again, the peak difference is also in the age group 20–24 yr. In contrast, the incidence rates for head and neck (including face) are higher in males than in females. Melanomas occurring on the arms/shoulders and trunk followed a similar bimodal pattern to the whole population as seen in Figure 2C.
The geographic locations do not affect the bimodal pattern
Geographic location is a known factor that impacts melanoma incidence rate (Haynes et al., 2008; Lee, 1997), which may be attributable to the difference in UV radiation and temperature. The average annual UV radiation for northern area and southern area is quite different according to US Environmental Protection Agency (http://www.epa.gov/sunwise/uvimonth.html); however, the bimodal distribution of NDs between males and females did not show a dramatic difference between the northern and southern areas (Figure 3E). These results further suggest that causes in addition to UV radiation exposure may be associated with the pathogenesis of melanoma
The bimodal pattern is observed in Caucasian and non-Caucasian populations
Caucasians have a higher incidence of melanomas than other ethnic groups due to their light skin color (hence, less protection from solar UV radiation) (Tadokoro et al., 2005). As shown in Figure 3F, Caucasians showed a typical bimodal difference in NDs along the age axis, with a peak in the 20–24 yr group. Non-Caucasians (including ‘Black’ and ‘other’ category in the SEER17 database) accounted for about 12% of the whole SEER17 population. The overall melanoma incidence rate for non-Caucasians is much lower than that in Caucasians. However, when we used the ND to compare different ethnic groups, we found a similar bimodal pattern for non-Caucasians as well (Figure 5F).
Figure 5.
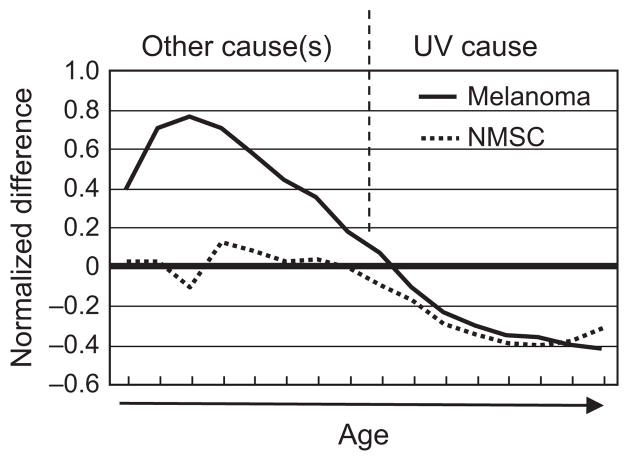
The bimodal etiology of melanoma. Melanomas in young population may have other causes than UV radiation while melanomas in older population may have a predominantly UV cause.
The bimodal gender difference is not observed in non-melanoma skin cancer (NMSC) in Nordic Cancer Registry data (1980–2006)
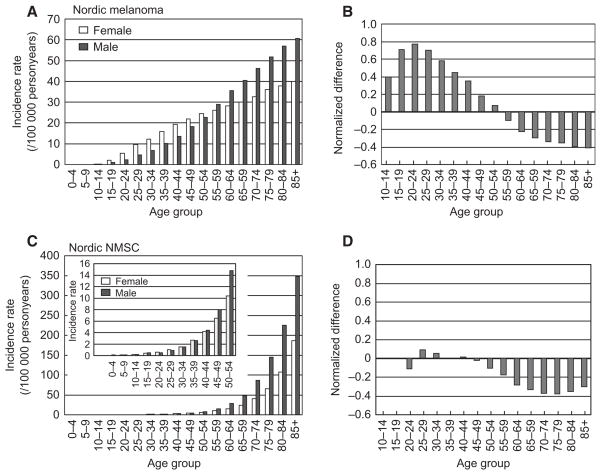
NMSC is another skin cancer that is caused by solar UV radiation. To compare the age- and sex-specific incidence rates between NMSC and melanoma, we used the Nordic Cancer Registry data because NMSC is not reportable in the SEER registries. As shown in Figure 4A, melanoma incidence rates increase with age for both genders in the Nordic Cancer Registry data (1980–2006). Again, it is higher in females than in males for groups younger than 50–54 yr; and the trend reverses in the 55–59 age group (Figure 4A). This represents 10 yr of age delay for the reversal of the trend as observed in US SEER17 population (compare with Figure 2C). However, the peak difference in melanoma incidence and the bimodal pattern is persistent. In contrast to melanoma, the incidence rates for NMSC did not show much difference in the younger age groups (<50 yr) (Figure 4C), and there was no bimodal pattern along the age axis (Figure 4D). Table 2 lists the age-specific IRs and RRs for females at different age groups (as compared to males) for NMSC and melanoma. While RR is about 1.0 for NMSCs at age 15–29 (reflecting a roughly equal incidence rate), RRs for melanomas in these age groups are greater than 2.0 (Table 2, in bold).
Figure 4.
The bimodal pattern was confirmed in Nordic population but not shown for Non-melanoma skin cancer. (A) age-specific melanoma incidence rates in the Nordic population for men and women (1980–2006). (B) The normalized gender difference in incidence rates in Nordic countries (1980–2006). (C) age-specific gender difference in NMSC in Nordic Cancer Registry. The figure within the panel is on a different scale showing subtle differences for younger age groups. (D) The normalized gender difference in incidence rates in NMSC in Nordic countries (1980–2006).
Table 2.
RRs for melanoma and NMSC in the Nordic population
| Age group | Incidence rate (melanoma)
|
Incidence rate (NMSC)
|
RR (F/M) Melanoma
|
RR (F/M) NMSC
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | F | M | F | RR | 95% CI | RR | 95% CI | |||
| 00–09 | 0.02 | 0.03 | 0.04 | 0.03 | 1.26 | 0.54 | 2.92 | 0.72 | 0.34 | 1.56 |
| 10–14 | 0.18 | 0.24 | 0.10 | 0.11 | 1.33 | 0.87 | 2.03 | 1.10 | 0.60 | 2.00 |
| 15–19 | 0.83 | 1.84 | 0.16 | 0.19 | 2.22 | 1.85 | 2.65 | 1.17 | 0.74 | 1.84 |
| 20–24 | 2.10 | 4.89 | 0.42 | 0.36 | 2.33 | 2.09 | 2.61 | 0.86 | 0.64 | 1.17 |
| 25–29 | 4.08 | 8.66 | 0.47 | 0.51 | 2.12 | 1.96 | 2.30 | 1.09 | 0.84 | 1.42 |
| 30–34 | 5.93 | 10.93 | 0.71 | 0.87 | 1.84 | 1.73 | 1.97 | 1.23 | 1.00 | 1.51 |
| 35–39 | 8.78 | 13.73 | 1.29 | 1.39 | 1.56 | 1.48 | 1.65 | 1.08 | 0.93 | 1.27 |
| 40–44 | 12.21 | 17.40 | 2.33 | 2.42 | 1.43 | 1.36 | 1.50 | 1.04 | 0.92 | 1.17 |
| 45–49 | 16.64 | 19.90 | 4.00 | 3.89 | 1.20 | 1.14 | 1.25 | 0.97 | 0.88 | 1.07 |
| 50–54 | 20.62 | 22.16 | 7.04 | 5.96 | 1.07 | 1.03 | 1.12 | 0.85 | 0.78 | 0.92 |
| 55–59 | 26.12 | 23.50 | 13.35 | 9.44 | 0.90 | 0.86 | 0.94 | 0.71 | 0.66 | 0.75 |
| 60–64 | 31.96 | 25.11 | 24.62 | 14.01 | 0.79 | 0.75 | 0.82 | 0.57 | 0.54 | 0.60 |
| 65–69 | 36.58 | 26.99 | 43.07 | 21.80 | 0.74 | 0.71 | 0.77 | 0.51 | 0.49 | 0.53 |
| 70–74 | 42.67 | 29.38 | 78.24 | 36.31 | 0.69 | 0.66 | 0.72 | 0.46 | 0.45 | 0.48 |
| 75–79 | 48.53 | 32.83 | 133.23 | 59.75 | 0.68 | 0.65 | 0.71 | 0.45 | 0.44 | 0.46 |
| 80–84 | 54.10 | 35.02 | 208.86 | 99.69 | 0.65 | 0.62 | 0.68 | 0.48 | 0.46 | 0.49 |
| 85+ | 57.15 | 37.31 | 324.76 | 175.74 | 0.65 | 0.61 | 0.69 | 0.54 | 0.53 | 0.56 |
| All age | 13.83 | 14.87 | 17.86 | 13.95 | 1.08 | 1.06 | 1.09 | 0.78 | 0.77 | 0.79 |
Discussion
Using the US SEER17 data set, we observed a significant bimodal difference in melanoma incidence rates between males and females along the age axis. This bimodal pattern of the normalized difference was confirmed in the Nordic melanoma cases but not observed in the Nordic NMSC cases. NMSC is known to be caused by UV radiation. If UV radiation is the only risk factor for both melanoma and NMSC, we would expect to observe a similar pattern for the age-specific rates in males and females. That is not the case. Hence, the unique bimodal pattern for the difference in male and female incidence rates in melanoma suggests that additional gender-related factors play a role in early onset melanoma etiology.
We propose that an etiology model in US population younger than 45 yr must include gender-related factors such as endogenous hormones, pregnancy-associated issues [such as immune suppression and reactive oxygen species (ROS)] in concert with UV radiation (Figure 5). However, after age 45–49 yr, UV-caused skin damage accumulates and contributes more to causation. (Figure 5). This is consistent with the observations that mutations need time to accumulate and exert their effects. In the HGF/SF melanoma mouse model, neonatal UV radiation caused melanomas to occur around 6 month of age (Noonan et al., 2003), which roughly equals a human age of 20, assuming a life span of 24 months for mice and 80 yr for humans. It has been calculated that, in the United States, only 25% of a person’s lifetime solar UV exposure is obtained before age 18 (Godar et al., 2003); hence, it is unlikely that melanoma incidence peak difference at age 20–24 would be caused by cumulative solar UV radiation in the population.
Melanoma incidence rate did not show a dramatic gender difference among children younger than 9 yr, the significant difference started during the onset of puberty (Table 1), which is also associated with dramatic sex hormonal changes. The peak difference of male and female rate was observed at age 20–24, which is also the peak pregnancy age for US women (Ventura et al., 2003). Again, when the difference between men and women incidence diminished, it was at age 45–49 when sex hormones begin to drop dramatically in females due to menopause (Nichols et al., 2006). A decrease in testosterone levels may also contribute to the observed increase in melanoma incidence rates in older men (Kaufman and Vermeulen, 2005). Furthermore, melanins play crucial roles in protecting skin from UV radiation, and it has been demonstrated that melanins are regulated by hormones in mammalian skin (Slominski et al., 2004). In addition, human melanocytes and human skin produce and metabolize steroids including sex hormones and exhibit endocrine activities (Slominski, 2009; Slominski and Wortsman, 2000). Therefore, it is a reasonable hypothesis to include gender-related hormones as contributing factors to pathogenesis.
However, a number of studies have indicated that exogenous hormones including oral contraceptives and hormonal replacement therapy are not risk factors for melanoma (Driscoll and Grant-Kels, 2007). Whether other endogenous hormone levels affect melanoma incidence is less well understood (Gupta and Driscoll, 2010). There have been a few studies examining the role of neuroendocrine factors and its effect in melanocytes (Slominski, 2009; Slominski et al., 2004) as well as the role of pregnancy in melanoma causation (Gallagher et al., 1985; Kaae et al., 2007; Karagas et al., 2006; Lambe et al., 1996; Osterlind et al., 1988). Pregnancy either had no impact or a reduced melanoma risk. However, pregnancy requires routine visiting to the medical office, providing better access to health care and maybe, skin examinations. In addition, pregnancy is associated with immune suppression (Loke and King, 2000) and increased ROS (Al-Gubory et al., 2010; Red-Horse et al., 2004), two risk factors for melanoma (Garibyan and Fisher, 2010; Ullrich, 2007). We have recently demonstrated that superoxide-generating enzyme NADPH Oxidase 1 (Nox1) is expressed in normal human melanocytes and increased in melanoma cells (Liu et al., 2012), which could be a source of ROS after UV radiation as well.
The observed difference in melanoma incidence rates may also reflect the difference between male and female skin and hair. Sex hormones can be synthesized locally by skin and play key roles in maintaining skin homeostasis and hair types (Dao and Kazin, 2007; Slominski and Wortsman, 2000). Females have more vellus hair and intermediately developed hair which are finer, lighter, and closer to the surface of the skin than males whose hairs are usually more course, darker, and deeper (Garcia et al., 2010). Hence, female hair follicles and the stem cell niche may be more susceptible to solar UV radiation.
Nordic countries receive less annual UV radiation than the United States which may account for the fact that the melanoma incidence rate is roughly equal to that of NMSC in the Nordic countries, while the melanoma rate in United States is estimated to be about 1/10 of NMSC (Rogers et al., 2010). The difference in the ratio of melanoma and NMSC rates also suggests that there are unique factors other than UV radiation that play a role in melanocyte carcinogenesis.
In summary, our hypothesis that melanomas in younger patients result from additional and perhaps multiple causative contributions compared with those in older patients may provide a foundation for further investigations of hormonal changes and other risk factors over life time developmental stages. Further understanding of how these factors interact with UV radiation should enhance our understanding of melanoma etiology and may present an opportunity for differential prevention and perhaps treatment for melanoma occurring at different ages.
Methods
Databases and population
The melanoma patient information in SEER17 database was retrieved using SEER*STAT software (version 7.0.4; Surveillance Research Program, National Cancer Institute, Bethesda, MD, USA). All records of skin melanoma were retrieved, total 228 035 cases from 1973 to 2008. The data were imported into Statistical Analysis Software (SAS) system, and analysis was performed using SAS9.2 software (SAS Institute Inc., Cary, North Carolina, USA). The US population data were downloaded from SEER website (http://seer.cancer.gov/popdata/download.html) and processed by SAS9.2. The sums of age-specific populations from all counties covered by SEER registries were calculated and used as a base for age-specific melanoma incidence rate calculation. The northern area include Alaska, Detroit, Iowa, Utah, Connecticut, San Francisco-Oakland, San Jose-Monterey, New Jersey, and Seattle-Puget Sound area – roughly all SEER registry covered regions north of latitude 37°N; the southern area include the rest of SEER registry covered regions including Atlanta, Greater Georgia, Rural Georgia, Kentucky, New Mexico Los Angeles, Hawaii, and Louisiana. The Greater California registry was not included in this analysis because of its longitudinal spanning, which includes the North Region, the Central region, the Sacramento region, the Tri-County region, the Desert Sierra region, and Imperial, San Diego, and Orange Counties.
The age-specific melanoma and non-melanoma skin cancer (NMSC) incidence rates in Nordic countries were calculated using NORDCAN software (http://www.ancr.nu/nordcan_on_the_web.asp). The Nordic countries include Denmark, Faroe Islands, Finland, Iceland, Norway, and Sweden, covering diagnosis years from 1980 to 2006. Total melanoma new cases during that period was 100 836 (53 250 women and 47 586 men) and total NMSC new cases was 110 163 cases (48 865 women and 61 298 men). The Nordic population from 1980 to 2006 was downloaded from NORDCAN website: (http://www-dep.iarc.fr/NORDCAN/english/frame.asp). Nordic age-specific melanoma incidence rates were used to validate the bimodal gender difference observed in US SEER17 database; and the age-specific NMSC incidence rates were calculated for a comparison with that of melanoma.
Statistical analysis
Poisson distribution-based methods were used to calculate the significance of the difference between male and female incidence rates in each age group according to (Sahai and Kurshid, 1996). Relative risk (RR) for females as compared to males at each age group was calculated by using female rates divided by male rates. The 95% confidence interval of RRs and NDs were also calculated according to Saihai and Kurshid based on Poisson distribution (Sahai and Kurshid, 1996). The NDs were calculated as outlined in the results section, and the 95% confidence intervals were computed by delta method.
Significance.
Over the past several decades, the incidence rate of melanoma continues to increase in the United States and solar UV radiation has been considered to be the crucial or only etiological factor. In this study, we present evidence that UV may play a less pivotal role in early onset melanomas, as gender-related factors appear to play an important role in younger age individuals. This observation challenges the paradigm that UV is the only causative factor in melanoma etiology and may provide a base for other prevention strategies.
Acknowledgments
This research is supported in part by CA62230 to F.L.M and the Waltmar and Oxnard Foundations to FLM, and by UC Irvine School of Medicine Faculty Research Grant to F.L. and F.L.M..
References
- Ahmad N, Kumar R. Steroid hormone receptors in cancer development: a target for cancer therapeutics. Cancer Lett. 2011;300:1–9. doi: 10.1016/j.canlet.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Al-Gubory KH, Fowler PA, Garrel C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int J Biochem Cell Biol. 2010;42:1634–1650. doi: 10.1016/j.biocel.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Bradford PT, Anderson WF, Purdue MP, Goldstein AM, Tucker MA. Rising melanoma incidence rates of the trunk among younger women in the United States. Cancer Epidemiol Biomarkers Prev. 2010;19:2401–2406. doi: 10.1158/1055-9965.EPI-10-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caini S, Gandini S, Sera F, Raimondi S, Fargnoli MC, Boniol M, Armstrong BK. Meta-analysis of risk factors for cutaneous melanoma according to anatomical site and clinico-pathological variant. Eur J Cancer. 2009;45:3054–3063. doi: 10.1016/j.ejca.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Coelho SG, Hearing VJ. UVA tanning is involved in the increased incidence of skin cancers in fair-skinned young women. Pigment Cell Melanoma Res. 2009;23:57–63. doi: 10.1111/j.1755-148X.2009.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordera F, Jordan VC. Steroid receptors and their role in the biology and control of breast cancer growth. Semin Oncol. 2006;33:631–641. doi: 10.1053/j.seminoncol.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–2147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- Dao H, Jr, Kazin RA. Gender differences in skin: a review of the literature. Gend Med. 2007;4:308–328. doi: 10.1016/s1550-8579(07)80061-1. [DOI] [PubMed] [Google Scholar]
- Dennis LK, Lynch CF, Sandler DP, Alavanja MC. Pesticide use and cutaneous melanoma in pesticide applicators in the agricultural heath study. Environ Health Perspect. 2010;118:812–817. doi: 10.1289/ehp.0901518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll MS, Grant-Kels JM. Hormones, nevi, and melanoma: an approach to the patient. J Am Acad Dermatol. 2007;57:919–931. doi: 10.1016/j.jaad.2007.08.045. quiz 932–6. [DOI] [PubMed] [Google Scholar]
- Fears TR, Tucker MA. Re: sun exposure and mortality from melanoma. J Natl Cancer Inst. 2005;97:1789–1790. doi: 10.1093/jnci/dji410. author reply 1791. [DOI] [PubMed] [Google Scholar]
- Fuller BB, Meyskens FL., Jr Endocrine responsiveness in human melanocytes and melanoma cells in culture. J Natl Cancer Inst. 1981;66:799–802. [PubMed] [Google Scholar]
- Gallagher RP, Elwood JM, Rootman J, Spinelli JJ, Hill GB, Threlfall WJ, Birdsell JM. Risk factors for ocular melanoma: Western Canada Melanoma Study. J Natl Cancer Inst. 1985;74:775–778. [PubMed] [Google Scholar]
- Garcia AM, Mclaren CE, Meyskens FL., Jr Melanoma: is hair the root of the problem? Pigment Cell Melanoma Res. 2010;24:110–118. doi: 10.1111/j.1755-148X.2010.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garibyan L, Fisher DE. How sunlight causes melanoma. Curr Oncol Rep. 2010;12:319–326. doi: 10.1007/s11912-010-0119-y. [DOI] [PubMed] [Google Scholar]
- Godar DE, Urbach F, Gasparro FP, Van Der Leun JC. UV doses of young adults. Photochem Photobiol. 2003;77:453–457. doi: 10.1562/0031-8655(2003)077<0453:udoya>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Gupta A, Driscoll MS. Do hormones influence melanoma? Facts and controversies Clin Dermatol. 2010;28:287–292. doi: 10.1016/j.clindermatol.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Hausauer AK, Swetter SM, Cockburn MG, Clarke CA. Increases in melanoma among adolescent girls and young women in California: trends by socioeconomic status and UV radiation exposure. Arch Dermatol. 2011;147:783–789. doi: 10.1001/archdermatol.2011.44. [DOI] [PubMed] [Google Scholar]
- Haynes R, Pearce J, Barnett R. Cancer survival in New Zealand: ethnic, social and geographical inequalities. Soc Sci Med. 2008;67:928–937. doi: 10.1016/j.socscimed.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2008. National Cancer Institute; Bethesda, MD: 2010. [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- Kaae J, Andersen A, Boyd HA, Wohlfahrt J, Melbye M. Reproductive history and cutaneous malignant melanoma: a comparison between women and men. Am J Epidemiol. 2007;165:1265–1270. doi: 10.1093/aje/kwm015. [DOI] [PubMed] [Google Scholar]
- Karagas MR, Zens MS, Stukel TA, et al. Pregnancy history and incidence of melanoma in women: a pooled analysis. Cancer Causes Control. 2006;17:11–19. doi: 10.1007/s10552-005-0281-y. [DOI] [PubMed] [Google Scholar]
- Kassi E, Moutsatsou P. Glucocorticoid receptor signaling and prostate cancer. Cancer Lett. 2011;302:1–10. doi: 10.1016/j.canlet.2010.10.020. [DOI] [PubMed] [Google Scholar]
- Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26:833–876. doi: 10.1210/er.2004-0013. [DOI] [PubMed] [Google Scholar]
- Lambe M, Thorn M, Sparen P, Bergstrom R, Adami HO. Malignant melanoma: reduced risk associated with early childbearing and multiparity. Melanoma Res. 1996;6:147–153. [PubMed] [Google Scholar]
- Lee JA. Declining effect of latitude on melanoma mortality rates in the United States. A preliminary study. Am J Epidemiol. 1997;146:413–417. doi: 10.1093/oxfordjournals.aje.a009294. [DOI] [PubMed] [Google Scholar]
- Lee JA, Storer BE. Excess of malignant melanomas in women in the British Isles. Lancet. 1980;2:1337–1339. doi: 10.1016/s0140-6736(80)92401-0. [DOI] [PubMed] [Google Scholar]
- Leiter U, Garbe C. Epidemiology of melanoma and nonmelanoma skin cancer–the role of sunlight. Adv Exp Med Biol. 2008;624:89–103. doi: 10.1007/978-0-387-77574-6_8. [DOI] [PubMed] [Google Scholar]
- Lens M, Bataille V. Melanoma in relation to reproductive and hormonal factors in women: current review on controversial issues. Cancer Causes Control. 2008;19:437–442. doi: 10.1007/s10552-008-9110-4. [DOI] [PubMed] [Google Scholar]
- Liu F, Gomez Garcia AM, Meyskens FL., Jr NADPH oxidase 1 overexpression enhances invasion via matrix metalloproteinase-2 and epithelial-mesenchymal transition in melanoma cells. J Invest Dermatol. 2012;132:2033–2044. doi: 10.1038/jid.2012.119. [DOI] [PubMed] [Google Scholar]
- Loke YW, King A. Immunology of implantation. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14:827–837. doi: 10.1053/beog.2000.0122. [DOI] [PubMed] [Google Scholar]
- Markovic SN, Erickson LA, Rao RD, et al. Malignant melanoma in the 21st century, part 1: epidemiology, risk factors, screening, prevention, and diagnosis. Mayo Clin Proc. 2007;82:364–380. doi: 10.4065/82.3.364. [DOI] [PubMed] [Google Scholar]
- Meyskens FL, Jr, Salmon SE. Inhibition of human tumor colony formation by retinoids. Ann N Y Acad Sci. 1981;359:414. doi: 10.1111/j.1749-6632.1981.tb12776.x. [DOI] [PubMed] [Google Scholar]
- Meyskens FL, Yang S. Thinking about the role (largely ignored) of heavy metals in cancer prevention: hexavalent chromium and melanoma as a case in point. Recent Results Cancer Res. 2011;188:65–74. doi: 10.1007/978-3-642-10858-7_5. [DOI] [PubMed] [Google Scholar]
- Nichols HB, Trentham-Dietz A, Hampton JM, Titus-Ernstoff L, Egan KM, Willett WC, Newcomb PA. From menarche to menopause: trends among US Women born from 1912 to 1969. Am J Epidemiol. 2006;164:1003–1011. doi: 10.1093/aje/kwj282. [DOI] [PubMed] [Google Scholar]
- Noonan FP, Dudek J, Merlino G, De Fabo EC. Animal models of melanoma: an HGF/SF transgenic mouse model may facilitate experimental access to UV initiating events. Pigment Cell Res. 2003;16:16–25. doi: 10.1034/j.1600-0749.2003.00014.x. [DOI] [PubMed] [Google Scholar]
- Osterlind A, Tucker MA, Stone BJ, Jensen OM. The Danish case-control study of cutaneous malignant melanoma. III Hormonal and reproductive factors in women. Int J Cancer. 1988;42:821–824. doi: 10.1002/ijc.2910420603. [DOI] [PubMed] [Google Scholar]
- Perry JK, Emerald BS, Mertani HC, Lobie PE. The oncogenic potential of growth hormone. Growth Horm IGF Res. 2006;16:277–289. doi: 10.1016/j.ghir.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Purdue MP, Freeman LE, Anderson WF, Tucker MA. Recent trends in incidence of cutaneous melanoma among US Caucasian young adults. J Invest Dermatol. 2008;128:2905–2908. doi: 10.1038/jid.2008.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Red-Horse K, Drake PM, Fisher SJ. Human pregnancy: the role of chemokine networks at the fetal-maternal interface. Expert Rev Mol Med. 2004;6:1–14. doi: 10.1017/S1462399404007720. [DOI] [PubMed] [Google Scholar]
- Rogers HW, Weinstock MA, Harris AR, Hinckley MR, Feldman SR, Fleischer AB, Coldiron BM. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283–287. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- Sahai H, Kurshid A, editors. Statistics in Epidemiology: Methods Techniques and Applications. CRC Press; 2000 NW Corporate Blvd, Boca Raton, Florida: 1996. [Google Scholar]
- Slominski A. Neuroendocrine activity of the melanocyte. Exp Dermatol. 2009;18:760–763. doi: 10.1111/j.1600-0625.2009.00892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21:457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- Tadokoro T, Yamaguchi Y, Batzer J, Coelho SG, Zmudzka BZ, Miller SA, Wolber R, Beer JZ, Hearing VJ. Mechanisms of skin tanning in different racial/ethnic groups in response to ultraviolet radiation. J Invest Dermatol. 2005;124:1326–1332. doi: 10.1111/j.0022-202X.2005.23760.x. [DOI] [PubMed] [Google Scholar]
- Ullrich SE. Sunlight and skin cancer: lessons from the immune system. Mol Carcinog. 2007;46:629–633. doi: 10.1002/mc.20328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura SJ, Abma JC, Mosher WD, Henshaw S. Revised pregnancy rates, 1990–97, and new rates for 1998–99: United States. Natl Vital Stat Rep. 2003;52:1–14. [PubMed] [Google Scholar]